Abstract
Cutaneous leishmaniasis (CL) is a major public health problem in tropical and subtropical countries worldwide. Treatment of CL by pentavalent antimony compounds remains a challenge because of limited efficacy, toxic side effects and drug resistance. In the present study, in vitro antileishmanial and cytotoxic activity of garlic extracts against promastigote forms of Leishmania tropica and murine macrophages was evaluated by colorimetric cell viability (MTT) assay. The results revealed that the methanolic and aqueous extracts of garlic were effective in inhibiting promastigote growth of L. tropica with IC50 (50 % inhibitory concentrations) values 12.3 and 19.2 µg/ml, respectively. In addition, methanolic and aqueous extracts of garlic showed low cytotoxicity against murine macrophages with CC50 (cytotoxicity concentration for 50 % of cells) values 291.4 and 348.2 µg/ml, respectively. Findings of present study were the first step in the search for new antileishmanial drugs. However, further works are required to evaluate exact effect of these extracts in volunteer human subjects.
Keywords: Promastigote, Murine macrophage, MTT, Cutaneous leishmaniasis, Cytotoxicity
Introduction
Cutaneous leishmaniasis (CL) is a protozoan infection caused by protozoa of the genus Leishmania. The disease is characterized by chronic skin lesions, leaving permanent scars with deformation of the infected area (WHO 2010). CL is a public health problem on a global level because it affects 1.5 million people annually, worldwide (Desjeux 2004). In Iran, both epidemiological forms of this skin disease are present; anthroponotic CL (ACL) and zoonotic CL (ZCL) caused by Leishmania tropica and Leishmania major, respectively (Sharifi et al. 2012). At present, treatment of leishmaniasis by antimonials drugs such as meglumine antimoniate and sodium stibogluconate, remains a challenge because of limited efficacy, toxic side effects and drug resistance (Croft et al. 2006). Thus, these factors render development of new effective antileishmanial drugs as a requirement. From last decades, natural products and their compounds have been the most productive source for new drug development (Rocha et al. 2005). Garlic (Allium sativum) is a perennial bulb-forming plant that belongs to the family of Liliaceae. It has been known to possess dietary and medicinal properties (Eja et al. 2007). Up to now, antioxidant (Harris et al. 2001), antitumor (Milner 1996), antiviral (Weber et al. 1992; Meng et al. 1993; Waldman 1993), antifungal (Ghannoum 1990; Cai 1991), antibacterial (Ross et al. 2001; Martin and Ernst 2003), antiprotozoal (Soffar and Mokhtar 1991; Harris et al. 2000; Behnia et al. 2008), and antihelminthic (Abdel-Salam et al. 2008; Moazeni and Nazer. 2010) effects of garlic have been shown. However, to the best of our knowledge and according to a survey of the literature, the effect of garlic extract on L. tropica remains mainly unexplored. Therefore, the present study was aimed to evaluate the in vitro antileishmanial potentials of various extracts of garlic (Allium sativum) against L. tropica as pathogenic parasitic strain.
Materials and methods
Chemicals
Meglumine antimoniate (MA, Glucantime) as a control drug was purchased from Rhône, Poulenc, France. Penicillin and streptomycin were obtained from Alborz pharmacy, Karaj, Iran, and were stored at room temperature (25 °C) until testing. MTT powder [3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyl tetrazolium bromide)], fetal calf serum (FCS) and RPMI-1640 medium with l-glutamine were prepared from Sigma-Aldrich (St. Louis, MO, USA). All the other chemicals and solvents were of analytical grade.
Parasite strain
A standard strain of L. tropica (MHOM/IR/2002/Mash2) was obtained from the Center for Research and Training in Skin Diseases and Leprosy (Tehran, Iran). The parasite was cultured in NNN medium, subcultured in RPMI-1640, supplemented with penicillin (200 IU/ml), streptomycin (100 μg/ml), and 15 % heat-inactivated FCS.
Plant collection and authentication
The garlic bulbs were collected from the farms in the Baft district of Kerman province, South East Iran in May 2013. The identities were confirmed by the botanist at the Botany Department of Shahid Bahonar University, Kerman, Iran.
Preparation of extracts
To prepare the methanolic and aqueous extracts, garlic bulbs were peeled, chopped into small pieces, dried under shade, and ground mechanically using a commercial electrical blender. To prepare the methanolic and aqueous extracts, 100 g of dry garlic powder was added to 400 ml of pure methanol and water respectively and then mixed gently for 1 h using a magnetic stirrer. The obtained solution was left at room temperature for 24 h. The solution was stirred again and filtered and then the solvent was removed by evaporation in a rotating evaporator. The remaining semisolid material was then freeze-dried. The obtained residue (5.5 g) was placed into a sterile glass container and stored at 4 °C for further use.
Antileishmanial effects against promastigote forms
In this investigation, colorimetric cell viability (MTT) assay was used to evaluate leishmanicidal effects of garlic extracts on promastigote forms of L. tropica according to the method described elsewhere (Mahmoudvand et al. 2014a). Initially, 100 μl of the promastigotes (106cells/ml) harvested from logarithmic growth phase was added to a 96-well microtiter plate. Then, 100 μl of different concentrations (0–200 µg/ml) of each plant extract was added to each well and incubated at 25 °C ± 1 °C for 72 h. After incubation with the extracts, 10 μl of MTT solution (5 mg/ml) was added to each well and incubated at 25 °C for 4 h. Then, cold isoprppanol was added as a solvent for foramazan crystals to produce purple color. Finally, absorbance was measured by an ELISA reader (BioTek-ELX800) at 490 nm. The promastigotes were cultured in the complete medium with no drug used as positive control and with no promastigotes and drugs as blank. 50 % inhibitory concentrations (IC50 values) were also calculated by probit test in SPSS software.
Isolation of peritoneal murine macrophages
In order to evaluate cytotoxic activity of garlic extracts, peritoneal murine macrophages were collected from male healthy BALB/c mice (4–8 weeks old) by injecting 2–5 ml of cold RPMI-1640 medium into mouse peritoneal cavity. Then, the aspirated macrophages were washed twice and re-suspended in RPMI-1640 medium. The experimental procedures carried out in this survey were in compliance with Standard Guidelines of Ethical Committee of Kerman University of Medical Sciences (Kerman, Iran) for Care and Use of Laboratory Animals.
Cytotoxic activity
To determine cytotoxic effects of garlic extracts, the CC50 (cytotoxicity concentration for 50 % of cells) determination of extracts on peritoneal macrophage cells was carried out by colorimetric cell viability (MTT) assay using the method described by Mahmoudvand et al. (2014b). Briefly, the macrophage cells were plated at 106 cells/ml in 96-well Lab-Tek (Nunc, USA) and left to adhere for 2 h at 37 °C in 5 % CO2. Non-adherent cells were removed by washing with medium after 2 h of incubation in similar conditions. In the next step, 190 μl of complete RPMI medium was added to each well and later 10 μl of dilutions of the extracts (previously prepared in medium) was added. The macrophages were treated with the extracts from 50–1,000 μg/ml for 72 h in 37 °C in 5 % CO2.
Statistical analyses
In this study, all the experiments were repeated in triplicate. We used SPSS software, ver. 17, (SPSS Inc., Chicago) for data entry and statistical analysis and differences between the groups were determined using one-way analysis of variance (ANOVA) test. Moreover, to compare IC50 values of the groups, t test was performed. P value of less than 0.05 was considered statistically significant. The selectivity index (SI) calculated based on the equation of CC50 for peritoneal macrophage cells/IC50 for promastigote forms of L. tropica was used to compare toxicity and activity of the crude extracts, as described by Weninger et al. (2001).
Results
Antileishmanial effects
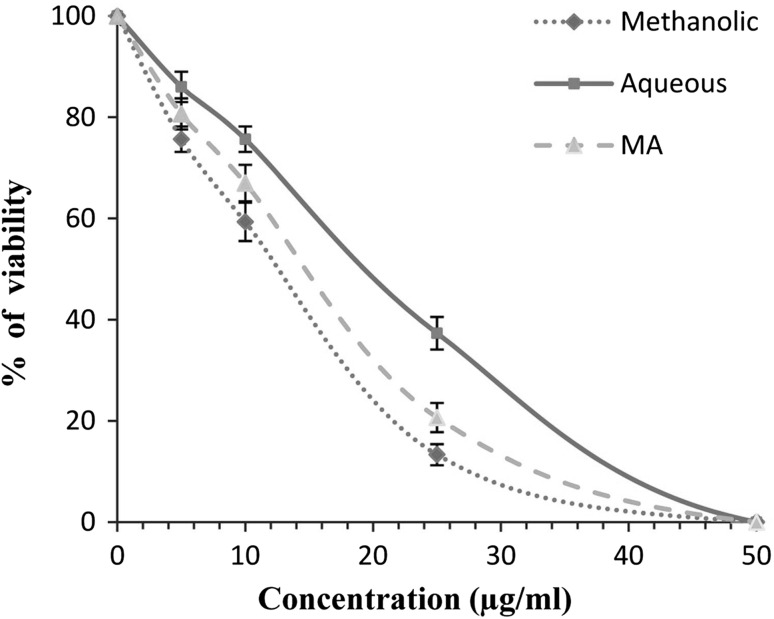
Antileishmanial effects of various extracts of garlic against promastigote forms of L. tropica were determined by MTT assay. As shown in Fig. 1, various extracts of garlic particularly methanolic extract were found to have potent antileishmanial effects against promastigote forms of L. tropica based on a dose-dependent manner (P < 0.05). The measured IC50 values for methanolic and aqueous extracts were 12.3 and 19.2 µg/ml against promastigote forms of L. tropica, respectively. In addition, MA as control drug demonstrated high antileishmanial effects with IC50 value of 15.4 µg/ml against promastigote forms of L. tropica.
Fig. 1.
Inhibitory effects of methanolic and aqueous extracts of garlic against the growth rate of promastigote forms of L. tropica. Data are expressed as the mean ± SD (n = 3)
Cytotoxic activity
The garlic extracts exhibited very low cytotoxicity against on peritoneal murine macrophages. The CC50 values of methanolic and aqueous extracts against peritoneal macrophage cells were 291.4 and 348.2 µg/ml, respectively. In addition, The SI values of methanolic and aqueous extracts of garlic were show in Table 1.
Table 1.
CC50 values of methanolic and aqueous extracts of garlic on peritoneal macrophage cells and their IC50 and selectivity index (SI) values against promastigote forms of L. tropica
| Samples | ICc50 (μg/ml) | CCb50 (μg/ml) | SId |
|---|---|---|---|
| Methanolic extract | 12.3 ± 1.15 | 291.4 ± 3.08 | 23.7 |
| Aqueous extract | 19.2 ± 2.51 | 348.2 ± 4.6 | 18.1 |
| MAa | 15.4 ± 2.51 | 283.6 ± 4.6 | 18.4 |
Data are expressed as the mean ± SD (n = 3)
aMeglumine antimoniate (MA, Glucantime) as a control drug
bConcentration of extracts that caused 50 % mortality in BALB/c mice peritoneal macrophages
cConcentration of extracts that caused 50 % inhibitory in promastigote forms of L. tropica
dSelectivity index (CC50/IC50)
Discussion
Previous studies revealed that medicinal plant extracts due to having less side effects, low cost and high availability are a successful approach to control a wide range of diseases, such as leishmaniases. As mentioned, according to the previous experimental studies antiviral, antifungal, antibacterial, antiprotozoal, and antihelminthic effects of garlic extracts have been reported. In this investigation, we indicated high potential methanolic and aqueous extracts of garlic as a natural source for the production of new antileishmanial drugs on an in vitro model. Our findings exhibited potent antileishmanial effects of garlic various extracts particularly methanolic extract against promastigote forms of L. tropica with IC50 values 12.3 and 19.2 µg/ml, respectively. While, this value for MA as control drug was 15.4 µg/ml against promastigote forms of L. tropica. In line with our results, Wabwoba et al. (2010) revealed that garlic extract had significantly better (P < 0.001) leishmanicidal activity against both species (IC50 34.22 μg/ml to L. major, 37.41 μg/ml to L. donovani) than pentostam as control drug after 48 h incubation. Moreover, they showed that treatment with the extract, daily for 28 days led to a significant reduction (P < 0.05) in footpad swelling in BALB/c mice similar with standard drugs. These differences of values between present study and study conducted by Wabwoba et al. (2010) might be related to time of incubation, type of control drug, species of Leishmania and methodology. The results of present study also showed that SI values the methanolic and aqueous extracts of garlic was more than ten which indicated their safety to the macrophages and specificity to the parasite based to Weninger et al. (2001) In consistent with our findings Wabwoba et al. (2010) showed that garlic extract have low toxicity (IC50 > 450 μg/ml) against Vero cells. Therefore, the present results were in agreement with those of previous studies in demonstrating antileishmanial and cytotoxic activities of garlic extracts. To conclude, Findings of present study revealed high leishmanicidal activity garlic extracts against L. tropica that indicated the potential of garlic as a natural source for the production of new antileishmanial agent for use in cutaneous leishmaniasis. However, further studies will be needed to confirm these results by checking the garlic extracts in volunteer human.
Acknowledgments
We would like to thank Ms. Razieh Tavakil for cultivation of promastigotes of L. tropica.
Conflict of interest
The authors declare that there is no conflict of interest in this study.
References
- Abdel-Salam AM, Ammar N, Abdel-Hamid AZ. Effectiveness of probiotic Labneh supplemented with garlic or onion oil against Schistosoma mansoni in infected mice. Int J Dairy Sci. 2008;3(2):97–104. doi: 10.3923/ijds.2008.97.104. [DOI] [Google Scholar]
- Behnia M, Haghighi A, Komeilizadeh H, Tabaei SJ, Abadi A. In vitro antiamoebic activity of Iranian Allium sativum in comparison with metronidazole against Entamoeba histolytica. Iran J Parasitol. 2008;3(4):32–38. [Google Scholar]
- Cai Y. Anticryptococcal and antiviral properties of garlic. Cardiol Pract. 1991;9:11. [Google Scholar]
- Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19(1):11–26. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Eja ME, Asikong BE, Abriba C, Arikpo GE, Anwan EE, Enyi-Idoh KH. A comparative assessment of the antimicrobial effects of garlic (Allium sativum) and antibiotics on diarrheagenic organisms. Southeast Asian J Trop Med Public Health. 2007;38(2):343–348. [PubMed] [Google Scholar]
- Ghannoum MA. Inhibition of Candida adhesion to buccal epithelial cells by an aqueous extract of Allium sativum (garlic) J Appl Bacteriol. 1990;68:163–169. doi: 10.1111/j.1365-2672.1990.tb02562.x. [DOI] [PubMed] [Google Scholar]
- Harris JC, Plummer S, Turner MP, Lloyd D. The microaerophilic flagellate Giardia intestinalis: Allium sativum (garlic) is an effective antigiardial. J Microbiol. 2000;146:3119–3127. doi: 10.1099/00221287-146-12-3119. [DOI] [PubMed] [Google Scholar]
- Harris JC, Cottrell SL, Plummer S, Lloyd D. Antimicrobial properties of Allium sativum (garlic) Appl Microbiol Biotechnol. 2001;57:282–286. doi: 10.1007/s002530100722. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H, Sharifi I, Harandi MF, Shokohi M, Shakibaie M, Riabi TR et al (2014a) Anti-leishmania effects of methotrexate (MTX) alone and incombination with meglumine antimoniate (MA) against Iranian isolate of sensitive and MA-resistant Leishmania tropica: an in vitro assay. Asian Pacific J Trop Med 4:412–420
- Mahmoudvand H, Sharififar F, Sharifi I, Ezatpour B, Harandi MF, Makki, Jahanbakhsh S (2014b) In Vitro inhibitory effect of Berberis vulgaris (Berberidaceae) and its main component, berberine against different Leishmania species. Iran J Parasitol 9(1):28–36 [PMC free article] [PubMed]
- Martin KW, Ernst E. Herbal medicines for treatment of bacterial infections: a review of controlled clinical trials. J Antimicrob Chemother. 2003;51:241–246. doi: 10.1093/jac/dkg087. [DOI] [PubMed] [Google Scholar]
- Meng Y, Lu D, Guo N, Reed E, Zhou GZ, Zhang LB. Anti-HCMV effect of garlic components. Virol Sin. 1993;8:147–150. [Google Scholar]
- Milner JA. Garlic: its anticarcinogenic and antitumorigenic properties. Nutr Rev. 1996;54:82–86. doi: 10.1111/j.1753-4887.1996.tb03823.x. [DOI] [PubMed] [Google Scholar]
- Moazeni M, Nazer A (2010) In vitro Effectiveness of Garlic (Allium sativum) Extract on Scolices of Hydatid Cyst. World J Surg 34:2677–2681 [DOI] [PubMed]
- Rocha LG, Almeida JR, Macedo RO, Barbosa-Filho JM. A review of natural products with antileishmanial activity. Phytomedicine. 2005;12:514–535. doi: 10.1016/j.phymed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Ross ZM, Ogara EA, Hill DJ, Sleightholme HV, Maslin DJ. Antimicrobial properties of garlic oil against human enteric bacteria: evaluation of methodologies and comparisons with garlic oil sulfides and garlic powder. Appl Environ Microbiol. 2001;67(1):475–480. doi: 10.1128/AEM.67.1.475-480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi F, Sharifi I, Zarean M, Parizi MH, Aflatoonian M, Harandi MF, et al. Spatial distribution and molecular identification of Leishmania species from endemic foci of South-eastern Iran. Iran J Parasitol. 2012;7(1):45–52. [PMC free article] [PubMed] [Google Scholar]
- Soffar SA, Mokhtar GM. Evaluation of the antiparasitic effect of aqueous garlic (Allium sativum) extract in hymenolepiasis nana and giardiasis. J Egypt Soc Parasitol. 1991;21:497–502. [PubMed] [Google Scholar]
- Wabwoba B, Anjili CO, Ngeiywa M, Ngure PK, Kigondu EM, Ingonga J, Makwail J (2010) Experimental chemotherapy with Allium sativum (Liliaceae) methanolic extract in rodents infected with Leishmania major and Leishmania donovani. J Vector Borne Dis 47:160–167 [PubMed]
- Waldman RH. Demonstration of antiviral activity of garlic extract against human cytomegalovirus in vitro. Chin Med J. 1993;106:93–96. [PubMed] [Google Scholar]
- Weber ND, Anderson DO, North JA, Murray BK, Lawson LD, Hughes BG. In vitro virucidal activity of Allium sativum (garlic) extract and compounds. Planta Med. 1992;58:417–423. doi: 10.1055/s-2006-961504. [DOI] [PubMed] [Google Scholar]
- Weninger B, Robledo S, Arango GJ, Deharo E, Arango R, Munoz V, et al. Antiprotozoal activities of Colombian plants. J Ethnopharmacol. 2001;78:193–200. doi: 10.1016/S0378-8741(01)00346-4. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2010) Control of the leishmaniasis. WHO (Technical Report Series 949) Geneva, p 5–12