Abstract
Leishmaniasis is a major infectious disease caused by protozoan parasites of the genus Leishmania. Despite of many efforts toward vaccine against Leishmania no effective vaccine has been approved yet. DNA vaccines can generate more powerful and broad immune responses than conventional vaccines. In order to increase immunity, the DNA vaccine has been supplemented with adjuvant. In this study a new nano-vaccine containing TSA recombinant plasmid and poly(methylmethacrylate) nanoparticles (act as adjuvant) was designed and its immunogenicity tested on BALB/c mouse. After three intramuscular injection of nano-vaccine (100 μg), the recombinant TSA protein (20 μg) was injected subcutaneously. Finally as a challenge animals were infected by Leishmania major. After the last injection of nano-vaccine, after protein booster injection, and also after challenge, cellular immune and antibody responses were evaluated by ELISA method. The findings of this study showed the new nano-vaccine was capable of induction both cytokines secretion and specific antibody responses, but predominant Th1 immune response characterized by IFN-γ production compared to control groups. Moreover, results revealed that nano-vaccine was effective in reducing parasite burden in the spleen of Leishmania major-infected BALB/c mice. Base on results, current candidate vaccine has potency for further studies.
Keywords: TSA, Leishmania major, Poly(methylmethacrylate), Immune response
Introduction
Leishmaniasis, caused by an intracellular protozoan parasite, is widespread in many parts of the world with about 12 millions infected cases. There are 1.5–2 millions of new cases of cutaneous leishmaniasis and 500,000 cases of visceral leishmaniasis reported annually. Infection with HIV/AIDS can increase the risk of developing leishmaniasis by 100–1,000 folds (Webb et al. 1998; Mendez et al. 2002; Campos-Neto et al. 2002). Chemotherapeutics are available but show high toxicity, costs and are prone to resistance development due to prolonged treatment period. So many studies have been developed to prepare a protective vaccine. Development of either new anti-Leishmania drugs or a vaccine is an attractive alternative. Immunity against reinfection is acquired following cutaneous infection with Leishmania spp., suggesting that prophylactic immunization is feasible (Gradoni 2001; Campos-Neto et al. 2001; Handman 2001). A number of Leishmania vaccine candidates, including killed parasites, crude parasite fractions, recombinant Leishmania antigens, and antigen-encoding DNA, have been investigated in murine models. But in spite of several tested vaccine protocols no protective vaccine against any clinical leishmaniasis has been produced commercially (Saldarriaga et al. 2006; Ahmed et al. 2009). DNA vaccination is a new immunization strategy that has many potential advantages over other vaccine strategies. The major advantage of DNA vaccine is induction the expression of antigens, which are unaltered in their protein structure and antigenicity (Zadeh-Vakili et al. 2004; Sambrook et al. 1989).Most of the works have focused on different antigens Among the vaccine candidates, TSA (Thiol-Specific Antioxidant protein) has been introduced as one of the predominant vaccine candidates. TSA is L major recombinant protein homologue to eukaryotic Thiol-Specific-Antioxidant protein with molecular weight of 22.1 KDa is composed of 200 amino acids and placed in the chromosome of 15. TSA is expressed in L. major promastigote and amastigote (Rafati et al. 2006a, b; Mendez et al. 2001; Monnerat et al. 2004). TSA DNA vaccine stimulated high titers of specific IgG2a antibody, high levels of IFN-γ and low levels of IL-4, phenotypic markers of Th1 responses, which are the type of immune responses required for the control of this parasite. Many efforts to develop effective Leishmania vaccine have been limited due to lack of an appropriate adjuvant. Nanoparticles are solid very small fragment ranging in size from 1 to 1,000 nm (1 μm). They consist of macromolecular materials and can be used therapeutically or prophylactically, for example, as an adjuvant in vaccines or drug carriers, in which the active principle is dissolved, drew or encapsulated, or to which the active principle is adsorbed or chemically attached. Nanoparticles are able to enter antigen-presenting cells by different pathways, thereby regulating the immune response to the antigen. Their properties also make them appropriate for the delivery of antigens at mucosal surfaces and for intradermal administration. It is generally agreed that the adjuvanticity of nanoparticles and microparticles is affected by particle sizes, which in turn affect the type of immune responses caused by antigens carried by particles. Particulate carriers can serve as an effective antigen delivery system and, thus, improve and/or facilitate the uptake of antigens by antigen-presenting cells such as dendritic cells or macrophages. Particle-based antigen carriers may attend as a depot for controlled release of antigen, thereby increasing the availability of antigens to the immune cells. Poly(methylmethacrylate) (PMMA) is a synthetic polymer approved by the Food and Drug Administration for specific human clinical applications such as the bone cement. In vivo, PMMA particles are phagocytosable and have the potential to initiate strong immune responses by stimulating the production of inflammatory cytokines (Mutiso et al. 2010; Lou et al. 2009; O’Hagan 2000; Stieneker et al. 1995) The purpose of this work was DNA-vaccine efficacy in the presence PMMA adjuvant comparing to absence of it. We evaluated the usefulness of PMMA as a nano-adjuvant with DNA vaccine encoding TSA antigen of L. major in BALB/c mice in order to optimize the efficacy of the vaccine against leishmaniasis.
Methods
L. major promastigotes
The MHRO/IR/75/ER (an Iranian strain to be isolated by Nadim et al. in 1964) of L. major was provided by Pasteur Institute of Iran. Promastigotes were grown at 26 °C in RPMI1640 medium (Sigma®) supplemented with 10 % heat inactivated fetal calf serum (Gibco®, BRL), and 100 μg/ml gentamicin (Sigma®). Stationary phase of the promastigotes were harvested at a density of 1 × 106/ml.
Plasmid constructions
The TSA recombinant plasmid DNA was prepared in a previous study (Tabatabaie et al. 2007) transformed into E. coli DH5-α and purified by plasmid extraction Kit (Bioneer, Germany), dissolved in sterile deionizer distilled water and stored at −20 °C until use. Then the EndoFree plasmid purification Giga Kit (Qiagen, CA, USA) was used according to the manufacturer’s instructions. DNA concentrations were measured by absorbance at 260 nm. The OD260/280 ratios for the purified DNA were 1.80–1.95, indicating that the preparations were free from protein contamination.
Preparation of vaccine
The PMMA polymeric nanoparticles used as adjuvant were produced by gamma irradiation polymerization method in the absence of antigen (Kreuter 1995; O’Hagan 2000). In order to prepare the nano-vaccine candidate, pcDNA3/TSA recombinant plasmid was loaded to PMMA nanoparticles. In brief, 10 Mm EDAC 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC) was added to 1 Mm of PMMA nanoparticles solution and incubated for 10 min at room temperature with gentle stirring. After that, 1 ml Plasmid DNA solution of 100 μg/ml was added (equal volumes of the two solution were mixed) and then left in the cold room overnight. For purification, the solution was further subjected to extensive dialysis. The resulting PMMA-plasmid DNA nanoparticles were preserved in suspension form in double-distilled water. Size of nanoparticles were determined using a Zeta Sizer (Malvern, UK) (data not shown). The TSA recombinant peptide booster (22 KD) was gifted by Miss Narges Khabbaz zade Tehrani from Faculty of Basic Sciences, Science and Research Branch of Islamic Azad University of Tehran.
Immunization and experimental infection of the mice
Inbred Female BALB/c (6–8 weeks old) mice were purchased from Lab. Animal Center (Pasteur Institute, Karaj, Iran) and handled in accordance with the National Animal Care and Use protocol at Iran University of Medical Sciences. Mice were divided into two test (T) and three control (C) groups (20 mice/group) and respectively received DNA Vaccine (pcDNA3/TSA), nano-vaccine (pcDNA3/TSA + PMMA) and pcDNA3 (as control group) in dose of 100 μg. Another control groups were injected with PMMA and PBS. The mice in each group were anesthetized with 25 μl g−1 of mixture of ketamine 10 % and xylazine 2 % via intraperitoneal (i.p.). All injections were done intramuscularly (i.m.) and the injection sites were immediately pulsed using tweezer-type electrodes (CUY650, Sonidel Limited®, Ireland) to administer eight 60 V pulses each of 20 ms duration with a 200 ms interval using a BTX ECM830 generator (Harvard Apparatus, USA). The mice were immunized (i.m.) injection into both quadriceps’s with 100 μl of PBS, 50 μl in each anterior tibialis muscle (Ahmed et al. 2009). Three inoculations were employed with the same DNA and PMMA doses and the same immunization schedule was applied at 3 weeks interval. Two weeks after the last injection of nano-vaccines, 20 μg booster peptide plus incomplete Freund’s adjuvant was injected subcutaneously. Three weeks after final immunization mice were challenged with 1 × 106 promastigotes of L. major (strain MHRO/IR/75/ER) at the base of tail by the intradermal route (Sasaki et al. 2003). Two weeks after the last injection of nano-vaccine, two weeks after the peptide booster injection and five weeks after parasite challenge the animals were sacrificed and the spleens and serum samples were harvested for immunological analysis.
Lymphocyte proliferation assay
Spleen was taken from each individual mouse, dissected and resuspended in sterile cold PBS containing 2 % FBS. RBCs were lysed with lysis buffer, and single-cell suspension was adjusted to 3 × 106 cells/ml in RPMI 1640 (Gibco, Germany) supplemented with 10 % FBS, 4 mM l-glutamine, 1 mM sodium pyruvate, 50 µm 2ME, 100 μg/ml streptomycin and 100 IU/ml penicillin. One hundred µl of the cell suspension was dispensed into 96-well flat-bottom culture plates and stimulated with 10 μg/ml of recombinant TSA protein (expressed in E. coli cells) as antigen recall. Phytohemagglutinin-A (5 μg/ml, Gibco) was used as a positive control. Un-stimulated wells were used as the negative controls and complete culture medium was used as blank. All experiments were done in triplicate. After 72 h of culture, 100 μl of 5-bromo-2-deoxy-uridine (Brdu) labeling solution was added into each well and incubation continued for 18 h. The plates were then centrifuged and after removing culture medium wells were dried and fixed with 100 μl of fixation/permeabilization buffer. Subsequently, 100 μl of anti-Brdu antibody was added to each well and the plates were washed four times and tetramethylbenzidine (TMB) substrate was added. The reaction was stopped by adding 100 μl of 2 N H2SO4. Optical density (OD) for each well was measured at 450 nm. Stimulation Index (SI) was calculated according to the formula: OD of stimulated wells/OD of un-stimulated wells.
Cytokines assay before and after the challenge infection with L. major
A total number of 3 × 106 cells from single-cell suspension of each mouse spleen were placed onto the wells of a 24-well plate, stimulated in vitro with 10 μg/ml of recombinant TSA protein and incubated at 37 °C in 5 % CO2. Seventy two hrs post antigen recall, supernatants were collected and centrifuged at 300×g for 10 min and stored at −70 °C for cytokine analysis. IFN-γ and IL-4 cytokines were quantified using commercial ELISA Kits (Mabtech sweden) according to the manufacturer’s instructions. The quantity of each cytokine was reported as pg/ml according to the plotted standard curve.
ELISA of total antibodies and IgG1, IgG2a subclasses
To evaluate the humoral immune responses, before and after challenge with L. major, sera of experimental groups were collected and specific antibodies were determined by an optimized indirect ELISA method. Briefly, 100 μl of 10 μg/ml of antigen in PBS buffer were added into 96-well ELISA Maxisorp plates (Nunc, Naperville, IL) and incubated 24 h at 37 °C. The wells washed with PBS containing 0.05 % Tween 20 (washing buffer) and blocked 1 h at 37 °C with 5 % skimmed milk in PBS (blocking buffer). Plates were washed with washing buffer and 100 μl of 1/100 diluted sera were added to each wells and incubated at 37 °C for 2 h. The wells washed five times with washing buffer and incubated for 2 h with 100 μl of 1/7,000 dilution of anti mouse conjugated to HRP (Sigma, USA). The wells washed five times and incubated 30 min with 100 μl of TMB substrate in the dark and reaction was stopped with 2 N H2SO4 and color density was measured at A 450 nm with ELISA plate reader. Specific IgG1 and IgG2a subclasses were detected using goat anti mouse IgG1 and IgG2a secondary antibodies (Sigma, USA) according to the manufacture’s instruction.
Determination of parasite burden
Three mice from each group were sacrificed and parasite burden draining spleens was determined using the limiting dilution method (Ahmed et al. 2009). Briefly, seven weeks after challenge a piece of spleen was excised, weighed and then homogenized with a tissue grinder in 2 ml of RPMI 1640 medium (Gibco, Germany) supplemented with 20 % heat-inactivated fetal calf serum and Gentamicin (0.1 %).Under sterile conditions, the serial dilutions were prepared in wells of 96 well micro titration plates. After 7 days of incubation at 26 °C, the plates were examined with an inverted microscope at a magnification of 40×. The presence or absence of mobile promastigotes was recorded in each well. The final titer was the last dilution for which the well contained at least one parasite. The number of parasite per gram was calculated in the following way: (Rafati et al. 2006a, b; Buffet et al. 1995).
Statistical analysis
One-way ANOVA statistical test was used to assess the significance of the differences among various groups and Post Hoc LSD test was used to compare the means of different treatment groups. Results with P < 0.05 were considered to be statistically significant.
Results
Lymphocyte proliferation assay
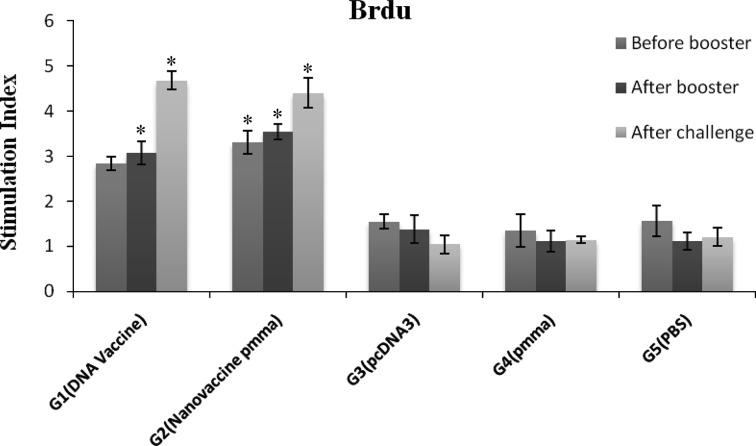
To assess the immune-potency of designed nano-vaccine, BALB/c mice intramuscularly received 3 injection of the vaccine with simultaneous electroporation pulses followed by an injection of recombinant protein. Analysis of lymphocyte proliferation using Brdu method indicated that after three injection of DNA vaccine and nano-vaccine (before booster injection) proliferation responses of lymphocytes markedly were increased in immunized mice with pcTSA + PMMA, which was significantly higher level of proliferation activity as compared to the control (P < 0.001) and DNA vaccine groups (P < 0.014). After booster injection no significant difference between vaccinated groups (pcTSA + PMMA and pcTSA) was observed (P = 0.337) but the difference of proliferative activity between test (T) and control (C) groups was statistically significant (P < 0.001). After challenge with L. major, no significant difference between immunization groups was observed (P < 0.549), but both vaccinated groups induced high level of proliferation responses as compared to the control groups (P < 0.001) (Fig. 1).
Fig. 1.
Lymphocyte proliferation responses following after immunization and after challenge. Mice were immunized with DNA vaccine with or without PMMA (n = 5 mice per group) in DNA prime/peptide boost strategy. Three groups of mice were injected with pcDNA3 vector, PMMA or PBS as negative controls (n = 4 mice per group). Proliferative responses was done triplicate for individual mice and evaluated with Brdu method as mentioned in Materials and Methods. Data represent mean ± SD (95 % C.I.). *P < 0.001 for pcTSA + PMMA group before peptide booster injection which was significantly higher level of proliferation activity than the other groups. *P < 0.001 for vaccinated groups compared to all control groups following the booster injection and after challenge
IL-4 and IFN-γ cytokine pattern
In another attempt to evaluate the pattern of cytokine secretion due to the vaccination, culture of individual mouse splenocytes was in vitro re-stimulated with recombinant TSA protein, expressed and purified in E. coli cells (data not shown). Collected supernatants were monitored for the amount of IFN-γ and IL-4 to determine the type (T helper 1 vs. T helper 2) of induced immune responses. The results showed that before booster injection, nano-vaccine induced high level of IFN-γ in comparison with DNA vaccine (P = 0.336) and control groups (P < 0.005), After booster injection IFN-γ secretion level in the vaccinated groups was significant difference as compared with control groups (P < 0.001) and immunization of mice with DNA vaccine alone increased IFN-γ producing lymphocytes as compared with nano-vaccine, though this increase was no significant difference (P = 0.234). After challenge level of IFN-γ markedly increased in immunized mice with nano-vaccine (pcTSA + PMMA), which was significantly higher than DNA vaccine (PcTSA) and control groups (P < 0.001) (Fig. 2a). Immunization with DNA vaccine formulated with PMMA nanoparticles showed significant increase in IL-4 producing lymphocytes compared to DNA vaccine alone (P < 0.001). After booster injection IL-4 level markedly increased in the group of mice immunized with pcTSA + PMMA, which was significantly higher than PcTSA (P = 0.023) and control groups (P < 0.025). After challenge with L. major no statistically significant difference between PcTSA + PMMA and PcTSA groups was observed (P > 0.733) (Fig. 2b).
Fig. 2.
Cytokine production [IFN-γ (a) and IL-4 (b)] by spleen cells of BALB/c mice after immunization periods (before and after booster injection) and after challenge. Mice were immunized with DNA vaccine with or without PMMA (n = 5 mice per group) in DNA prime/peptide boost strategy. Three groups of mice were injected with pcDNA3 vector, PMMA or PBS as negative controls (n = 4 mice per group). Cytokine analyses were monitored using ELISA method during the study as mentioned in materials and methods. Experiments were carried out in duplicate for individual mice. Values represent mean ± SD (95 % C.I.). *P < 0.005 for nano-vaccine group compared to all other groups before booster injection. *P < 0.001 for immunized groups compared to the control groups after booster injection. *P < 0.001 for pcTSA + PMMA group compared to all other groups after challenge with L. major (Fig. 2a). *P < 0.025 for nano-vaccine group compared to all other groups before and after peptide booster injection. *P < 0.011 for vaccinated groups compared to PBS group after challenge (Fig. 2b)
Antibody response
Results of total antibodies in the experimental groups show that before recombinant peptide booster injection immunized group with nano-vaccine, significantly increased total antibodies as compared to the control groups (P < 0.003). Following the booster injection and after challenge, mice were immunized with DNA vaccine and boosted with recombinant peptide significantly increased total antibodies as compared to control groups (groups 3, 4 and 5) (P < 0.031). Immunization of mice with vaccine candidate formulated with PMMA (group 1) after booster injection and also after challenge showed high level of induction of total antibodies as compared to the negative control groups but no statistical significant difference was observed (P > 0.059) (Fig. 3a, b, c).
Fig. 3.
Specific antibody production against TSA recombinant protein in BALB/c mice immunized with DNA vaccine and nano-vaccine before booster injection (a), following booster injection (b) and after challenge (c) Specific total IgG, IgG1 and IgG2a were measured by ELISA method as mentioned in "Methods" section. Sera from each group were diluted 1:200 and evaluated for the presence of IgG1 and IgG2a. Specific changes of IgG1 levels during the study (d). Specific IgG2a level changes in the study (e). Detection was done with the substrate TMB and optical density at 450 nm was determined. All data represent mean ± SD (95 % C.I.). The results indicated the significant difference of total antibody between G2 and control groups after 3 injection of vaccine (i.m) (*P < 0.003) (a). After peptide booster injection and after challenge the significant difference of total antibody was observed between G2 and control groups (*P < 0.048) (b, c). *P < 0.041 for DNA vaccine group compared to all other groups after booster injection (d, e). *P < 0.030 for vaccinated group compared to control groups following the booster injection (e). *P < 0.018 for G1 and G2 compared to control groups after challenge with L. major (d). *P < 0.001 for DNA vaccine/peptide booster compared to other groups after challenge (e). There was no significant difference among groups before booster injection (P > 0.05)
Results of IgG isotyping showed that following the booster injection IgG1 level in the group of mice which received DNA vaccine was significantly increased compared to nano-vaccine and control groups (P = 0.012 and P < 0.002 respectively). Moreover all vaccine immunized groups (groups 1, 2) significantly increased IgG2a isotype as compared to the control groups (groups 3, 4, 5) (P < 0.030) and immunization of mice with DNA vaccine candidate (group 1) significantly increased IgG2a titer as compared to nano-vaccine group (group 2) (P = 0.041). After challenge IgG1 levels in vaccinated groups showed increased significantly compared to control groups (P < 0.018) and high level of IgG2a in mice were immunized with DNA vaccine/peptide booster in comparison to control groups was observed (P < 0.001) (Fig. 3d, e).
Splenic parasite burden
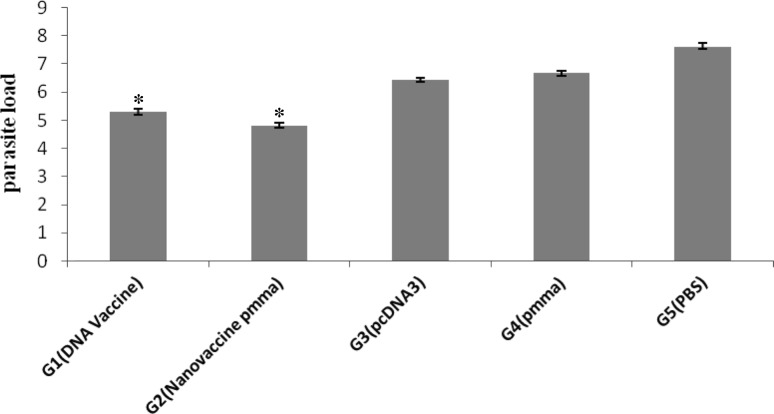
Protection against leishmaniasis was assessed by measurement of parasite burden in the spleen of infected animals. The splenic parasite burden following by immunization and 7 weeks challenge with L. major in all experimental groups showed that the number of viable parasites in the spleen was significant difference between vaccinated and unvaccinated groups. Significant difference was observed between two vaccinated groups (P < 0.001) and the mice immunized with nano-vaccine (pcTSA + PMMA) showed significantly lowest number of live parasite compared to the other groups (P < 0.001) (Fig. 4).
Fig. 4.
Parasite burden at 7 weeks after challenge in vaccinated (DNA vaccine, nano-vaccine) in prime/boost strategy and control groups (vector only/PMMA and PBS). The total numbers of viable parasites presented in the spleen of the infected animals were calculated as (parasite dilution per tissue weight). Values represent mean ± SD for individual mice (n = 3). The parasite burden in the spleen of vaccinated mice was significantly less than in the control mice (*P < 0.001)
Discussion
In recent years, significant progress has been made in the identification of vaccine candidates which can induce a protective response against leishmaniasis, but no effective vaccine is available yet. A number of vaccine strategies have been tested, ranging from killed parasites to recombinant antigens or DNA vaccines (Campos-Neto et al. 2001; Mauel 2002; Webb et al. 1996; Ahmed et al. 2004). DNA vaccines effectively engage both MHC-I and MHC-II pathways allowing for the induction of CD8+ and CD4+ T cells whereas antigen present in soluble form, such as recombinant protein, generally induces only antibody responses. DNA vaccines are easy to produce and can induce strong, long lasting and powerful humeral and cellular immunity. Using the prime-boost immunization strategy was a method which was able to affect the quality and quantity of immune responses (Ivory and Chadee 2004; Kwissa et al. 2003; Rafati et al. 2006a, b). Moreover, some approaches such as application of in vivo electroporation that improve the efficacy of DNA vaccine is needed to be considered (Buchan et al. 2005; Miyazaki and Aihara 2002), In the present study a new nano-vaccine containing TSA DNA plasmid was designed and evaluated for it’s immunogenicity in BALB/c mice. TSA (thiol-specific antioxidant antigen) is a immune-dominant antigen of L. major and is antigenic in both murine and human systems and is constitutive expressed in both promastigote and amastigote life stages (Fatemeh et al. 2012). Immunization of BALB/c mice with a TSA plasmid DNA confers high levels of protective immunity. This DNA vaccine induces CTL activity and solid protection. It also stimulates high titers of specific IgG1, IgG2a antibodies. Further, it also induces a predominant IFN-γ production and low levels of IL-4, phenotypic markers of Th1 responses. The recombinant leishmanial antigens LmSTI1 and TSA have been shown that they can induce excellent protection in both murine and nonhuman primate models of human cutaneous leishmaniasis. It seems that the use of an adjuvant and/or delivery system is necessary for almost any modern vaccine particularly vaccines against leishmaniasis (Crowther 2013; Badiee et al. 2013). In this research PMMA nanoparticles was utilized as adjuvant to improve specific humoral and cellular immune responses to our candidate vaccine. PMMA nanoparticle adjuvant achieved good antibody responses and good protection against challenge with a number of antigens. Additionally, they also seem to lead to a higher stability of the vaccines containing this type of adjuvant PMMA nanoparticles are polymeric particulate adjuvant for vaccines. These nanoparticles can easily be manufactured in a reproducible manner in the described particle sizes and with specific surface properties. Among the numerous advantages of the nanoparticles can be pointed simple and easy to produce, process low degradation, safety of adverse effects during use of them. The Use of Poly methylmethacrylate adjuvant for Split Influenza vaccines showed safety record and excellent and stronger protection. In this research the animals were sacrificed and their spleen cells were obtained and cultured in the presence of specific antigen. To evaluate the proliferative responses of lymphocytes Brdu test was used. Brdu-test is commonly used in the detection of proliferating cells in tissues (Special living tissues) and is Sensitive, Accurate, Uptodate technique and capable comparative with thymidine radioactive hence Bromodeoxyuridine (BrdU) competes with thymidine (TdR). Results showed that immunization with nano-vaccine formulated with PMMA before and after booster injection induced higher level of lymphocyte proliferation as compared to the DNA vaccine group so nano-formulation of vaccines with PMMA could increase lymphocyte proliferation activity against candidate vaccine. Recombinant TSA protein elicited in vitro proliferative responses from peripheral blood mononuclear cells of human leishmaniasis patients. In the study of Kreuter et al., it was observed that application of HIV-1 Tat antigen formulated with PMMA particles induced proliferation responses of lymphocytes (Kreuter 1996; Kreuter et al. 1976; Voltan et al. 2007). Culture supernatants from stimulated spleen cells were collected and IFN-γ and IL-4 concentration were measured by ELISA test. Cytokine analysis of experimental groups revealed that candidate nano-vaccine increased IFN-γ cytokine compared to DNA vaccine alone in the immunized groups, before booster injection and after challenge. After booster injection level of IFN-γ cytokine in vaccinated groups considerably increased in comparison with before booster injection. Moreover, before and after booster injection IL-4 titer showed increase in the group of mice immunized with nano-vaccine as compare to DNA vaccine alone. These findings amend previous studies by revealing that nano-vaccine and prime boost strategy induce Th1 and Th2 pattern of immune responses (Badiee et al. 2013; Danesh-Bahreini et al. 2011; Rafati et al. 2006a, b). Also, the findings of this study showed that immunization of mice with nano-vaccine or DNA vaccine enhanced total antibody responses as compare with control groups. Before booster injection high level of total antibody was observed in mice immunized with nano-vaccine and following the booster injection and after infection with L. major the level of total specific antibody in the group of mice immunized with DNA vaccine was higher than the other vaccinated group. Previous findings demonstrated that PMMA nanoparticle enhanced humoral responses in Hiv-2 Split Whole virus and there was safety of adverse effects during use of them (Stieneker et al. 1995). Other study suggested that PMMA adjuvant may represent an attractive alternative to increase the efficacy of candidate vaccines toward antibody production (Kreuter et al. 1976). While exploring IgG isotypes our results revealed that both specific IgG1 and IgG2a were augmented. Considering that IgG1 is a Th2 marker and IgG2a is a Th1 marker (Campos-Neto 2005), these funding indicated that after peptide booster injection level of IgG1 and IgG2a isotypes in immunized groups (nano-vaccine and DNA vaccine) increased. Studies of Campos-Neto et al. showed that Immunization of BALB/c mice with a TSA plasmid DNA induced high titers of specific IgG1, IgG2a antibodies against Leishmania (Campos-Neto et al. 2002). The highest reduction in parasite burden was observed in immunized mice with pcTSA + PMMA and using prime-boost vaccination regimen resulted in enhanced protection against Leishmania infection. The results of previous studies showed that use of nanoparticles (Mutiso et al. 2010; Badiee et al. 2013; Danesh-Bahreini et al. 2011) and prime boost strategy enhanced protective immunity in animal models of Leishmania infection (Tewary et al. 2005). In this study, we demonstrated that PMMA can effect on efficacy of a DNA vaccine encoding TSA against L. major infection and elicits humoral and cellular immune responses to the antigen delivered. The vaccine formulation described here may be an excellent candidate for further vaccine development against Leishmania.
References
- Ahmed SB, Bahloul C, Robbana C, Askri S, Dellagi K. A comparative evaluation of different DNA vaccine candidates against experimental murine leishmaniasis due to L. major. Vaccine. 2004;22:1631–1639. doi: 10.1016/j.vaccine.2003.10.046. [DOI] [PubMed] [Google Scholar]
- Ahmed SB, Touihri L, Chtourou Y, Dellagi K, Bahloul C. DNA based vaccination with a cocktail of plasmids encoding immunodominant Leishmania (Leishmania) major antigens confers full protection in BALB/c mice. Vaccine. 2009;27:99–106. doi: 10.1016/j.vaccine.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Badiee A, Heravi Shargh V, Khamesipour A, Jaafari MR. Micro/nanoparticle adjuvants for antileishmanial vaccines: present and future trends. Vaccine. 2013;31:735–749. doi: 10.1016/j.vaccine.2012.11.068. [DOI] [PubMed] [Google Scholar]
- Buchan S, Gronevik E, Mathiesen I, King CA, Stevenson FK, Rice J. Electroporation as a “prime/boost” strategy for naked DNA vaccination against a tumor antigen. J Immunol. 2005;174:6292–6298. doi: 10.4049/jimmunol.174.10.6292. [DOI] [PubMed] [Google Scholar]
- Buffet PA, Sulahian A, Garin YJ, Nassar N, Derouin F. Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob Agents Chemother. 1995;39:2167–2168. doi: 10.1128/AAC.39.9.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Neto A (2005) What about Th1/Th2 in cutaneous leishmaniasis vaccine discovery? Brazilian J Med Biol Res = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica [et al] 38:979–984 [DOI] [PubMed]
- Campos-Neto A, Porrozzi R, Greeson K, Coler RN, Webb JR, Seiky YA, et al. Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infect Immun. 2001;69:4103–4108. doi: 10.1128/IAI.69.6.4103-4108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Neto A, Webb JR, Greeson K, Coler RN, Skeiky YA, Reed SG. Vaccination with plasmid DNA encoding TSA/LmSTI1 leishmanial fusion proteins confers protection against Leishmania major infection in susceptible BALB/c mice. Infect Immun. 2002;70:2828–2836. doi: 10.1128/IAI.70.6.2828-2836.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther JR. ELISA. Theory and practice. Methods Mol Biol. 2013;1995(42):1–218. doi: 10.1385/0-89603-279-5:1. [DOI] [PubMed] [Google Scholar]
- Danesh-Bahreini MA, Shokri J, Samiei A, Kamali-Sarvestani E, Barzegar-Jalali M, Mohammadi-Samani S. Nanovaccine for leishmaniasis: preparation of chitosan nanoparticles containing Leishmania superoxide dismutase and evaluation of its immunogenicity in BALB/c mice. Int J Nanomed. 2011;6:835–842. doi: 10.2147/IJN.S16805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemeh G, Fatemeh T, Zohreh S, Abdolhosein D, Mohammad Zahir H, Mehdi M. Cloning of a recombinant plasmid encoding thiol-specific antioxidant antigen (TSA) gene of Leishmania major and expression in the Chinese hamster ovary cell line. Malays J Med Sci. 2012;19:15–19. [PMC free article] [PubMed] [Google Scholar]
- Gradoni L. An update on antileishmanial vaccine candidates and prospects for a canine Leishmania vaccine. Vet Parasitol. 2001;100:87–103. doi: 10.1016/S0304-4017(01)00486-1. [DOI] [PubMed] [Google Scholar]
- Handman E. Leishmaniasis: current status of vaccine development. Clin Microbiol Rev. 2001;14:229–243. doi: 10.1128/CMR.14.2.229-243.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivory C, Chadee K. DNA vaccines: designing strategies against parasitic infections. Genet Vaccines Ther. 2004;2:17. doi: 10.1186/1479-0556-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter J. Nanoparticles as adjuvants for vaccines. Pharm Biotechnol. 1995;6:463–472. doi: 10.1007/978-1-4615-1823-5_19. [DOI] [PubMed] [Google Scholar]
- Kreuter J. Nanoparticles and microparticles for drug and vaccine delivery. J Anat. 1996;189(Pt 3):503–505. [PMC free article] [PubMed] [Google Scholar]
- Kreuter J, Mauler R, Gruschkau H, Speiser PP. The use of new polymethylmethacrylate adjuvants for split influenza vaccines. Exp Cell Biol. 1976;44:12–19. doi: 10.1159/000162849. [DOI] [PubMed] [Google Scholar]
- Kwissa M, Lindblad EB, Schirmbeck R, Reimann J. Codelivery of a DNA vaccine and a protein vaccine with aluminum phosphate stimulates a potent and multivalent immune response. J Mol Med. 2003;81:502–510. doi: 10.1007/s00109-003-0452-9. [DOI] [PubMed] [Google Scholar]
- Lou PJ, Cheng WF, Chung YC, Cheng CY, Chiu LH, Young TH. PMMA particle-mediated DNA vaccine for cervical cancer. J Biomed Mater Res Part A. 2009;88:849–857. doi: 10.1002/jbm.a.31919. [DOI] [PubMed] [Google Scholar]
- Mauel J. Vaccination against Leishmania infections. Curr Drug Targets Immune Endocr Metabol Disord. 2002;2:201–226. doi: 10.2174/1568008023340631. [DOI] [PubMed] [Google Scholar]
- Mendez S, Gurunathan S, Kamhawi S, Belkaid Y, Moga MA, Skeiky YA, et al. The potency and durability of DNA- and protein-based vaccines against Leishmania major evaluated using low-dose, intradermal challenge. J Immunol. 2001;166:5122–5128. doi: 10.4049/jimmunol.166.8.5122. [DOI] [PubMed] [Google Scholar]
- Mendez S, Belkaid Y, Seder RA, Sacks D. Optimization of DNA vaccination against cutaneous leishmaniasis. Vaccine. 2002;20:3702–3708. doi: 10.1016/S0264-410X(02)00376-6. [DOI] [PubMed] [Google Scholar]
- Miyazaki J, Aihara H. Gene transfer into muscle by electroporation in vivo. Methods Mol Med. 2002;69:49–62. doi: 10.1385/1-59259-141-8:049. [DOI] [PubMed] [Google Scholar]
- Monnerat S, Martinez-Calvillo S, Worthey E, Myler PJ, Stuart KD, Fasel N. Genomic organization and gene expression in a chromosomal region of Leishmania major. Mol Biochem Parasitol. 2004;134:233–243. doi: 10.1016/j.molbiopara.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Mutiso JM, Macharia JC, Gicheru MM. A review of adjuvants for Leishmania vaccine candidates. J Biomed Res. 2010;24:16–25. doi: 10.1016/S1674-8301(10)60004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hagan DT. Methods in molecular medicine, vaccine adjuvants: preparation methods and research protocols. Totowa: Humana Press; 2000. [Google Scholar]
- Rafati S, Salmanian AH, Hashemi K, Schaff C, Belli S, Fasel N. Identification of Leishmania major cysteine proteinases as targets of the immune response in humans. Mol Biochem Parasitol. 2001;113:35–43. doi: 10.1016/S0166-6851(00)00377-7. [DOI] [PubMed] [Google Scholar]
- Rafati S, Zahedifard F, Nazgouee F. Prime-boost vaccination using cysteine proteinases type I and II of Leishmania infantum confers protective immunity in murine visceral leishmaniasis. Vaccine. 2006;24:2169–2175. doi: 10.1016/j.vaccine.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Rafati S, Ghaemimanesh F, Zahedifard F. Comparison of potential protection induced by three vaccination strategies (DNA/DNA, Protein/Protein and DNA/Protein) against Leishmania major infection using Signal Peptidase type I in BALB/c mice. Vaccine. 2006;24:3290–3297. doi: 10.1016/j.vaccine.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Saldarriaga OA, Travi BL, Park W, Perez LE, Melby PC. Immunogenicity of a multicomponent DNA vaccine against visceral leishmaniasis in dogs. Vaccine. 2006;24:1928–1940. doi: 10.1016/j.vaccine.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sasaki S, Takeshita F, Xin KQ, Ishii N, Okuda K. Adjuvant formulations and delivery systems for DNA vaccines. Methods. 2003;31:243–254. doi: 10.1016/S1046-2023(03)00140-3. [DOI] [PubMed] [Google Scholar]
- Stieneker F, Kersten G, van Bloois L, Crommelin DJ, Hem SL, Lower J, et al. Comparison of 24 different adjuvants for inactivated HIV-2 split whole virus as antigen in mice. Induction of titres of binding antibodies and toxicity of the formulations. Vaccine. 1995;13:45–53. doi: 10.1016/0264-410X(95)80010-B. [DOI] [PubMed] [Google Scholar]
- Tabatabaie F, Ghaffarifar F, Sharifi Z, Dalimi A, Zavaran Hoseini A. Cloning and sequencing of Leishmania major thiol-specific antioxidant antigen (TSA) gene. Iranian J Parasitol. 2007;2:30–41. [Google Scholar]
- Tewary P, Jain M, Sahani MH, Saxena S, Madhubala R. A heterologous prime-boost vaccination regimen using ORFF DNA and recombinant ORFF protein confers protective immunity against experimental visceral leishmaniasis. J Infect Dis. 2005;191:2130–2137. doi: 10.1086/430348. [DOI] [PubMed] [Google Scholar]
- Voltan R, Castaldello A, Brocca-Cofano E, Altavilla G, Caputo A, Laus M, et al. Preparation and characterization of innovative protein-coated poly(methylmethacrylate) core-shell nanoparticles for vaccine purposes. Pharm Res. 2007;24:1870–1882. doi: 10.1007/s11095-007-9310-8. [DOI] [PubMed] [Google Scholar]
- Webb JR, Kaufmann D, Campos-Neto A, Reed SG. Molecular cloning of a novel protein antigen of Leishmania major that elicits a potent immune response in experimental murine leishmaniasis. J Immunol. 1996;157:5034–5041. [PubMed] [Google Scholar]
- Webb JR, Campos-Neto A, Ovendale PJ, Martin TI, Stromberg EJ, Badaro R, et al. Human and murine immune responses to a novel Leishmania major recombinant protein encoded by members of a multicopy gene family. Infect Immun. 1998;66:3279–3289. doi: 10.1128/iai.66.7.3279-3289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadeh-Vakili A, Taheri T, Taslimi Y, Doustdari F, Salmanian AH, Rafati S. Immunization with the hybrid protein vaccine, consisting of Leishmania major cysteine proteinases Type I (CPB) and Type II (CPA), partially protects against leishmaniasis. Vaccine. 2004;22:1930–1940. doi: 10.1016/j.vaccine.2003.11.014. [DOI] [PubMed] [Google Scholar]