Abstract
The study was conducted with the objective to estimate the prevalence of gastrointestinal strongyles and identifying the prevailing strongyle species Gamo-Gofa Zone. A total of 598 animals (241 sheep and 357 goats) and 45 animals (20 sheep and 25 goats) were examined coproscopically and by post mortem, respectively. The overall coproscopic prevalence of strongylosis in the study area was 51.4 %. Strongyles were more prevalent in sheep than goats (95 % CI is 74.6–84.8 % and 58.6–68.6 % for sheep and goats, respectively; P < 0.05). A higher prevalence (P < 0.05) of strongyles infection was recorded in the midland and highland than the lowland, and in wet season than the dry season. The mean fecal egg count was found to be significantly higher (P < 0.05) in the midland area (908.7 ± 94.5) and in wet season (1,033.7 ± 84.2). The post mortem examination result indicated that the overall prevalence of gastrointestinal strongyles was 97.7 %; and a total of 11 strongyle species were identified of which Trichostrongylus axei, Teladorsagia species, Trichostrongylus colubriformis and Haemonchus contortus were the dominant and with higher load. During this study infection with multiple parasites, 3 and more 3, species were recorded in about 68 % goats and 85 % sheep. This study revealed the very high strongyle prevalence and higher proportion of multiple parasitism both in sheep and goats. Hence, it suggests the need to the institution of various control measures like strategic anthelmintic treatment for efficient utilization of the available small ruminant resources.
Keywords: Prevalence, Small ruminant, Faecal egg count, Strongyle, Post mortem, Gamo-Gofa, Ethiopia
Introduction
The impact of gastrointestinal parasitic infection is greater in sub-Saharan Africa in general and Ethiopia in particular due to the availability of a wide range of agro-ecological factors suitable for diversified hosts and parasite species (Lebbie et al. 1994). Gastrointestinal parasites infections are recognized as a major constraint to ruminant production throughout the tropics and elsewhere (Githiori et al. 2004). The economic losses due to helminthosis of ruminants include a decrease in food intake and lesser weight gains, lowered fertility, reduced work capability, unintentional culling, lower milk production, treatment costs, and mortality in heavily parasitized animals (Lebbie et al. 1994).
The prevalence of ruminant gastrointestinal helminthes reported from various parts of the world varied from 0.72 to 84.1 % (Khan et al. 2010; Fikru et al. 2006; Wang et al. 2006; Waruiru et al. 2005; Nginyi et al. 2001). The risk factors influencing the prevalence and distribution of gastrointestinal helminthes include: age, sex, weather condition and husbandry or management practices (Miller et al. 1998). In Ethiopia, the reported prevalence of small ruminant gastrointestinal helminthes range from 50.4 to 84.1 % (Bersissa et al. 2011; Asha and Wossene 2007; Fikru et al. 2006). Even though various studies had been conducted on small ruminant gastrointestinal helminthosis, the prevailing species of the parasites rarely identified.
Therefore, the current study was done to estimate the prevalence and identify the strongyle species of small ruminants in various agro-ecologies and season through coproscopic and post mortem examination in Gamo-Gofa zone, Southern Ethiopia.
Materials and methods
Study areas
The study was carried out between January 2010 and August 2011 at Gamo Gofa zone in three selected districts with three different agro-ecologies: Chencha, highland, which obtains mean maximum and minimum rainfall of 2,400 and 600 mm, respectively. The mean maximum and minimum temperature of the area were 30 and 5 °C, respectively. Kucha, midland receives mean maximum and minimum rainfall of 1,050 and 560 mm respectively, and annual average maximum and minimum temperature of 29 and 17 °C, respectively. Mirab-Abaya especially the lowland part receives an annual rainfall of 500–580 mm (Gamo-Gofa zone BRD 2010/11).
Study design and sampling
A cross-sectional study was undertaken in the three selected districts, representative of the three agroecological areas, of Gamo-Gofa zone. Both coproscopic and post mortem examinations were employed for the study. During the study putative risk factors like species, sex, age, site and season were considered with respect to helminthic prevalence.
The study districts were selected purposely, and study animals were selected by systematic random sampling technique. From selected animals faecal samples were taken directly from the rectum of animals using glove. The collected faeces were placed in air-tight universal bottle containing 10 % formalin. On the day of collection the samples were transported to Sodo Regional Veterinary Laboratory for coprological investigation. Parasitological examination was done by sedimentation and flotation techniques following the standard procedures (Hansen and Perry 1994). Those faecal samples positive for strongyle eggs were further examined quantitatively with modified McMaster technique (Urquhart et al. 1996; Hansen and Perry 1994); and the degree of infection was determined according to Shah-Fischer and Say (1989) and Soulsby (1982).
A total of 45 animals, 20 sheep and 25 goats, were slaughtered, and then gastrointestinal strongyles recovered and counted as described by Hansen and Perry (1994). The recovered parasites were taken to Sodo Regional Veterinary Laboratory and identified (MAFF 1984).
Sample size
The number of animals required for the study was determined by taking into consideration of 85 % prevalence of small ruminant helminthosis (Asha and Wossene 2007) and 5 % desired absolute precision and the formula given by Thrusfield (2005). Accordingly 241 sheep and 358 goats were selected and studied.
Data management and analysis
Collected data were entered into Microsoft Excel spread sheet, and then summarized by using descriptive statistics like mean, and percentage; and Chi square test was employed to test the effect of the considered risk factors on the prevalence of gastrointestinal helminthes. The mean EPG faeces and worm burden were analyzed by using ANOVA and t test. For the analysis STATA 11.0 software was used.
Results
Coproscopic prevalence
A total of 598 faecal samples were collected from small ruminant (241 Sheep and 357 Goats) for coprological examination. The overall coprological prevalence of strongyles was 70.1 %, and the prevalence in sheep and goats was 79.7 and 63.6 %, respectively (Table 1). Season, agro-ecology, animal species, sex and age of the animals were considered as risk factor of gastrointestinal strongyles infection. The result of risk factors analysis is presented in Table 1.
Table 1.
Effect of risk factors on coprological prevalence of strongyles in small ruminant
| Risk factors | Number examined | Prevalence | Std. E | 95 % CI | χ2 | P Value |
|---|---|---|---|---|---|---|
| Animal species | ||||||
| Goat | 357 | (63.6 %) | 0.03 | 58.6–68.6 | 1 | |
| Sheep | 241 | (79.7 %) | 0.03 | 74.6–84.8 | 17.743 | 0.000 |
| Sex | ||||||
| Male | 137 | 65.0 % | 0.04 | 56.9–73.0 | 1 | |
| Female | 461 | 71.6 % | 0.02 | 67.5–75.7 | 2.027 | 0.137 |
| Age | ||||||
| <1 year | 51 | 38 (74.5 %) | 0.06 | 62.4–86.6 | 1 | |
| >1 year | 547 | 381 (69.7 %) | 0.02 | 65.8–73.5 | 0.525 | 0.469 |
| Site | ||||||
| Lowland | 202 | 95 (47.0 %) | 0.04 | 40.1–53.9 | ||
| Midland | 179 | 152 (84.9 %) | 0.03 | 79.6–90.1 | ||
| Highland | 217 | 172 (79.3 %) | 0.03 | 73.9–84.7 | 77.542 | 0.000 |
| Season | ||||||
| Dry | 284 | 178 (62.7 %) | 0.03 | 57.0–68.3 | 1 | |
| Wet | 314 | 241 (75.8 %) | 0.02 | 72.1–81.4 | 14.087 | 0.000 |
Quantitative faecal examination
The quantitative faecal examination revealed that faecal egg counts were highly variable, ranging from 100 to 9,500 EPG, and the overall mean EPG was 759.9 (Table 2). The t, F and P value in Table 2 were computed after the data for faecal egg counts were transformed into the logarithmic form. The analysis for the degree of infection were summarized and presented in Table 3.
Table 2.
Egg per gram of faeces by species, agro-ecology and season of studied animals
| Risk factor | Positive for strongyle egg | Mean EPG ± SE | t or F Value | P value |
|---|---|---|---|---|
| Species | ||||
| Sheep | 192 | 792.5 ± 89.1 | 0.902 | 0.367 |
| Goat | 227 | 736.5 ± 68.7 | ||
| Sex | ||||
| Male | 90 | 840.7 ± 124.7 | 1.088 | 0.277 |
| Female | 329 | 738.2 ± 60.7 | ||
| Age | ||||
| <1 year | 38 | 1,126.5 ± 186.7 | 1.251 | 0.211 |
| >1 year | 381 | 724.1 ± 56.8 | ||
| Site | ||||
| Lowland | 95 | 420.9 ± 56.1 | ||
| Midland | 152 | 908.7 ± 94.5 | 4.605 | 0.000 |
| Highland | 172 | 821.1 ± 99.1 | ||
| Season | ||||
| Dry | 177 | 354.5 ± 32.1 | 6.878 | 0.000 |
| Wet | 242 | 1,033.7 ± 84.2 | ||
t Value or F Value and P Value computed after logarithmic [Log10(x + 1)] transformation
Table 3.
Mean EPG of gastrointestinal nematodes infection and the degree of infection
| Species | Degree of infection | Frequency | Proportion (%) | Mean EPG | Std. E | 95 % CI |
|---|---|---|---|---|---|---|
| Sheep | Mild | 129 | 59.2 | 389.9 | 19.6 | 351.4–428.4 |
| Moderate | 33 | 15.1 | 1,028.0 | 20.3 | 988.1–1,068.0 | |
| Severe | 56 | 25.7 | 2,692.9 | 280.1 | 2,141.6–3,244.1 | |
| Goat | Mild | 70 | 68.0 | 416.8 | 23.6 | 370.1–463.4 |
| Moderate | 24 | 23.3 | 1,002.1 | 29.4 | 944.0–1,060.2 | |
| Severe | 9 | 8.7 | 1,755.6 | 127.1 | 1,504.6–2,006.5 | |
| Both | Mild | 199 | 62.0 | 399.4 | 15.2 | 369.6–429.1 |
| Moderate | 57 | 17.8 | 1,017.0 | 17.0 | 983.7–1,050.5 | |
| Severe | 65 | 20.2 | 2,563.1 | 244.9 | 2,081.7–3,044.4 |
Post mortem examination
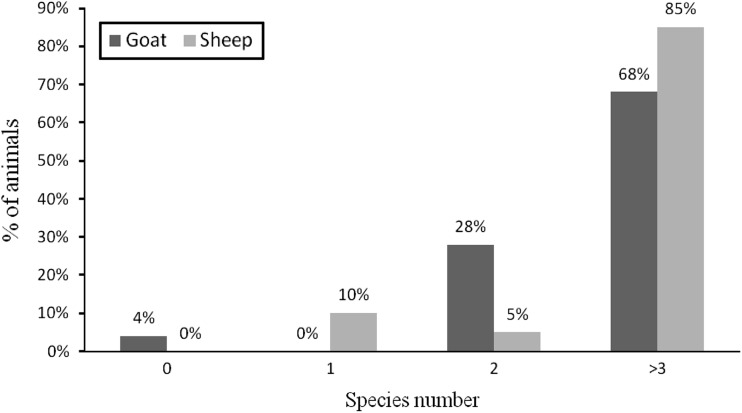
From a total of 45 small ruminants (20 sheep and 25 goats) slaughtered, gastrointestinal strongyles were recovered from 44 animals (97.8 %), 24 goats (96.0 %) and 20 sheep (100 %). The recovered gastrointestinal strongyles species and their mean burden are shown in Table 4. The study revealed mixed infection with three and more than three strongyle species in 68 % goats and 85 % sheep (Fig. 1). Teladorsagia species were recovered only in sheep coming from highland area during wet season, whereas Haemonchus contortus, Trichostrongylus axei and Trichostrongylus colubriformis recovered in both sheep and goats coming from all agro-ecological zones during wet and dry periods.
Table 4.
Mean burden of adult strongyle species recovered by through postmortem
| Species of strongyle | Sheep | Goats | ||
|---|---|---|---|---|
| Wet season (n = 9) | Dry season (n = 11) | Wet season (n = 15) | Dry season (n = 10) | |
| Haemonchus contortus | 1,522.9 | 2,651.3 | 1,306.4 | 875.1 |
| Trichostrongylus axei | 5,004.0 | 3,103.7 | 2,762.5 | 1,150.4 |
| T. colubriformis | 3,857.1 | 2,428.6 | 2,363.6 | 4,407.2 |
| T. probolurus | 193.6 | – | – | – |
| Bunostomum trigonocephalum | 144.0 | 105.2 | 142.5 | 1,918.2 |
| Oesophagostomum columbianum | 71.3 | 238.4 | 117.8 | 34.7 |
| T. ovis | 38.3 | 10.6 | 13.4 | 6.8 |
| Teladorsagia spp | 4,603.8 | 4,375 | – | 1,487 |
| S. papillosus | 276.6 | – | – | – |
Fig. 1.
Proportion of animals with single and mixed type of infection
Discussion
Both in sheep and goats the prevalence of gastrointestinal strongyles was high both in dry and wet seasons, and in all agroecological zones. The prevalence of strongyles was significantly higher in wet season (χ2 = 17.743, P < 0.05) than dry the season. Moreover, the prevalence of strongyles was significantly higher in sheep (χ2 = 14.087, P < 0.05) than in goats. There was statistically significant difference in the prevalence of strongyles infection between the agro-ecological zones, which was higher in highland and midland (χ2 = 77.542, P < 0.05) than in the lowland. This study result, especially in sheep is consistent with the reports of Fikru et al. (2006) and Waruiru et al. (2005). The observed significant prevalence difference between sheep than goats was accounted to the variation in the feeding behaviour of the two species. Similar to the current study result Yasser et al. (2009) and Fikru et al. (2006) also reported higher overall prevalence in wet season than dry season. Hot humid conditions of tropical regions are favorable for the development, survival and transmission of free stage infective larvae of the parasitic nematodes (Urquhart et al. 1996; Hansen and Perry 1994).
A higher mean EPG was recorded in wet season (P < 0.05) than dry season. This observation is in a general agreement with the report of Nginyi et al. (2001) and Pandey et al. (1994). The overstocking grazing practice of communal pasture land of African countries including Ethiopia is a responsible cause for the availability of infective larvae of nematodes on pasture during the warm wet season (Hansen and Perry 1994). The observed significant difference in the mean EPG between different agro-ecological zones (F = 4.605, P < 0.05) and season (F = 6.878, P < 0.05) is in line with the report of Fikru et al. (2006) and Kumba et al. (2003). Highest mean EPG was recorded during wet season in the midland area. Hot and humid tropical environment positively influence the survival and development of infective larval stage of most parasites (Radostits et al. 2007; Urquhart et al. 1996).
The post mortem examination revealed that sheep and goats infection by multiple gastrointestinal helminthes was very common. About 85 and 70.8 % of sheep and goats harboured more than two parasites, respectively. During the post mortem examination higher proportion and burden of H. Contortus, T. Colubriformis and T. axei were recorded in both species, in all the study areas and seasons, which is in a agreement with the report of Bersissa et al. (2011) and Asha and Wossene (2007). In a general agreement with the current finding Haileleul (2002) and Molalegne et al. (2011) also reported Bunostomum species in small ruminants from central and southern parts of the country.
The finding of Teladorsagia species in those sheep coming from highland area is in line with the report of Asha and Wossene (2007). Teladorsagia is a temperate parasite which is adapted to cool environment (Radostits et al. 2007). Moreover, higher proportion Haemonchus contortus, Trichostrongylus axei and Trichostrongylus colubriformis were recovered from both sheep and goats. The report of Molalegne et al. (2011), Asha and Wossene (2007), Haileleul (2002) and Achenef (1997) were in agreement with what we observed. According to Radostits et al. (2007) these parasites are more prevalent in warm and moist climatic conditions.
Multiple infections with more than one species of helminth were the most frequent among examined animals in the study area. This observation is in consistent with the report of Bersissa et al. (2011), Asha and Wossene (2007), Fikru et al. (2006) and Haileleul (2002) from Ethiopia, and various parts of the world (Yasser et al. 2009; Waruiru et al. 2005; Wang et al. 2006; Agyei 2003 and Nahed-Toral et al. 2003).
Conclusion and recommendation
The results of this study revealed the existence of diversified helminths of small ruminants in all agro-ecological zones of the study areas. The higher prevalence of gastrointestinal strongyles and multiple parasitisms can be a causes of illness and failure of productivity in small ruminants.
Therefore, there is a need to design feasible strategy for the control of gastrointestinal helminthosis in sheep and goats the study areas. Improved animal husbandry practices especially better animal feeding systems (i.e. dry period supplement) could also be helpful.
Acknowledgments
We like to express our gratitude to Arba-Minch University Research and Development Coordination Directorate and Wolaita Sodo Regional Veterinary Laboratory for their great support.
Reference
- Achenef B (1997) Observation of ovine gastrointestinal nematodosis and coenuroses in sheep population on Ethiopian highland, Debre-Berhan, North Showa. DVM thesis, Addis Ababa University Faculty of Veterinary Medicine, Debre-Zeit, Ethiopia
- Agyei AD. Epidemiological studies on gastrointestinal parasitic infections of lambs in the costal savanna regions of Ghana. Trop Anim Health Prod. 2003;35:207–217. doi: 10.1023/A:1023339328589. [DOI] [PubMed] [Google Scholar]
- Asha A, Wossene A. Gastrointestinal nematodosis of small ruminants in different agro-ecological zones in southern Ethiopia. Ethiop Vet J. 2007;11(1):89–91. [Google Scholar]
- Bersissa K, Tigist T, Teshale S, Reta D, Bedru H. Helminth of sheep and goats in Central Oromia (Ethiopia) during the dry season. J Anim Vet Adv. 2011;10(14):1845–1849. doi: 10.3923/javaa.2011.1845.1849. [DOI] [Google Scholar]
- Fikru R, Teshale S, Reta D, Yosef K. Epidemiology of gastrointestinal parasites of ruminants in Western Oromia, Ethiopia. Int J Appl Res Vet Med. 2006;4:51–57. [Google Scholar]
- Gamo-Gofa zone BRD (2010/11) Gamo-Gofa zone Bureau of Rural Development Annual report
- Githiori JB, Hogland J, Waller PJ, Baker RL. Evaluation of anthelmintic properties of some plants used as livestock dewormers against Haemonchuscontortus infection in sheep. Parasitology. 2004;129:245–253. doi: 10.1017/S0031182004005566. [DOI] [PubMed] [Google Scholar]
- Haileleul N (2002) Study on prevalence of gastrointestinal helminthes in small ruminants in and around Wolaita Sodo, Southern Ethiopia. DVM thesis, Addis Ababa University Faculty of Veterinary Medicine, Debre-Zeit, Ethiopia
- Hansen J, Perry B. The epidemiology, diagnosis and control of helminth parasites of ruminants, a handbook. Nairobi: International Laboratory for Research on Animal Diseases; 1994. p. 171. [Google Scholar]
- Khan MN, Sajid MS, Iqbal Z, Hussain A. Gastrointestinal helminthiasis: prevalence and associated determinants in domestic ruminants of district Toba Tek Singh. Punjab Pak Parasitol Res. 2010;107:787–794. doi: 10.1007/s00436-010-1931-x. [DOI] [PubMed] [Google Scholar]
- Kumba FF, Katjivena H, Kauta G, Lutaaya E. Seasonal evolution of fecal egg output by gastrointestinal worms in goats on communal farms in eastern Namibia. Onderstepoort J Vet Res. 2003;70(4):265–271. doi: 10.4102/ojvr.v70i4.291. [DOI] [PubMed] [Google Scholar]
- Lebbie SHB, Rey B, Irungu EK (1994) Small ruminant Research and Development in Africa: In: Proceedings of the second biennial conference of the African small ruminant research network, ILCA, pp 1–6
- MAFF (1984) Manual of Veterinary Investigation Laboratory Techniques, London vol. 2:162–184
- Miller JE, Bahirathan M, Lemarie SL, Hembry FG, Kearney MT, Barras SR. Epidemiology of gastrointestinal nematode parasitism in Suffolk and gulf COAST native sheep with special emphasis on relative susceptibility to Haemonchus contortus infection. Vet Parasitol. 1998;74:55–74. doi: 10.1016/S0304-4017(97)00094-0. [DOI] [PubMed] [Google Scholar]
- Molalegne B, Yeshitla A, Kidist B. Abomasal and small intestinal nematodes ruminants slaughtered in different restaurants in Hawassa. Vet Res. 2011;4(2):39–44. [Google Scholar]
- Nahed-Toral J, Lopez-Tirado Q, Mendoza-Mart’inez G, Aluja-Schunemann A, Tirgo-Tavera FJ. Epidemiology parasitosis in the Tzotzil sheep production system. Small Rumin Res. 2003;49:199–206. doi: 10.1016/S0921-4488(03)00076-2. [DOI] [Google Scholar]
- Nginyi JM, Duncan JL, Mellor DJ, Stear MJ, Wanyangu SW, Bain RK, Gatongi PM. Epidemiology of parasitic gastro-intestinal nematode infections of ruminants on smallholder farms in central Kenya. Res Vet Sci. 2001;70:33–39. doi: 10.1053/rvsc.2000.0438. [DOI] [PubMed] [Google Scholar]
- Pandey VS, Ndao M, Kumar V. Seasonal prevalence of gastrointestinal nematodes in communal land goats from the highveld of Zimbabwe. Vet Parasitol. 1994;51:241–248. doi: 10.1016/0304-4017(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Radostits OM, Gay CC, Hinchcliff KW, Constable PD. Veterinary medicine, a text book of the disease of cattle, sheep pigs, Goats and horses. 10. London: W.B Sounders Company Ltd; 2007. pp. 1541–1583. [Google Scholar]
- Shah-Fischer M, Say R (1989) Manual of tropical veterinary parasitology. CAB International, The Technical Center for Agricultural and Rural Cooperation (CTA)
- Soulsby EJL. Helminthes, arthropods and protozoa of domesticated animals. 7. London: Bailliere Tindall; 1982. [Google Scholar]
- Thrusfield M. Veterinary epidemiology. 3. Oxford: Blackwell Science Ltd; 2005. pp. 232–245. [Google Scholar]
- Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW. Veterinary parasitology. 2. Hoboken: Blackwell Science; 1996. [Google Scholar]
- Wang CR, Qiu JH, Zhu XQ, Han XH, Ni HB. Survey of helminths in adult sheep in Heilogjiang Province, People’s Republic of China. Vet Parasitol. 2006;140:378–382. doi: 10.1016/j.vetpar.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Waruiru RM, Mutune MN, Otieno RO. Gastrointestinal parasite infections of sheep and goats in a semi-arid area of Machakos District, Kenya. Bull Anim Health Prod Afr. 2005;53(1):25–34. [Google Scholar]
- Yasser MG, Mohammed HN, Ahemd HA, Ahemd H (2009) An epidemiological study of protozoan and nematode parasites of ruminants in tropical semi-arid district of Somaliland (northern of Somalia)