Abstract
Leishmaniasis is one of the neglected infectious diseases included in the World Health Organization’s list of the top guns of antimicrobial resistance. Miltefosine is the first and the only available oral effective therapy for leishmaniasis. For fear of its potential resistance, identification of alternative, effective and safe drugs is urgently needed. Therefore, in view of azithromycin promising activity against a number of Leishmania species, this work was carried out to evaluate the efficacy of oral azithromycin alone versus its combination with miltefosine against experimental Old World Cutaneous leishmaniasis thus, can provide another alternative oral therapy or for the first time an oral combination therapy for leishmaniasis. The experiment were carried out on Swiss strain albino mice which were treated either with miltefosine for 20 days, Azithromycin for 20 days or both drugs in combination therapy for shorter duration of 10 days. Efficacy of azithromycin mono and combination therapy with miltefosine was evaluated clinically, parasitologically and by examination of the cutaneous lesions by Transmission Electron Microscopy. The current work demonstrated superior activity of oral azithromycin over oral miltefosine in the treatment of experimentally infected mice with Leishmania major (MHOM/IL/81/FEBNI). Unfortunately, oral combination therapy of azithromycin and miltefosine for short duration though, induced dramatic clinical improvement yet, relapse rapidly developed after cessation of therapy. Oral azithromycin could be a promising oral antileishmanial agent. Further research is recommended to investigate its leishmanicidal activity against other Leishmania species thus; another alternative oral therapy for leishmaniasis can be rapidly available.
Keywords: Leishmania major, Miltefosine, Azithromycin, In vivo, Experimental, Cutaneous leishmaniasis
Introduction
Leishmaniasis, caused by infection with Leishmania parasites, represents one of the major worldwide health threats. It is transmitted by the bite of infected sandflies and includes a spectrum of diseases occurring throughout Asia, Africa, and the Americas. The disease manifestations are determined predominantly by the host’s immune response and the parasite species (Murray et al. 2005).
The prevalence of leishmaniasis has been reported approximately 12 million cases with an annual mortality rate of 60,000. Around 2 million new cases of leishmaniasis are detected every year out of which 50,000 cases were diagnosed as visceral leishmaniasis (Croft et al. 2006). Endemic areas constitute foci of anthroponotic transmission of the parasite, which increases the chances for the fast spreading of drug resistant parasites once these have been generated (Shaw 2007). A growing interest by developed countries was prompted by increased frequency of leishmaniasis cases among HIV patients and overseas travelers (Desjeux 2004; Croft and Olliaro 2011).
Current control of leishmaniasis is reliant mainly on chemotherapy of patients, but unfortunately the range of drugs available is relatively limited to pentavalent antimonials, various amphotericin B lipid formulations and a variety of other drugs such as pentamidine, miltefosine, and paromomycin (Alvar et al. 2012). The high toxicity of the antimonial formulation has been implicated in the death of several patients under treatment (Ahasan et al. 1996). Furthermore, the emergence of antimony-resistant strains has been reported (Boelaert et al. 2002; Hadighi et al. 2007; Mittal et al. 2007). Amphotericin B is the second line treatment in India and has been used in different formulations, such as the liposomal formulation known as Ambisome, which has shown a >95 % cure rate in patients of India suffering from the disease (Sundar and Chakravarty 2010; Sundar et al. 2010). However, the high cost of this treatment limits a wider use. Pentamidine is another antileishmanial drug used in endemic areas where antimonials are inefficient, however from 1983 its therapeutic efficacy has decreased significantly till 70 %, decreasing its use in different areas. The search for an effective oral antileishmanial drug spans two decades. The introduction of miltefosine, the first oral antileishmanial drug, is an encouraging new development, although there are safety concerns with women of childbearing age (Davies et al. 2004; Ramesh et al. 2011).
Azithromycin is a semi-synthetic macrolide antibiotic chemically related to erythromycin and clarithromycin. Like all macrolide antibiotics, it prevents bacteria from growing by interfering with their ability to make proteins. Its antiprotozoal activity has been shown both in vitro and in vivo as a mono or combination therapy against Toxoplasma gondii (Blais et al. 1993), Cryptosporidium parvum (Hicks et al. 1996), Acanthamoeba (Schuster and Visvesvara 1998), and Plasmodium (Noedl et al. 2006). As it concentrates in tissues and especially in macrophages, and may reach levels 100 to 200 times higher than those in serum (Gladue et al. 1989) therefore, its potential role as antileishmanial agent was evaluated. Krolewiecki et al. (2002) demonstrated that, azithromycin has antileishmanial activity following its subcutaneous adminstration in BALB\c mice infected with Leishmania major. Moreover, Sinagra et al. (2007) reported significant activity of its oral adminstration against Leishmania (Viannia) braziliensis however, no activity against infections with Leishmania (Leishmania) amazonensis in golden hamsters could be observed. Recently, azithromycin and a structuraly similar antibiotic clarithromycin showed in vitro activity against promastigotes and amastigotes of L. tropica (Balcioğlu et al. 2012).
Miltefosine was reported in a previous study carried out by the same authors (Eissa et al. 2012) to be effective in the treatment of experimental CL caused by L. major (MHOM/IL/81/FEBNI), the same strain used in this study. The wide use of miltefosine increases the fear of drug resistance emergence, which could be probably a matter of time unless preventative measures are taken. The experts agree that we need to improve surveillance for emerging antimicrobial resistance problems, to prolong the useful life of antimicrobial drugs, to develop new drugs, and to improve vaccines. The chances of drug resistance can sometimes be minimized by using multiple drugs simultaneously. The combined therapy works because individual mutations can be independent and may tackle only one drug at a time; if the individuals are still killed by the other drugs, then the mutations can not persist (en.wikipedia.org/wiki/Drug_resistance). Moreover Pérez-Victoria et al. (2003) reported that, the therapeutic window for miltefosine is very narrow as parasite resistance caused by a single point mutation can be selected easily in vitro. Therefore, it is important to protect miltefosine from development of resistance by testing its combination with other antileishmanial drugs.
In view of azithromycin promising value against a number of Leishmania species, this work was carried out to evaluate the efficacy of oral azithromycin alone versus its combination with miltefosine against experimental Old World CL. The work aimed in the first place to provide another oral therapeutic alternative to miltefosine and testing their combination for shorter treatment duration. This could provide for the first time an oral combination therapy which makes the treatment of leishmaniasis more effective, attractive and available to all sections of the society and at the same time reduce treatment duration and toxicity, improves compliance, and prolongs the useful therapeutic life-span of both drugs.
Materials and methods
Leishmania strain and its maintenance
Leishmania major strain (MHOM/IL/81/FEBNI) was maintained both in vitro and in vivo in the laboratory of Medical Parasitology Department, Faculty of Medicine, Alexandria University, Egypt. In vitro maintenance was performed by serial passage on N.N.N. medium; Offutt’s modification every 7–10 days. While, in vivo maintenance was done by inoculation into the foot pad of Swiss strain albino mice to avoid any diverse effect of continuous in vitro cultivation that may lead to loss of strain virulence (Nolan and Herman 1985). The Leishmania strain used in this study was kindly provided by Prof. Dr. Tamas Laskay (Professor of Immunology, Innate Immunity Research Unit, Institute for Medical Microbiology and Hygiene, University of Lübeck, Germany).
Culture media
N.N.N. medium supplemented with 100 IU/ml of sodium penicillin G, and 100 µg/ml of streptomycin sulfate was used for in vitro maintenance of the L. major strain and mass cultivation of leishmania promastigotes required for animal inoculation (McCartry-Burke et al. 1991).
Drugs
a- Miltefosine (Milteforan®) 2 % veterinary oral solution was kindly supplied by Dr. Paolo Bianciardi, Scientific advisor, Virbac, Italy.
b- Azithromycin (Zithromax®) Pfizer, Cairo, Egypt 250 mg in tablets form was purchased from a local pharmacy.
Animal inoculation and grouping
All animal studies presented here have been approved by the local government based on national regulations for animal experimentation and was approved by the Ethics Committee of the Faculty of Medicine, Alexandria University, Egypt.Experiments were carried out on 100 pathogen free laboratory bred Swiss strain albino mice, 4–5 weeks old and weighing 20–25 g. They were purchased from the animal house, Medical Parasitology Department, Faculty of Medicine, Alexandria University, Alexandria, Egypt. Leishmania major promastigotes in the stationary phase were harvested from N.N.N. medium on the 7th day of culture. Counting the parasites following immobilization in 1 % formalin was performed using an Improved Double Neubauer Rulling Haemocytometer at 40× objective lens under light microscope. The parasites were adjusted to the required concentration.
Animals used in this study were categorized into three main groups; groups I and II served as control and control therapy respectively while group III represented the mono and combined therapy.
Group I-control infected, non treated group
Fourty mice were infected by intradermal injection of 20 × 106 stationary-phase promastigotes into the right hind footpad (Eissa et al. 2011).
Group II-control infected, treated group (miltefosine treated control group)
Twenty mice were infected as in group I. They were treated orally with miltefosine at the third week post infection in a dose of 20 mg/kg/day for 20 successive days. The required dose was equivalent to 0.03 ml of the provided drug formula without dilution and given through oral gavage (Eissa et al. 2012).
Group III-experimental treated group
Fourty mice were infected as in group I& II. They were divided into two equal subgroups:
Group (IIIa)-azithromycin treated group (mono-therapy)
They were treated orally with azythromycin at the third week post infection in a dose of 200 mg/kg/day for 20 days. The dose required for each mouse was adjusted to 0.1 ml of distilled water and given through oral gavage. It was administrated at least 1 h before or 2 h after meals to ensure its absorption and avoid its binding to food.
Group (IIIb)-azithromycin and miltefosine treated group (combination therapy)
Both drugs were given in doses similar to group IIIa and group II but, for a shorter duration of only 10 days.
Animals of all studied groups were divided equally into two subgroups according to the time of sacrifice; at the end of treatment (Barret et al. 1999), and 1 month following treatment. Animals of the control infected non treated group (group I) were sacrificed simultaneously as well.
Efficacy of azithromycin mono and combination therapy with miltefosine (group IIIa & IIIb) was assessed by the following parameters in comparison to those of the control groups (group I & II)
Lesion size
Clinical response was evaluated by careful observation of the inoculated footpad regarding the size and severity of the cutaneous lesions. The size of the resulting lesions was assessed by weekly measurement of the increase in footpad thickness with the contra lateral footpad using a dial manual micrometer (RABONE, Swiss made), while the progression of the cutaneous lesion developmentwas assessed as regards redness, swelling, crust formation, ulceration, gangrene and autoamputation (Krolewiecki et al. 2002).
Quantification of parasite burden
Parasite load of the affected cutaneous lesion was determined using a quantitative limiting-dilution assay (Titus et al. 1985). The infected footpad was removed aseptically between the heel and toes. The weight was determined and then cut into several pieces in sterile saline supplemented with penicillin/streptomycin containing 10 % FCS and homogenized in a grinder. The homogenate were settled in aliquots to remove large debris. Serial dilutions of the homogenate were prepared in 96-well plates containing blood agar medium. A minimum of ten replicate wells for each dilution was prepared, taking the precautions one should take in any serial dilutions as changing pipettes and avoiding cross-contaminations between dilutions. Finally, a piece of parafilm was laid at the top of the open plate to keep its humidity, and then incubated at 25 °C for 10 days. The interpretation was done by scoring the highest dilution with moving parasites under inverted microscopy. Dissemination was studied by removal of the draining lymph nodes, liver and spleen of infected and infected treated groups. Impression smears were made from these organs, fixed with methanol and stained with Giemsa stain for examination under oil immersion lens. Dissemination was also evaluated by tissue culturing in N.N.N. medium for 7 days, after which the presence of live parasites was determined (Sinagra et al. 2007).
Transmission electron microscope (TEM) study
Parts of the cutaneous lesions were sliced into strips about 1 × 1 × 1 mm in cold 1.5 % glutaraldehyde fixative. These slices were placed in phosphate-buffered osmium tetroxide, dehydrated, and then embedded in Epoxy resins. Semi thin sections were made using Riechert Jung microtome and stained with toluidine blue for light microscopy. Ultrathin sections were cut and stained with uranyl acetate and lead citrate, then examined under Joel TEM (Hayat 1981).
Statistical analysis
The collected, revised and verified data were analyzed by the Statistical Package for Social Science (SPSS) program, version 15.0 for Windows. Data were described using median, minimum and maximum. P value for Kruskal–Wallis test between different studied groups was detected. The significance between each two groups was assessed by Mann–Whitney test.
Results
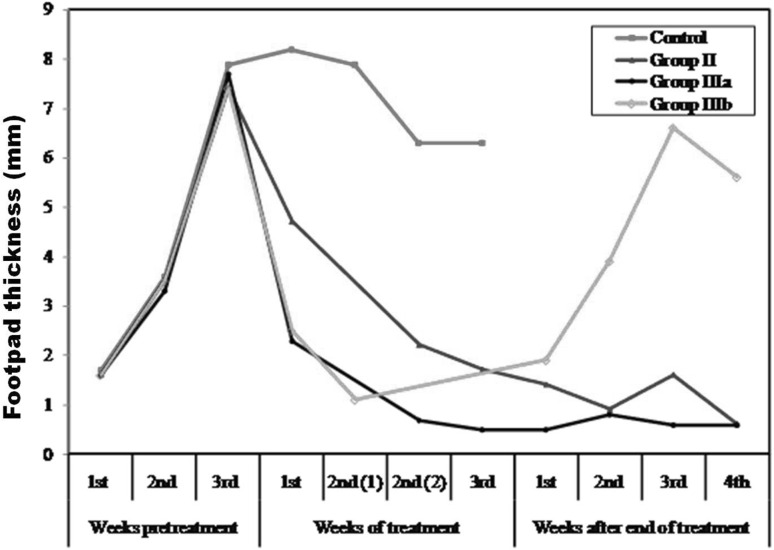
Swelling of the inoculated footpads of all studied groups started at the end of the first week post infection, and increased progressively till reach its maximum at the third week post infection, the time where treatment started before the development of irreversible lesions. The difference in footpad thickness in comparison with the contra-lateral side was weekly calculated. In infected non treated mice (group I), lesion size was slightly increased up to the fifth week. Later on, lesions sizes could not be measured due ulceration, gangrenous changes and auto-amputation of the inoculated foot pads by the 8th week post infection. Miltefosine treated mice (group II) showed, statistically significant gradual decrease in the lesion size when compared to infected non treated group (groupI). At the end of miltefosine therapy, the mean difference in footpad thickness with contra lateral site was statistically non significant (Table 1) (Fig. 1). Clinically, animals’ foot pads were apparently normal in texture, size, and color. One month after therapy, no signs of relapse could be observed.
Table 1.
The footpad lesion size (mm) among the different studied groups along the experiment duration
| Weeks post infection | Group I | Group II | Group IIIa | Group IIIb | p |
|---|---|---|---|---|---|
| Weeks pretreatment | |||||
| 1st | 1.50 (1.0–3.0) | 2.0 (0.0–3.0) | 1.0 (1.0–3.0) | 1.50 (1.0–3.0) | 0.936 |
| 2nd | 4.0 (3.0–4.0) | 3.50 (3.0–4.0) | 3.0 (3.0–4.0) | 3.50 (3.0–4.0) | 0.603 |
| 3rd | 8.0 (6.0–10.0) | 8.0 (5.0–9.0) | 8.0 (6.0–9.0) | 7.0 (6.0–9.0) | 0.859 |
| Weeks of treatment | |||||
| 1st | 8.0 (7.0–10.0) | 5.0 (3.0–6.0) | 2.50 (1.0–3.0) | 2.0 (2.0–4.0) | <0.001* |
| Sig. with | I*** | I***, II*** | I***, II** | ||
| 2nd (1) | 8.0 (6.0–10.0) | – | – | 1.0 (0.0–2.0) | – |
| Sig. with | – | – | I*** | ||
| 2nd (2) | 6.50 (4.0–10.0) | 2.0 (1.0–3.0) | 1.0 (0.0–1.0) | – | <0.001* |
| Sig. with | I*** | I***, II** | |||
| 3rd | – | 1.50 (0.0–3.0) | 0.50 (0.0–1.0) | – | – |
| Sig. with | II** | – | |||
| Weeks after end of treatment | |||||
| 1st | – | 1.0 (1.0–2.0) | 0.50 (0.0–1.0) | 2.0 (1.0–3.0) | 0.001* |
| Sig. with | II** | IIIa** | |||
| 2nd | – | 1.0 (0.0–2.0) | 0.50 (0.0–2.0) | 4.0 (3.0–5.0) | <0.001* |
| Sig. with | NS | II***, IIIa*** | |||
| 3rd | – | 1.0 (1.0–3.0) | 1.0 (0.0–1.0) | 6.50 (5.0–9.0) | <0.001* |
| Sig. with | II** | II***, IIIa*** | |||
| 4th | – | 1.0 (0.0–1.0) | 0.50 (0.0–2.0) | 6.0 (5.0–6.0) | <0.001* |
| Sig. with | NS | II***, IIIa*** | |||
Data were described using median, minimum and maximum
p: p value for Kruskal–Wallis test between different studied groups
The significance between each two groups was assessed by Mann–Whitney test; * Significant at P < 0.05; ** significant at P < 0.01; *** significant at P < 0.001 Group I: infected non treated mice; Group II: infected treated mice with miltefosine for 20 days; Group IIIa: infected treated mice with azithromycin for 20 days; Group IIIb: infected treated mice with combination of miltefosine and azithromycin for 10 days; 2nd (1): corresponding to 10 days of receiving therapy; 2nd (2): corresponding to 15 days of receiving therapy
NS no significance
Fig. 1.
The mean footpad lesion size of the different studied subgroups
As regards the experimental group (azithromycin treated group), animals that were treated only with azithromycin (group IIIa- monotherapy) showed, rapid onset of decreased footpads thickness. The mean lesion size was gradually decreased. Two weeks after therapy, no significant differences were demonstrated with those of contra-lateral side. Clinically, the animals’ footpads were completely normal whether at the end of therapy or 1 month after its cessation. On the other hand, oral combination therapy of azithromycin and miltefosine for short duration of only 10 days (group IIIb) showed significant difference in lesion size with the contra lateral side. However, in comparison with treated groups (group II & group IIIa), the decrease in lesion size was a bit lower than those achieved by azithromycin treated group, but was superior to miltefosine treated group. After cessation of treatment, close clinical observation of treated footpads demonstrated signs of relapse. Gradual progressive development of the lesions, crust formation and ulceration at the end of the experiment were noticed. Marked significant difference in lesion size with the contralateral side was observed (Table 1) (Fig. 1).
Parasite load recovered from lesions were evaluated by limiting dilution assay. At the end of treatment, all regimen (miltefosine, azithromycin and combination therapy) succeeded to induced significant reduction in parasite burden with the highest reduction in azithromycin treated group (groupIIIa-monotherapy) where parasites could hardly be detected in the early dilution series. At the end of therapy, there was no significant difference in parasite load between miltefosine treated control group (group II) and combination therapy group (group IIIb). One month after the end of therapy, reliable parasite counts could not be obtained for control non treated group because of gangrenous changes of the lesions and occurrence of partial or total autoamputation of the foot pad. Whereas, in other groups it was observed that in azithromycin monotherapy (group IIIa) no significant difference in parasite burden when compared with those achieved immediately after therapy stoppage. Unfortunately, those received combination therapy (group IIIb), there was statistically significant increase in the parasite load as compared to those achieved by monotherapy groups (group II & group IIIa) (Table 2).
Table 2.
The mean parasite load (lesion) of the different studied groups at the end of treatment and 1 month later
| Group I | Group II | Group IIIa | Group IIIb | p | ||
|---|---|---|---|---|---|---|
| C1 | C2 | |||||
| End of treatment | 107 (106–107) | 107 (107–107) | 103 (102–104) | 101 (101–102) | 104 (103–105) | <0.001* |
| Sig. with | C2*** | C2***,II*** | C1***, IIIa*** | |||
| 1 month post treatment | – | – | 102 (101–103) | 101 (101–102) | 106 (105–106) | 0.002* |
| Sig. with | II** | II***, IIIa*** | ||||
Data were described using median, minimum and maximum
C1 control infected non treated group corresponding to group IIIb, C2 control infected non treated group corresponding to group II and group IIIa; Group II: infected treated mice with miltefosine for 20 days; Group IIIa: infected treated mice with azithromycin for 20 days; Group IIIb: infected treated mice with combination of miltefosine and azithromycin for 10 days. * Significant at P < 0.05; ** significant at P < 0.01; *** significant at P < 0.001
In the present study, dissemination of L major (MHOM/IL/81/FEBNI) amastigotes to different organs could not be observed whether in mice sacrificed at the 6th or 10th week post infection. Impression smears prepared from liver, spleen and lymph nodes of infected non-treated control (group I) were negative. These finding was confirmed by tissue culturing on N.N.N. medium and histopathological examination of H&E stained sections of these organs (data not shown).
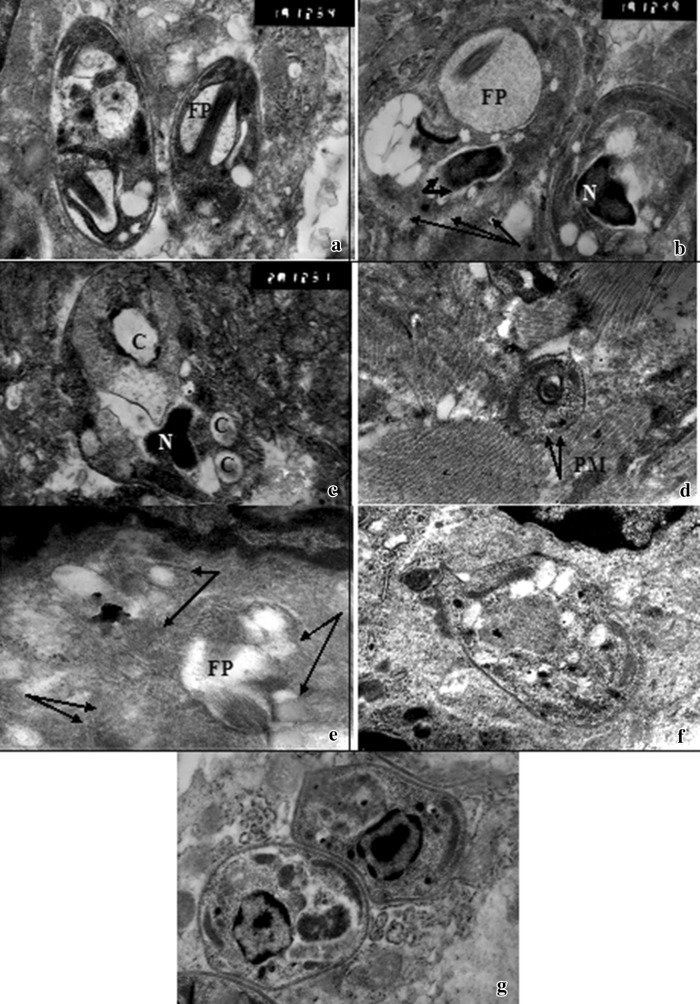
Ultrathin structural changes of amastigotes in miltefosine treated control mice were detected. The main drug target was amastigotes plasma membrane. Furthermore, absence of the nuclear envelops and abnormal nuclear chromatin distributions were detected. Numerous non-membrane bounded cavities were evident (Fig. 2a–c). On the other hand, amastigotes of AZM treated group could hardly be detected and showed dramatic morphological and structural changes. They were variable from discrete forms to digestion of intra cellular compartments with total disruption of parasite structure (not shown). Disfigurement of parasite contour, vacuolization of the cytoplasm were observed. In addition, AZM induced alterations at the plasma membrane (Fig. 2d–f). These ultra structural changes were not detected in the control untreated group (Fig. 2g)
Fig. 2.
Transmission electron micrograph (TEM) of cutaneous lesions of infected treated mice a, b, c Infected mice treated with miltefosine (group II) showing a L. amastigotes with flagellar knot inside dilated flagellar pocket (FP) (15,000×). b L. amastigotes with extensive dilatation of flagellar pocket (FP), disintegration of plasma membrane (arrows), abnormal nuclear chromatin condensation (N), and absence of nuclear membrane (thick short arrows) (15,000). c L. amastigotes with homogenous nucleus (N), cytoplasmic vaculization, and multiple membrane bound cavities (C) (20,000×). d, e, f Infected mouse treated with azithromycin (group IIIa) showing d L. amastigotes with disintegration of plasma membrane (PM) (arrows) (7,500×). e L. amastigotes with ill defined parasite contour and content(arrows) but with evident flagellar pocket (FP) (15,000×). f L. amastigotes with cytoplasmic vacuolization (13,000×). g L. amastigotes of infected untreated control group
Discussion
Despite good knowledge on the epidemiology leishmaniasis, it still remains uncontrollable. Hence, efforts should be made to develop newer drugs which should be cheap, less toxic and easy to administer. Emergence of drug resistance along with lack of standard molecular markers for its detection makes the situation worse.
Miltefosine is the first effective oral treatment for both visceral and cutaneous leishmaniasis (Berman and Lane 2008). For fear of its potential resistance, studies on identification of alternative effective and safe drugs or its use in combination therapy are going on.
The current work demonstrated that azithromycin, the safe and the highly tolerated antibiotics, is an effective oral treatment for experimentally infected mice with Leishmania major (MHOM/IL/81/FEBNI). The choice of the dose of azithromycin used in this study was based on the usual dose used in previous studies against different protozoa and proved to be effective and well tolerated by animals (Allam et al. 1999; Sadaka et al. 1999; Hicks et al. 1996). In the current work, its oral administration as a monotherapy in a dose of 200 mg/kg for 20 consecutive days proved to be highly effective in achieving complete clinical cure. The rapid onset of azithromycin in achieving this goal is probably due to its immunomodulatory activity through preventing the production of pro-inflammatory mediators and cytokines (Ianaro et al. 2000). It induced statistically significant reduction in parasitic load. Moreover, no signs of relapse could be observed in mice sacrificed one month after cessation of treatment. Krolewiecki et al. (2002) demonstrated that treatment of L. major (MHOM/IL/80/Friedlin) infected BALB/cByJ mice with 100–200 mg/kg azithromycin by subcutaneous route on the same day of infection, 5 times a week for 4–8 weeks, resulted in reduced swelling of the inoculated foot pad and the lesions parasite load. Whereas, no effect was detected in the treated C57BL/6 J mice. The latter mice strain could be considered resistant as similarly, amphotericin B was reported to be ineffective in treatment of L.tropica C57BL/6 mice (Panosian et al. 1984).
The high efficacy of azithromycin reported in this study could be related to the different administration route, the initiation time of the therapy and the used mice strain. Contrasting to these findings, in an area endemic for L. major in Syria, no cures were recorded among 45 patients with CL using ten-day cycles of azithromycin (Daoud and Boushi 2006). On the other hand, oral azithromycin at 450 mg/kg had no activity in golden hamsters against infections with L.amazonensis. For infections due to L.braziliensis, azithromycin demonstrated significant activity relative to untreated controls, but inferior to meglumine antimoniate, for controlling lesion size. Neither drug was able to totally eliminate parasites from the lesions (Sinagra et al. 2007). In addition, the previously reported clinical experiences include an uncontrolled study that involved 20 patients with CL in an area endemic for L. (Viannia) braziliensis treated with variable doses of azithromycin for two to ten days with a cure rate of 85 % (Prata et al. 2003). Three cases of mucosal leishmaniasis with contraindications for the use of antimonials were successfully treated with three cycles of 10 days of azithromycin in Brazil (Silva-Vergara et al. 2004). This indicates that the response of leishmaniasis to azithromycin treatment seems to be variable, depending on several factors such as, the causative species, different strains of each species and the affected geographical areas. Thus it is evident that chemotherapy of CL needs to be evaluated in each endemic region, even if the “same” species of Leishmania causes disease in these locales (Escobar et al. 2002; Filho et al. 2008).
Eissa et al. (2012) proved the in vivo efficacy of oral miltefosine in a daily dose of 20 mg/kg for 20 days against L. major (MHOM/IL/81/FEBNI) in Swiss albino mice model. The drug achieved complete clinical cure of skin lesions but parasitologically, no complete elimination of the parasite at the lesions site was detected. The present experiment demonstrated that oral azithromycin when given as monotherapy, was significantly superior to miltefosine in reduction of the parasite burden. This indicates that, Leishmania major could be more sensitive to azithromycin than miltefosine. This finding was compatible with previous researches, in vitro data showed that the sensitivity of different Leishmania species to miltefosine was variable, the rank order was L. donovani > L. aethiopica > L. tropica > L. mexicana > L. panamensis > L. major (Escobar et al. 2002). This variation was attributed to the variation in both membrane sterol and phospholipid concentration in different Leishmania species, which are important targets for miltefosine to perform its leishmanicidal activity.
Efforts are needed to develop combination therapy with other drugs that have tolerability, compatibility, and prospective immunomodulatory roles to shorten the duration of treatment, ensure the persistent therapeutic effect, and prolong the effective life of the drug (Jha 2006; Loiseau and Bories 2006; Sundar and Chakravarty 2008).
It can potentially broaden the spectrum, increase the activity of the drug by additive or synergistic action, decrease the duration and dosage, reduces the side effects and thereby reduces the cost of treatment and the emergence of drug resistance. Among the anti-leishmanials, miltefosine and paromomycin were found appropriate for combination therapy because of less interference with other drugs. The use of different dose regimen in combination therapy of antileishmanial drugs was applied by many researchers (Hailu et al. 2010; Sundar et al. 2011; Musa et al. 2012). They all showed that combination treatment is safe and effective in visceral leishmaniasis (VL), offering a significant reduction in treatment duration. A randomized, controlled clinical trial was performed by Hailu et al. (2010) to compare three treatment regimens for VL in East Africa: paromomycin sulphate (PM) at 15 mg/kg/day for 21 days versus sodium stibogluconate (SSG) at 20 mg/kg/day for 30 days; and the combination of both dose regimens for shorter duration therapy of 17 days. Furthermore, a control trial was carried out in East Africa for the treatment of VL comparing paramomycin (PM) 20 mg/kg/day for 21 days monotherapy with PM and sodium stibogluconate combination(PM 15 mg/kg/day and SSG 20 mg/kg/day for 17 day) and SSG (30 mg/kg/day for 30 days) alone. The 17 day combination regimen found equally efficacious and safer in comparison to 30 days regimen (Musa et al. 2012). In a trial to achieve this goal, in this study, combination of miltefosine with azitheromycin in their effective doses for shorter duration therapy of only 10 successive days was tested against L. major. In response to this combination, dramatic clinical improvement of lesions with significant reduction of parasite burden was achieved but rapid relapse after stoppage of treatment was observed. Whereas, Aguiar et al. (2009) investigated the activity of miltefosine alone, given at a dose of 25 mg/kg/day for the treatment of experimental cutaneous leishmaniasis caused by L. major versus its combination with topical 10 % paromomycin gel twice a day for 10 days, reported statistically significant reduction in lesion size and parasite burden in the skin, with complete healing of ulcers, as compared with those treated with oral miltefosine alone. de Morais-Teixeira et al. (2014) evaluated the in vitro interactions between paromomycin sulphate and different antileishmanial drugs including azitheromycin and miltefosine against New World leishmaniasis. They provided a preclinical dataset that supports future in vivo studies on multidrug treatment schedules as in vitro synergism was observed for the combinations of paromomycin with all tested antileishmanial drugs. In the current study combination therapy of azithromycin and miltefosine for short duration though, induced dramatic clinical improvement yet, relapse rapidly developed after cessation of therapy. The mechanisms behind such rapid relapse needs to be further investigated
Dissemination of L. major to visceral organs of reticulo-endothelial system in infected non treated mice along the duration of the experiment could not be observed. This finding was contradicted with another study which reported the detection of the parasite of the same cutaneous Leishmania species (L. major) in spleen of infected BALB/c mice (Aguiar et al. 2009). This difference could be attributed to different mouse model. However, in another study done by the same authors, dissemination of this L.major strain in Swiss albino mice was observed to occur only in immunosuppresded mice (data under publications).
Electron microscopy is an important approach for confirming the postulated drug-target interactions. It can identify not only the target organelles but also ultrastructural changes that characterize distinct forms of programmed cell death (Adade and Souto-Padrón 2010). Miltefosine treatment revealed the strong possibility of an apoptosis-like mode of cell death in Leishmania promastigotes, as well as extra cellular and intracellular amastigotes (Paris et al. 2004). Miltefosine interferes with cellular carrier proteins; leading to depletion of essential nutrients and thus contribute to growth arrest and cell death (Berkovic et al. 1992). In addition, sphingomyelin biosynthesis has been shown to be inhibited by miltefosine, leading to increased levels of cellular ceramide; which triggers apoptosis (Wieder et al. 1999). On the other hands, the exact mechanism by which azithromycin induce activity against Leishmania is unknown. It is also possible that azithromycin has an immunomodulatory effect (Gerhardt et al. 2003), macrophage activation killing mechanism (Xu et al. 1996), and a direct killing effect (Blais et al. 1993). In the present work, its effect on the ultrastructure of the amastigotes was analyzed by electron microscopy. Azithromycin induced dramatic morphological alterations in the parasite structure and induced plasma membrane distortion till total destruction and parasite lyses. The formation of large vacuoles followed by a total destruction of the cell strongly suggests an autophagic-like or necrotic mechanism of cell death (Bera et al. 2003; Menna-Barreto et al. 2009; Marr et al. 2012). In fact, unlike apoptosis, autophagic cell death is a process characterized by the accumulation of autophagic vacuoles in the cytoplasm accompanied by extensive degradation of the Golgi apparatus, the polyribosomes and the endoplasmic reticulum, which precedes the destruction of the nucleus (Lefranc et al. 2007). Alterations at the plasma membrane and formation of autophagic structures, which could have been the result of recycling of abnormal membranes during the processing of damaged organelles, as what has been described in suramin-treated Trypanosoma rhodesiense (Macadam and Williamson 1974). The suggested direct effect of azithromycin against Leishmania in this experiment is considered as in vivo confirmatory extension with electron microscopic evidences to the in vitro direct inhibitory effect of azithromycin against L. major previously reported by Krolewiecki et al. (2002).
In conclusion, our data demonstrated superior activity of oral azithromycin over oral miltefosine in treatment of L. major infected mice. Unfortunately, oral combination therapy of azithromycin and miltefosine for short duration of 10 days though, induced dramatic clinical improvement yet, relapse rapidly developed after cessation of therapy. Further research is recommended to investigate the leishmanicidal activity of oral azithromycin against other Leishmania species thus, another alternative oral therapy for leishmaniasis can be rapidly available.
Acknowledgments
We are grateful to Prof. Dr. Tamas Laskay, Professor of Immunology, Research Unit, Institute for Medical Microbiology and Hygiene, University of Lubeck, Germany, for providing the Leishmania strain, Dr. Paolo Bianciardi, Scientific Advisor, Virbac, Italy, for providing miltefosine, and Mrs Nemat Ahmed, Senior Technician, Medical Parasitology Department, Faculty of Medicine, Alexandria University, Alexandria, Egypt, for excellent work with the culture of Leishmania.
Conflict of interests
We declare that we have no conflict of interest, as all authors do not have any financial relation with the commercial identities mentioned in the Materials and methods section of this manuscript.
References
- Adade CM, Souto-Padrón T. Contributions of ultrastructural studies to the cell biology of trypanosmatids: targets for anti-parasitic drugs. Open Parasitol J. 2010;4:178–187. doi: 10.2174/1874421401004010178. [DOI] [Google Scholar]
- Aguiar MG, Silva DL, Nunan FA, Nunan EA, Fernandes AP, Ferreira LAM. Combined topical paromomycin and oral miltefosine treatment of mice experimentally infected with Leishmania major leads to reduction in both lesion size and systemic parasite burdens. J Antimicrob Chemoth. 2009;64:1234–1240. doi: 10.1093/jac/dkp365. [DOI] [PubMed] [Google Scholar]
- Ahasan HA, Chowdhury MA, Azhar MA, Rafiqueuddin AK, Azad KA. Deaths in visceral leishmaniasis (Kala-azar) during treatment. Med J Malaysia. 1996;51:29–32. [PubMed] [Google Scholar]
- Allam SR, Sadaka HAH, Eissa MM. A Novel macrolide in mixed intestinal protozoal infection on immunosuppressed mice. J Med Res Inst. 1999;20(4):149–159. [Google Scholar]
- Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7:e3567. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcioğlu İC, Gırgınkardeşler N, Ok ÜZ, Özbılgın A, Özbel Y. The in vitro effects of azithromycin and clarithromycin on promastigotes and amastigotes of leishmania tropica. Kafkas Univ Vet Fak Derg. 2012;18:115–120. [Google Scholar]
- Barret MP, Mott-ram JC, Commbs GH. Recent advances in identifying and validating drug targets in Trypanosomes and Leishmanias. Trends Microbiol. 1999;7:82–88. doi: 10.1016/S0966-842X(98)01433-4. [DOI] [PubMed] [Google Scholar]
- Bera A, Singh S, Nagaraj R, Vaidya T. Induction of autophagic cell death in Leishmania donovani by antimicrobial peptides. Mol Biochem Parasitol. 2003;127:23–35. doi: 10.1016/S0166-6851(02)00300-6. [DOI] [PubMed] [Google Scholar]
- Berkovic D, Fleer EAM, Eibl H, Unger C. Effects of hexadecylphosphocholine on cellular function. Prog Exp Tumor Res. 1992;34:59–68. doi: 10.1159/000420832. [DOI] [PubMed] [Google Scholar]
- Berman J, Lane P. Treatment of leishmaniasis with miltefosine: 2008 status. Expert Opin Drug Met. 2008;4(9):1209–1216. doi: 10.1517/17425255.4.9.1209. [DOI] [PubMed] [Google Scholar]
- Blais J, Garneau V, Chamberland S. Inhibition of Toxoplasma gondii protein synthesis by azithromycin. Antimicrob Agents Ch. 1993;37:1701–1703. doi: 10.1128/AAC.37.8.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert M, Le-ray D, Van-der SP. How better drugs could change kala-azar control. Lessons from a cost-effectiveness analysis. Trop Med Int Health. 2002;7:955–959. doi: 10.1046/j.1365-3156.2002.00959.x. [DOI] [PubMed] [Google Scholar]
- Croft SL, Olliaro P. Leishmaniasis chemotherapy—challenges and opportunities. Clin Microbiol Infec. 2011;17:1478–1483. doi: 10.1111/j.1469-0691.2011.03630.x. [DOI] [PubMed] [Google Scholar]
- Croft SL, Seifert K, Yardley V. Current scenario of drug development for leishmaniasis. Indian J Med Res. 2006;123(3):399–410. [PubMed] [Google Scholar]
- Daoud S, Boushi L. Azithromycin, ineffective in the treatment of old-world cutaneous leishmaniasis. Int J Dermatol. 2006;45:1126–1128. doi: 10.1111/j.1365-4632.2006.02885.x. [DOI] [PubMed] [Google Scholar]
- Davies CR, Kaye PM, Croft SL, Sundar S. Leishmaniasis: new approaches to disease control. Brit Med J. 2004;326:377–382. doi: 10.1136/bmj.326.7385.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Morais-Teixeira E, Gallupo MK, Rodrigues LF, Romanha ÁJ, Rabello A. In vitro interaction between paromomycin sulphate and four drugs with leishmanicidal activity against three new world Leishmania species. J Antimicrobial Chemother. 2014;96(1):150–154. doi: 10.1093/jac/dkt318. [DOI] [PubMed] [Google Scholar]
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microb. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Eissa MM, Amer EI, El-Sawy SMF. Leishmania major: activity of tamoxifen against experimental cutaneous leishmaniasis. Exp Parasitol. 2011;128:382–390. doi: 10.1016/j.exppara.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Eissa MM, Amer EI, Mosallam SF, Gomaa MA, Baddour NM. Miltefosine for old world cutaneous leishmaniasis: an experimental study on Leishmania major infected mice. Alex J Med. 2012;48:261–271. doi: 10.1016/j.ajme.2012.04.003. [DOI] [Google Scholar]
- Escobar P, Matus S, Marques C, Croft SL. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), Et-18-och3 (eldefosine) and amphotericin B. Acta Trop. 2002;81(2):151–157. doi: 10.1016/S0001-706X(01)00197-8. [DOI] [PubMed] [Google Scholar]
- Filho AVC, Lucas IC, Sampaio RNR. Comparative study between oral miltefosine and parenteral antimonate of N-methyl glucamine in the treatment of experimental leishmaniasis caused by Leishmania amazonensis. Rev Soc Bras Med Trop. 2008;41(4):340–353. doi: 10.1590/s0037-86822008000400022. [DOI] [PubMed] [Google Scholar]
- Gerhardt SG, McDyer JF, Girgis RE, Conte JV, Yang SC, Orens JB. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am J Resp Crit Care. 2003;168:121–125. doi: 10.1164/rccm.200212-1424BC. [DOI] [PubMed] [Google Scholar]
- Gladue RP, Bright GM, Isaacson RE, Newborg MF. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob Agents Ch. 1989;33:277–282. doi: 10.1128/AAC.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadighi R, Boucher P, Khamesipour A, Meamar AR, Roy G, Ouellette M, Mohebali M. Glucantime-resistant Leishmania tropica isolated from patients with cutaneous leishmaniasis are sensitive to alternative antileishmania drugs. Parasitol Res. 2007;101:1319–1322. doi: 10.1007/s00436-007-0638-0. [DOI] [PubMed] [Google Scholar]
- Hailu A, Musa A, Wasunna M, Balasegaram M, Yifru S, Mengistu G, Hurissa Z, et al. Geographical variation in the response of visceral leishmaniasis to paromomycin in East Africa: a multicentre, open-label, randomized trial. PLoS Negl Trop Dis. 2010;4(10):e709. doi: 10.1371/journal.pntd.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat MA. Principles and techniques of electron microscopy. 2. Union: New Jersey University Park Press; 1981. Biological application; pp. 340–356. [Google Scholar]
- Hicks P, Zwiener RJ, Squires J, Savell V. Azithromycin therapy for Cryptosporidium parvum infection in four children infected with human immunodeficiencyvirus. J Pediatr. 1996;129:297–300. doi: 10.1016/S0022-3476(96)70258-5. [DOI] [PubMed] [Google Scholar]
- Ianaro A, Ialenti A, Maffia P, Sautebin L, Rombola L, Carnuccio R, Iuvone T, D’Acquisto F, Di Rosa M. Anti-inflammatory activity of macrolide antibiotics. J Pharmaco Exp Ther. 2000;292:156–163. [PubMed] [Google Scholar]
- Jha TK. Drug unresponsiveness & combination therapy for kala-azar. Indian J Med Res. 2006;123:389–398. [PubMed] [Google Scholar]
- Krolewiecki A, Leon S, Scott P, Abraham D. Activity of azithromycin against Leishmania major in vitro and in vivo. Am J Trop Med Hyg. 2002;67:273–277. doi: 10.4269/ajtmh.2002.67.273. [DOI] [PubMed] [Google Scholar]
- Lefranc F, Facchini V, Kiss R. Proautophagic drugs: a novel means to combat apoptosis-resistant cancers, with a special emphasis on glioblastomas. Oncologist. 2007;12:1395–1403. doi: 10.1634/theoncologist.12-12-1395. [DOI] [PubMed] [Google Scholar]
- Loiseau PM, Bories C. Mechanisms of drug action and drug resistance in Leishmania as basis for therapeutic target identification and design of antileishmanial modulators. Curr Top Med Chem. 2006;6:539–550. doi: 10.2174/156802606776743165. [DOI] [PubMed] [Google Scholar]
- Macadam RF, Williamson J. Drug effects on the fine structure of Trypanosoma rhodesiense: suramin, tryparsamide and mapharside. Ann Trop Med Parasitol. 1974;68:301–306. doi: 10.1080/00034983.1974.11686952. [DOI] [PubMed] [Google Scholar]
- Marr AK, McGwire BS, McMaster WR. Modes of action of Leishmanicidal antimicrobial peptides. Future Microbiol. 2012;7(9):1047–1059. doi: 10.2217/fmb.12.85. [DOI] [PubMed] [Google Scholar]
- McCartry-Burke C, Bates PA, Dwyer DM. Leishmania donovani: use of two different commercially available chemically defined media for the continuous in vitro cultivation of promastigotes. Exp Parasitol. 1991;73:385–387. doi: 10.1016/0014-4894(91)90112-A. [DOI] [PubMed] [Google Scholar]
- Menna-Barreto RF, Salomão K, Dantas AP, Santa-Rita RM, Soares MJ, Barbosa HS, de Castro SL. Different cell death pathways induced by drugs in Trypanosoma cruzi: an ultrastructural study. Micron. 2009;40:157–168. doi: 10.1016/j.micron.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Mittal MK, Rai S, Ashutosh S, Sundar S, Goyal N. Characterization of natural antimony resistance in Leishmania donovani isolates. Am J Trop Med Hyg. 2007;76:681–688. [PubMed] [Google Scholar]
- Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366(9496):1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- Musa A, Khalil E, Hailu A, Olobo J, Balasegaram M, Omollo R, et al. Sodium stibogluconate (SSG) and paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: a randomised controlled trial. PLoS Negl Trop Dis. 2012;6:e1674. doi: 10.1371/journal.pntd.0001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noedl H, Krudsood S, Chalermratana K, et al. Azithromycin combination therapy with arsenate or qunine for the treatment of uncomplicated Plasmodium falciparum malaria in adults: a randomized, phase 2 clinical trial in Thialand. Clin Infect Dis. 2006;43(10):1264–1271. doi: 10.1086/508175. [DOI] [PubMed] [Google Scholar]
- Nolan TJ, Herman R. Effects of long term in vitro cultivation on Leishmania donovani promastigotes. J Protozool. 1985;32(1):70–75. doi: 10.1111/j.1550-7408.1985.tb03015.x. [DOI] [PubMed] [Google Scholar]
- Panosian CB, Barza M, Szoka F, Wyler DJ. Treatment of experimental cutaneous leishmaniasis with liposome intercalated amphotericin B. Antimicrob Agents Ch. 1984;25:655–656. doi: 10.1128/AAC.25.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris C, Loiseau PM, Bories C, Breard J. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob Agents Chemother. 2004;48:852–859. doi: 10.1128/AAC.48.3.852-859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Victoria FJ, Castanys S, Ganarro F. Leishmania donovani resistance to miltefosine involves a defective inward translocation of the drug. Antimicrob Agents Chemother. 2003;47:2397–2403. doi: 10.1128/AAC.47.8.2397-2403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata A, Silva-Vergara ML, Costa L, Rocha A, Krolewiecki A, Silva JC, Paula EV, Pimenta FG, Jr, Giraldo LER. Efficacy of azithromycin in the treatment of cutaneous leishmaniasis. Rev Soc Bras Med Trop. 2003;36:65–69. doi: 10.1590/S0037-86822003000100010. [DOI] [PubMed] [Google Scholar]
- Ramesh V, Katara GK, Verma S, Salotra P. Miltefosine as an effective choice in the treatment of post-kala-azar derma leishmaniasis. Br J Dermatol. 2011;165:411. doi: 10.1111/j.1365-2133.2011.10402.x. [DOI] [PubMed] [Google Scholar]
- Sadaka HAH, Eissa MM, Allam SR, Baddour NM. Azithromycin in treatment of experimental toxoplasmosis. J Med Res Inst. 1999;20(3):68–78. [Google Scholar]
- Schuster FL, Visvesvara GS. Efficacy of novel antimicrobials against clinical isolates of opportunistic amebas. J Eukaryot Microbiol. 1998;45:612–618. doi: 10.1111/j.1550-7408.1998.tb04557.x. [DOI] [PubMed] [Google Scholar]
- Shaw J. The leishmaniases–survival and expansion in a changing world. A mini-review. Mem I Os Cr. 2007;102:541–547. doi: 10.1590/S0074-02762007000500001. [DOI] [PubMed] [Google Scholar]
- Silva-Vergara ML, Almeida-Silva L, Maneira FRZ, Silva AG, Prata A. Azithromycin in the treatment of mucosal leishmaniasis. Rev I Med Trop. 2004;46:175–177. doi: 10.1590/s0036-46652004000300011. [DOI] [PubMed] [Google Scholar]
- Sinagra A, Luna C, Abraham D, Iannella M, Riarte A, Krolewiecki AJ. The activity of azithromycin against Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis in the golden hamster model. Rev Soc Bras Med Trop. 2007;40:627–630. doi: 10.1590/S0037-86822007000600005. [DOI] [PubMed] [Google Scholar]
- Sundar S, Chakravarty J. Paromomycin in the treatment of leishmaniasis. Expert Opin Inv Drug. 2008;17:787–794. doi: 10.1517/13543784.17.5.787. [DOI] [PubMed] [Google Scholar]
- Sundar S, Chakravarty J. Liposomal amphotericin B and leishmaniasis: dose and response. J Global Infect Dis. 2010;2(2):159–166. doi: 10.4103/0974-777X.62886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. New Engl J Med. 2010;326(6):504–512. doi: 10.1056/NEJMoa0903627. [DOI] [PubMed] [Google Scholar]
- Sundar S, Sinha PK, Rai M, Verma DK, Nawin K, Alam S, Chakravarty J, Vaillant M, Verma N, Pandey K, Kumari P, Lal CS, Arora R, Sharma B, Ellis S, Strub-Wourgaft N, Balasegaram M, Olliaro P, Das P, Modabber F. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet 5. 2011;377(9764):477–486. doi: 10.1016/S0140-6736(10)62050-8. [DOI] [PubMed] [Google Scholar]
- Titus RG, Marchand M, Boon T, Louis JA. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Wieder T, Reutter W, Orfanos CE, et al. Mechanisms of action of phospholipid analogs as anticancer compounds. Prog Lipid Res. 1999;38:249–259. doi: 10.1016/S0163-7827(99)00004-1. [DOI] [PubMed] [Google Scholar]
- Xu G, Fujita J, Negayama K, Yuube K, Hojo S, Yamaji Y, Kawanishi K, Takahara J. Effect of macrolide antibiotics on macrophage functions. Microbiol Immunol. 1996;40:473–479. doi: 10.1111/j.1348-0421.1996.tb01097.x. [DOI] [PubMed] [Google Scholar]