Abstract
Toxoplasmosis is an important zoonotic disease with worldwide distribution. The infection is observed in an unusually wide range of warm-blooded animals, including most of the livestock and humans. Many studies have shown high prevalence of toxoplasmosis in man and animals in Iran. The present study was conducted to investigate the seroprevalence of toxoplasmosis in Turkoman horses in the North Khorasan Province. During 2011–2012, 100 blood samples from horses were collected and tested for antibodies against toxoplasmosis using indirect fluorescent antibody test. The seroprevalence of toxoplasmosis was detected in 14 % (14) horses. The antibodies titres were detected in the range of 1:20–1:160 dilution. The lowest and highest frequencies of toxoplasmosis were observed in the age groups of <1 year and 1–10 years, respectively (P < 0.05). No significant difference was observed between toxoplasmosis frequencies and gender and usage of horses. With regard to the high frequency of toxoplasmosis in the sampled horses, attention must be paid to the animal health for the control and prophylaxis of the disease.
Keywords: Toxoplasma, Serology, Horse, The North Khorasan Province
Introduction
Toxoplasma gondii infection is widely prevalent in man and animal worldwide and has been recognised as an important zoonotic disease (Dubey and Porterfield 1986).Both domestic and wild cats are definitive host of this organism and mammals, birds and humans are intermediate hosts (Tenter et al. 2000; Lindsay and Dubey 2007). The most common sources of infection in intermediate hosts are tissue cysts in meat and oocysts in water and vegetables. Horses are usually infected by the ingestion of oocysts in contaminated feed and water (Dubey and Porterfield 1986; Tassi 2007), with clinical toxoplasmosis being a rare condition (Rodostits et al. 2007). Horses are resistant to experimental infection with 1 × 104 or 1 × 105 oocysts. T. gondii can persist in edible tissues of horses for up to 476 days (Lindsay and Dubey 2007).The cyst of T. gondii has been isolated from the eyes, placenta and brain of aborted foetus in horses (Dubey and Porterfield 1986; Shappan and Ghazy 2007; Turner and Savva 1990, 1991). In France, three cases of acquired toxoplasmosis in humans have been reported, which were caused by the consumption of raw horse meat (Pomares et al. 2011).
Toxoplasma gondii infection stimulates both the cell mediated immunity and humoral immune response as antibody production. Cellular immunity is therefore the key component of the host’s immune reaction in the event of attack by Toxoplasma. The macrophages, T lymphocytes T (TL) and “natural killer” (NK) cells, on the one hand, and the cytokines, on the other, are the major elements involved in immune response (Filisetti and Candolfie 2004).
Antibodies play a minor role but remain the essential means for diagnosing toxoplasmosis in humans (Filisetti and Candolfie 2004). Parasite-specific IgM, IgA, IgE and IgG2 antibodies have been isolated from human patients, and detection of parasite specific antibodies is an effective diagnostic tool to distinguish newly infected individuals from those in the chronic stage of infection (Dupont et al. 2012). The prevalence of toxoplasmosis in the horses were detected by different serological tests including dye test (DT) in Saudi arabia, Turkey (Alanazi and Alyousif 2011; Karatepe et al. 2010), by modified agglutination test (MAT) in Argentina, Mexico, Iran, Tunisia, Portugal, Eygypt (Dubey et al. 1999; Alvarado-Esquivel et al. 2012; Raeghi et al. 2011; Hajialilo et al. 2010; Boughattas et al. 2011; Lopes et al. 2013; Shappan et al. 2012) by enzym linked immunosorbent assay (ELISA) in Eygypt (Shappan et al. 2012), by indirect hemagglutination test (IHA) in china (MiaoQ et al. 2013), by indirect fluorescent antibody test (IFAT) in Brazil and The South Korea (Locatelli-Dittrich et al. 2006; Evers et al. 2013; Gupta et al. 2002) by latex agglutination test (LAT) in Czech and Eygypt (Bártová et al. 2010; Shappan et al. 2012).The seroprevalence of toxoplasmosis in horses has been estimated to be a wide range from 0 to 90 % in different areas of the world (Tassi 2007) and from 11.5 to 72 % in Iran (Raeghi et al. 2011; Hajialilo et al. 2010). The Turkoman horse is an oriental breed, which is bred and found in the east of Caspian Sea, Iran. The present study was conducted to detect the seroprevalence of toxoplasmosis in Turkoman horses in the North Khorasan Province, Iran.
Materials and methods
Field study area
This study was conducted in the North Khorasan Province, located in the northeastern part of Iran at 36°37′–38°17′ N latitudes and 55°53′–58°20′ E longitudes, with an area of more than 28,400 km2. This province is situated next to the north-eastern border of Iran, near the southern Caspian Sea and south of Turkmenistan, has mountainous areas and receives about 250 mm of rainfall annually. The Turkmen tribes had settled in parts of this province and Turkoman horse rearing is common in the area. A total of 14 villages situated in the border between Iran and Turkmenistan were selected for sampling.
Sampling
Blood samples were collected from the horses from June to August 2011, this was due mainly to easier access to turkeman horses during June–August. Before sample collection, data, including age, gender and usage of horses, were recorded for each horse. The blood samples were collected in clean tubes from the jugular vein. The tubes with the samples were transported to the laboratory under cool condition. The clotted blood was centrifuged at 800×g for 10 min at room temperature and the sera were stored at −20 °C until the time of serological examination.
Indirect fluorescent antibody test (IFAT)
The serum samples were analysed for antibodies against T. gondii using IFAT as previously described by Razmi and Rahbari (2001). Briefly, the tachyzoites of the RH strain of T. gondii were fixed onto clean slides and stored at −20 °C in freezer (Pasteur Institute, Tehran, Iran). For each reaction, sera were diluted in PBS (1:20) and 5 μl of diluted sera were added over the slide holes. The slides were incubated for 30 min at room temperature in humid chamber and were washed three times for 5 min in PBS. The rabbit-anti-horse IgG conjugate (Fuller laboratories, Fullerton, California) was diluted in PBS (1:20) with 0.2 % filtered Evan’s blue and 5 μl of the solution were placed over the slide holes. Subsequently, incubation and washing steps were repeated once as outlined earlier. For fluorescence microscopy, the slides were mounted with buffered glycerine, covered with cover slips and examined at 400× magnification. For each slide, positive and negative controls were also used. If the sample was fluorescent positive, a subsequent serial dilution (1:20–1:640) was prepared and re-examined by applying IFAT. A Titre of ≥1:64 was accepted as positivity according to the previous studies in horses (Ghazy et al. 2007; Evers et al. 2013). We used a Zeiss Fluorescence microscope (Carl zeiss, Germany) for microscopy and a digital camera (Olympus, model c-7070 wide zoom, Japan) for photography.
Statistical analysis
Host-related factors such as type of infection, age, gender and activity were analysed by Chi square test to detect any significant relationship.
Results
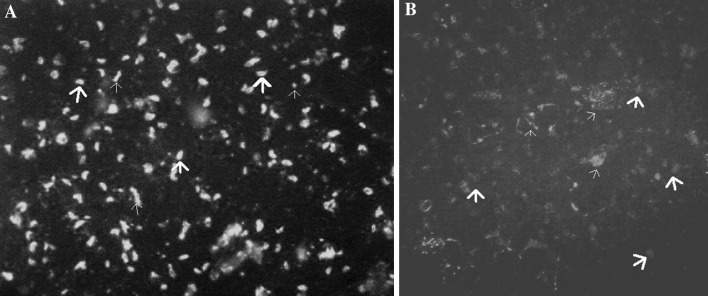
The antibody titres against T.gondii infection were detected in 1:20 dilution in 15 horses, 1:40 dilution in 13 horses, 1:80 dilution in 12 horses and 1:160 dilution in 2 horses. The results of immunoflurescence staining of tachyzoites with sera of infected and uninfected horses were shown in IFAT (Fig. 1a, b). Seropositivity was found in 14 (14 %) of the 100 horses by cut-off titre ≥1:64. The high and low seroprevalence was observed in the age groups of 1–10 years (P < 0.05). No significant relationship was observed between the seroprevalence of toxoplasmosis and gender and usage of the horses (Fig. 1; Table 1).
Fig. 1.
Immunofluorescenct staining of tachyzites of T.gondii with serum of infected horse (a) but not staining with serum of uninfected horse (b). Thick arrows point to tachyzites of T.gondii and thin arrows to artifacts
Table 1.
Seroprevalence of toxoplasmosis in 100 Turkoman horses with respect to age, activity and sex in the North Khorasan province, Iran
| Risk factors | Negative | Positive | Total | P values | |
|---|---|---|---|---|---|
| No | No | % | |||
| Age (year) | – | – | – | – | P < 0.05 |
| <1 | 7 | 0 | 0 | 7 | – |
| 1–10 | 64 | 14 | 17.9 | 78 | – |
| >10 | 15 | 0 | 0 | 15 | – |
| Activity | – | – | – | – | P > 0.05 |
| Racehorse | 59 | 8 | 11.9 | 67 | – |
| Stud | 27 | 6 | 18 | 33 | – |
| Gender | – | – | – | – | P > 0.05 |
| Male | 18 | 3 | 14.2 | 21 | – |
| Female | 68 | 11 | 13.9 | 79 | – |
| Total | 86 | 14 | 14 | 100 | – |
Discussion
Different serological methods have been used for the detection of antibodies against toxoplasmosis in man and animals. The IFAT has high sensitivity and specificity of serological diagnosis of toxoplasmosis in man when compared with other serological tests (Piergili Fioretti 2004; Wilson et al. 1990), while a comparative serological study about toxoplasmosis in horses showed that ELISA gave better results than IFAT.
IFAT revealed a seroprevalence of toxoplasmosis in 14 % of the horses examined. Recently, in Iran, seropositivity of toxoplasmosis was detected in 71 % of the racing horses in Qazvin area (Hajialilo et al. 2010) and 11.5 % of the horses in Urmea area (Raeghi et al. 2011). Similar studies have been conducted in other countries and the seroprevalence of toxoplasmosis in horses was noted to be 7.2 % (Karatepe et al. 2010)and 28 % (Zeynep et al. 2007) in Turkey, 31 % in Saudi Arabia (Alanazi and Alyousif 2011), 34 % in Czech (Bártová et al. 2010), 24 % in Spain (García-Bocanegra et al. 2012), 17 % in Tunisia (Boughattas et al. 2011) and 30–50 % in Egypt (Shappan et al. 2012). The difference in the results may be owing to the type of serological methods employed, climatic conditions, the age of animals and even to the hygienic condition of the farms and farm management.
In the present study, the highest antibody titre of 1:160 was detected by IFAT. Similar serum dilutions from 1:100 to 1:200 have also been reported in horses in Iran (Raeghi et al. 2011; Hajialilo et al. 2010), Argentina (Dubey et al. 1999) and South Korea (Gupta et al. 2002). The results obtained in the present study indicated the high seroprevalence of toxoplasmosis in older horses. These findings were predictable because the chance of oocyst ingestion in older horses is higher than that in younger horses. The results obtained in the study were in agreement with those obtained in a previous study conducted in Tunisia (Boughattas et al. 2011), which showed that the seroprevalence of toxoplasmosis in adult horses was significantly higher than that in young horses. However, this finding was in contrast to that reported in studies performed in Turkey (Karatepe et al. 2010) and Brazil (Finger et al. 2013). Furthermore, the difference in the seroprevalence of toxoplasmosis in male and female horses was not statistically significant. This result was in agreement with the previous findings obtained in Turkey (Karatepe et al. 2010) and Brazil (Karatepe et al. 2010), but is contradictory to those observed in Tunisia (Boughattas et al. 2011) and Egypt (Shappan et al. 2012). The Turkoman horses graze in the pasture and the seroprevalence of toxoplasmosis in the mare similar to that in horses with different activities. Previous studies have shown that the usage of horses with different feeding significantly affected the seroprevalence rate of infection (Alvarado-Esquivel et al. 2012; Kouam et al. 2010). In conclusion, in the present study, toxoplasmosis was found to be highly prevalent among Turkoman horses and horse-meat consumption increased the risk of transmission of this organism to the final host (Family: Felidae) and intermediate hosts (humans and other animals).
Acknowledgments
We are very grateful to Dr. Abdoljalil Ghiadi the manager of Turkoman horse breeding company (THBC) for his hospitality. We also thank to Mohammad Abedi and Dr. Ali Sarani for their hepling to samples collection and Mr H. Eshrati and Mr G.A. Azari for their technical assistance. This study was supported by a grant VPRTFM-22992.2 from The Vice President Research and Technology of Ferdowsi University of Mashhad, Iran.
References
- Alanazi AD, Alyousif MS. Prevalence of antibodies to Toxoplasma gondii in horses in Riyadh Province, Saudi Arabia. J Parasitol. 2011;97:943–945. doi: 10.1645/GE-2677.1. [DOI] [PubMed] [Google Scholar]
- Alvarado-Esquivel C, Rodríguez-Peña S, Villena I, Dubey JP. Seroprevalence of Toxoplasma gondii infection in domestic horses in Durango State, Mexico. J Parasitol. 2012;98:944–945. doi: 10.1645/GE-3174.1. [DOI] [PubMed] [Google Scholar]
- Bártová E, Sedlák K, Syrová M, Literák I. Neospora spp. and Toxoplasma gondii antibodies in horses in the Czech Republic. Parasitol Res. 2010;107:783–785. doi: 10.1007/s00436-010-1929-4. [DOI] [PubMed] [Google Scholar]
- Boughattas S, Bergaoui R, Essid R, Aoun K, Bouratbine A. Seroprevalence of Toxoplasma gondii infection among horses in Tunisia. Parasite Vectors. 2011;4:218–220. doi: 10.1186/1756-3305-4-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP, Beattie CP. Toxoplasmosis of animal and man. Bocaraton: CRC Press, Inc.; 1988. [Google Scholar]
- Dubey JP, Porterfield ML. Toxoplasma-like sporozoa in an aborted equine fetus. J Am Vet Med Assoc. 1986;188:1312–1313. [PubMed] [Google Scholar]
- Dubey JP, Venturini MC, Venturini L, McKinney J, Pecoraro M. Prevalence of antibodies to Sarcocystis neurona, Toxoplasma gondii and Neospora caninum in horses from Argentina. Vet Parasitol. 1999;86:59–62. doi: 10.1016/S0304-4017(99)00127-2. [DOI] [PubMed] [Google Scholar]
- Dupont CD, Christian DA, Hunter CA. Immune response and immunopathology during toxoplasmosis. Semin.Immunopathol. 2012;34:793–813. doi: 10.1007/s00281-012-0339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers F, Garcia JL, Navarro IT, Zulpo DL, de Nino BSL, de Ewald MPC, Pagliari S, de Almeida JC, Freire RL. Diagnosis and isolation of Toxoplasma gondii in horses from Brazilian slaughterhouses. Rev Bras Parasitol Vet. 2013;22:58–63. doi: 10.1590/S1984-29612013005000009. [DOI] [PubMed] [Google Scholar]
- Filisetti D, Candolfie E. Immune response to Toxoplasma gondii. Ann Ist Super Sanità. 2004;40:71–80. [PubMed] [Google Scholar]
- Finger MA, Villalobos EM, Lara MD, Cunha EM, de Barros Filho IR, Deconto I, Dornbusch PT, Ullmann LS, Biondo AW. Detection of anti-Toxoplasma gondii antibodies in carthorses in the metropolitan region of Curitiba, Paraná, Brazil. Rev Bras Parasitol Vet. 2013;22:179–181. doi: 10.1590/S1984-29612013005000001. [DOI] [PubMed] [Google Scholar]
- García-Bocanegra I, Cabezón O, Arenas-Montes A, Carbonero A, Dubey JP, Perea A, Almería S. Seroprevalence of Toxoplasma gondii in equids from Southern Spain. Parasitol Inter. 2012;61:421–424. doi: 10.1016/j.parint.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Ghazy AA, Shaapan RM, Abdel-Rahman EH. Comparative serological diagnosis of toxoplasmosis in horses using locally isolated Toxoplasma gondii. Vet Parasitol. 2007;145:31–36. doi: 10.1016/j.vetpar.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Gupta GD, Lakritz J, Kim JH, Kim DY, Kim JK, Marsh AE. Seroprevalence of Neospora, Toxoplasma gondii and Sarcocystis neurona antibodies in horses from Jeju island, South Korea. Vet Parasitol. 2002;106:193–201. doi: 10.1016/S0304-4017(02)00064-X. [DOI] [PubMed] [Google Scholar]
- Hajialilo E, Ziaali N, Harandi MF, Saraei M, Hajialilo M. Prevalence of anti-Toxoplasma gondii antibodies in sport horses from Qazvin. Iran. Trop Anim Health Prod. 2010;42:1321–1322. doi: 10.1007/s11250-010-9576-4. [DOI] [PubMed] [Google Scholar]
- Karatepe B, Babür C, Karatepe M, Kılıçm S. Seroprevalence of toxoplasmosis in horses in Niğde Provinceof Turkey. Trop Anim Health Prod. 2010;42:385–389. doi: 10.1007/s11250-009-9430-8. [DOI] [PubMed] [Google Scholar]
- Kouam MK, Diakou A, Kanzoura V, Papadopoulos E, Gajadhar AA, Theodoropoulos G. Seroepidemiological study of exposure to Toxoplasma, Leishmania, Echinococcus and Trichinella in equids in Greece and analysis of risk factors. Vet Parasitol. 2010;170:170–175. doi: 10.1016/j.vetpar.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Dubey JP. Toxoplasmosis in wild and domestic animals. In: Weiss LM, Kim K, editors. Toxpoplasma gondii. The Model Apicomplexan: Perspectives and Methods. 1. San Deigo: Elsevier Ltd.; 2007. pp. 133–152. [Google Scholar]
- Locatelli-Dittrich R, Dittrich JR, Richartz RR, Gasino Joineau ME, Antunes J, Pinckney RD, Deconto I, Hoffmann DC, Thomaz-Soccol V. Investigation of Neospora sp. and Toxoplasma gondii antibodies in mares and inprecolostral foals from Parana State, Southern Brazil. Vet Parasitol. 2006;135:215–221. doi: 10.1016/j.vetpar.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Lopes AP, Sousa S, Ribeiro AJ, Dubey JP, Silvestre R, Cotovio M, Schallig HDFH, Cardoso L, Corderio da-Silva A. Prevalence of antibodies to Leishmania infantumand Toxoplasma gondii in horses from the north of Portugal. Parasite Vectors. 2013 doi: 10.1186/1756-3305-6-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MiaoQ Wang X, She LN, Fan YT, Yuan FZ, Yang JF, Zhu XQ, Zou FC. Seroprevalence of Toxoplasma gondii in horses and donkeys in Yunnan Province, Southwestern China. Parasite Vectors. 2013;6:168. doi: 10.1186/1756-3305-6-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piergili Fioretti D. Problems and limitations of conventional and innovative methods for the diagnosis of Toxoplasmosis in humans and animals. Parassitologia. 2004;46:177–181. [PubMed] [Google Scholar]
- Pomares C, Ajzenberg D, Bornard L, Bernardin G, Hasseine L, Darde ML, Marty P. Toxoplasmosis and horse meat, France. Emerg Infect Dis. 2011;17:1327–1328. doi: 10.3201/eid1707.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeghi S, Akbari A, Sedghi A. Seroprevalence of Toxoplasma gondii in Sheep, Cattle and Horses in Urmia North-West of Iran. Iran J Parasitol. 2011;6:90–94. [PMC free article] [PubMed] [Google Scholar]
- Razmi GR, Rahbari S. Comparative study of three tests (modified direct agglutination test, indirect immunofluorescent antibody test and dye test) for detection of antibodies to Toxoplasma gondii in sheep sera. Iran J Vet Res. 2001;1:12–19. [Google Scholar]
- Rodostits OM, Gay CC, Hinchcliff KW, Constable PD. Veterinary Medicine. 10. London: Saunders-Elsiveier; 2007. [Google Scholar]
- Shappan RM, Ghazy AA. Isolation of Toxoplasma gondii from horse meat in Egypt. Pak J Biol Sci. 2007;10:174–177. doi: 10.3923/pjbs.2007.174.177. [DOI] [PubMed] [Google Scholar]
- Shappan RM, Abo-Eimaty AM, Abd-Eirazik KA, Abd-Eihafez SM. PCR and serological assays for detection of Toxoplasma gondii infection in sport horses in Cairo, Egypt. Asian J Anim Vet Adv. 2012;7:158–165. doi: 10.3923/ajava.2012.158.165. [DOI] [Google Scholar]
- Tassi P. Toxoplasma gondii infection in horses. A review. Parassitologia. 2007;49:7–15. [PubMed] [Google Scholar]
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Inter J Parasitol. 2000;30:1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CB, Savva D. Evidence of Toxoplasma gondii in an equine placenta. Vet Rec. 1990;127:96. [PubMed] [Google Scholar]
- Turner CB, Savva D. Detection of Toxoplasma gondii in equine eyes. Vet Rec. 1991;129:128. doi: 10.1136/vr.129.6.128-a. [DOI] [PubMed] [Google Scholar]
- Wilson M, Ware DA, Juranek DD. Serologic aspect of toxoplasmosis. J Am Vet Med Assoc. 1990;196:277–280. [PubMed] [Google Scholar]
- Zeynep G, Zafer K, Cahit B, Selçuk K. Investigation of Toxoplasma gondii Antibodies in Sport Horses Bred in Ankara Province. Türkiye Parazitol Derg. 2007;31:264–267. [PubMed] [Google Scholar]