Abstract
The present study was aimed at screening for the presence of protozoan’s among Cyprinid fishes collected from various fish ponds and farms in Jammu division of Jammu and Kashmir (J and K) state. Out of 75 fishes collected from local water bodies of Jammu division, only 35, (49.6 %) were infested with Trichodina. Trichodina infestations were studied in the period from November to February. In light infestation Trichodina was usually present on gills, fins and skin of apparently healthy fish. Clinical signs of Trichodiniasis appeared on fish with heavy infections and in presence of one or more stress factors including, rough handling during transportation from ponds, over crowdedness, malnutrition, high concentration of free ammonia and low oxygen concentration. Clinical signs of Trichodiniasis in fish such as sluggish movement, loss of appetite, black colouration, necrosis and ulcer on different parts of the body, detached scales and excessive accumulation of mucous in gill pouches were also observed.
Keywords: Fishes, Ectoparasite, Trichodina heterodentata, Morphology, Taxonomy
Introduction
Trichodinids are ciliate protozoans widely known as ectocommensals and are probably the most commonly encountered protozoan parasites on wild and cultured fishes in marine as well as freshwater environments (Urawa 1992; Basson and Van As 1994; Martins and Ghiraldelli 2008). To date, about 300 nominal Trichodinda species have been reported from different environments in the world (Tang and Zhao 2011, 2012). Many species are morphologically variable and show low host specificity which make their determination difficult (Lom and Dykova 1992). Because of their direct transmission the trichodinid ciliates are able to invade their hosts within a short period, especially fish that are kept under less than optimal conditions (Lom 1995).
The taxonomy of Trichodinids is based on the structure and the appearance of the adhesive disc and number and size of its constituents. All of these features can be revealed only by the silver impregnation technique of Klein and by this over 100 species have been described from fishes (Klein 1958). Consequently, the numerous species identified early without silver techniques are inadequately described (Lom and Dykova 1992). Today, ten genera are described within the family Trichodinidae. The genus (Trichodina Ehrenberg 1838) is the largest of this family with more than 200 species described from fish (Asmat et al. 2005).
Trichodinids reproduce by binary fission and it has been the subject of study since the previous century (Kruger et al. 1995). Most trichodinids are not pathogens, but when the relationship host/parasite/environment is broken by nutritional deficiency, poor water quality, infectious and/or parasitic diseases trichodinids may proliferate, being responsible for severe epidermal lesions and disease outbreaks, as reported by Madsen et al. (2000); Martins et al. (2002); Khan (2004); Huh et al. (2005). According To Khan (2004) outbreaks and mass mortality of Atlantic cod (Gadus morhua) associated with Trichodina murmanica infection was reported in a coastal embayment of Newfoundland. The lesions mostly induced by this parasite are hyperplasia and necrosis of the epidermal cells (Padnos and Nigrelli 1942; Davis 1947; Sarig 1971; Hassan 1999). The present study reports first time the presence of Trichodina heterodentata parasite from the gills of Cyprinid fishes from Jammu division of Jammu and Kashmir state.
Materials and methods
A number of 75 apparently healthy and naturally infected fishes of different species, (31 of Cyprinus carpio, 22 of Labeo rohita, 15 of Catla catla, 8 of Puntius ticto) were collected from November 2013 to February 2014 with the help of a drag net/hand net from different localities of Jammu division of J and K state.
External examination on the gills, fins and body surfaces of the fish for ectoparasites was first carried out using hand lens for detection of parasitic manifestations. Later, skin smears were taken using scalpel blade. The procedure was performed using a spatula by which the skin scrapings (smears) from the head to the tail were obtained. Thereafter, the scraped samples of mucus together with the tissues were placed on a Petri-dish containing 3 ml of 0.9 % saline solution and stirred using a mounted pin (Omeji et al. (2010); Bichi and Ibrahim (2009); Emere and Egbe (2006). Some drops of the mixed solution were collected using dropper, placed on a clean slide and examined under microscope.
Detection of parasites from the gills of the sampled fish was also made using the methods described by Omeji et al. (2010); Bichi and Ibrahim (2009); Emere and Egbe (2006). Gills were cut by scissors, placed in a Petri-dish and gill filaments were dissected using anatomical needle and examined under the microscope. Gill scrapings were placed on few drops of water previously placed onto glass slides then covered with cover-slide and examined under the microscope. The stomach and the intestine of each of the fish were cut opened, and contents washed into the Petri-dish containing the saline solution. The lining of the gut lumen was also scrapped out and placed in the saline solution. One to two drops of the preparation were placed on slide covered with slips and observed using a light binocular microscope for endoparasites.
The total numbers of trichodinids were determined by screening all body surfaces including the fins and gills using a light microscope at 10× × 100× magnification. For species identification and determination of infestation site, following total counts, samples of Trichodina were taken from each fish specimen and dry smears were made in accordance with Klein’s silver nitrate (AgNO3) method (Lom and Dykova 1992). The parasites were identified by making their sketches as observed on the binocular microscope and compared with the pictorial guide on fish parasites by Pouder et al. (2005).
Results
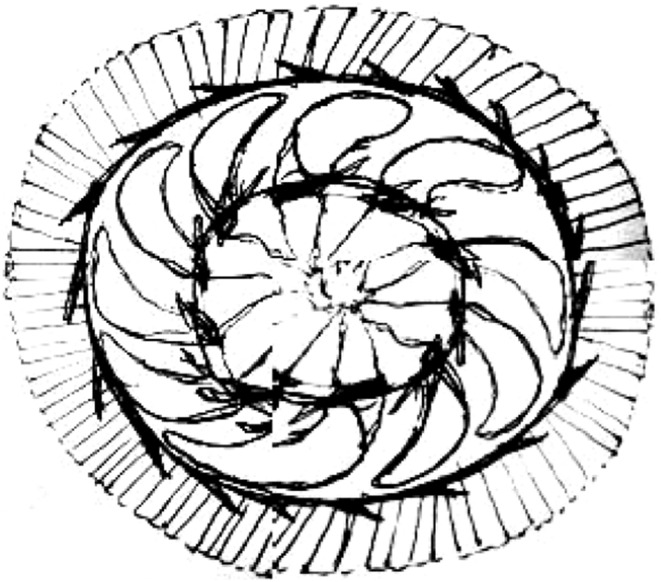
The current study is the first to report trichodinid fauna from the fish species (Cyprinus carpio, Labeo rohita,Catla catla, Puntius ticto.) captured from their natural environment in the Jammu division of J and K state. About 75 fishes were collected from different ponds and fish farms of Jammu division. After thorough examination of the collected fishes, a medium-sized trichodinid parasite with disc-shaped body diameter 57 ± 6.25 (48.3–77, 40); convex adoral surface, aboral side with slightly concave adhesive disc; centre of adhesive disc without granules after silver-impregnation was reported. The parasite has a horseshoe shaped macronucleus 42.6 ± 3 (40–47, 5) of external diameter and thickness 12.0 + 1.8 (10–14, 5). The parasite was compared with the already reported parasites described by different authors in different parts of world and after micrometric measurements, line drawing and with the help of prescribed keys was found to be T. heterodentata Fig. 1. The denticles of T. heterodentata are characterized by wide blade and having sickle-shape provided with apophysis for blade connection with the central part. The number of denticles vary from 13 to 26. In light infestation Trichodina Fig. 2 was usually present on gills, fins and skin of apparently healthy fish. Clinical signs of Trichodiniasis in fish were sluggish movement, loss of appetite, black colouration, necrosis and ulcer on different parts of the body, detached scales and excessive accumulation of mucous in gill pouches.
Fig. 1.
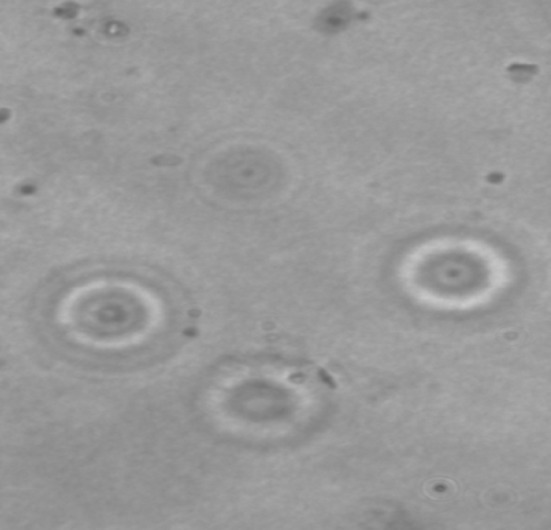
Images of Trichodina heterodentata stained with Giemsa stain
Fig. 2.
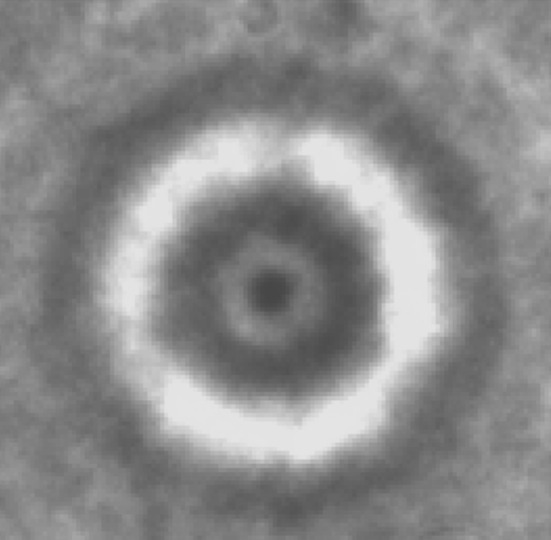
Images of Trichodina heterodentata stained with Giemsa stain
Discussion
Trichodinids are geographically a widely dispersed group of ectoparasites in freshwater, marine and euryhaline environments. Trichodinids are widely studied and well-documented parasites of fishes and their importance is reflected by the reported literature on several aspects of the biology of these parasites, such as distribution (Lom 1962; Lom and Hoffman 1964; Lom 1970; Gaze and Wootten 1998. Ozer and Erdem 1998, 1999; Ozer 2000, 2003a, b), behaviour (Ahmed 1977; Van As and Basson 1987; Ozer and Erdem 1999), the impact of environmental factors (Calenius 1980; Sanmartin Duran et al. 1991; Ozer and Erdem 1999) and their pathogenicity (Lom 1973).
About 70 species have been identified in marine fishes (Kinne 1984) and more than 112 from freshwater fishes worldwide (Lom and Dykova 1992). Some trichodinids including T. domerguei and T. tenuidens parasitising Gasterosteus aculeatus and Pungitius pungitius have been recorded in euryhaline waters (Calenius 1980). The morphological variations of denticle form and appearance of central circle in Trichodina parasite observed in this study are also in agreement with the statement of Lom and Stein (1966).
Host specificity in trichodinids was highly variable. In general, the severity of most ecto- and endoparasitic infections increases with the age of the host fish, possibly as a result of the greater accumulation period and/or the larger space for feeding and breeding of the parasite. Özer and Erdem (1998) noted a tendency to increase in the mean intensity of Trichodina spp. in relation to the length of common carp. Our findings on the intensity levels of Trichodina agree Fig. 3 with those reported by the above mentioned authors. Studies on the parasite fauna in farmed and wild fish in Jammu are quite rare Fig. 4. This was the first study conducted on the trichodinids of C. carpio, L. rohita,C. catla, P. ticto fish species found in the water bodies of Jammu division of J and K state.
Fig. 3.

Trichodina heterodentata stained with iron haematoxylin
Fig. 4.
Line drawing of Trichodina heterodentata
Acknowledgments
Thanks are due to Department of Science and Technology, Government of India for providing financial assistance. Authors are also thankful to Centre of Research for Development, University of Kashmir for providing the laboratory facility during the completion of this work.
Contributor Information
Towsief Ahmad Tantry, Phone: 918491078412, Email: tawseefahmad87@gmail.com.
Ruqeya Nazir, Email: ruqeya.ku@gmail.com.
References
- Ahmed ATA (1977) Morphology and life history of Trichodina reticulata from goldfish and other carps. Fish Pathol 12:21–31
- Basson L, Van As JG (1994) Trichodinid ectoparasites (Ciliophora: Peritrichida) of wild and cultured freshwater fishes in Taiwan, with notes on their origin. Syst Parasitol 28:197–222
- Bichi AH, Ibrahim, AA (2009) A survey of ecto and intestinal parasites of tilapia zillii (gervias) in tiga lake, kano, Northern Nigeria, Bayero. J Pure Appl Sci 2(1):79–82 Center, Tribhuvan University, Nepal: 61–109
- Calenius G. Parasites of fish of Finland.III. Ciliates of the family Urceolariidae. Acta Acad Abo Ser B. 1980;40(3):1–16. [Google Scholar]
- Davis HS (1947) Studies of the protozoan parasites of freshwater fish. U.S. Fish Wild-Life Service. Fish Bull 41:1–29
- Ehrenberg CG (1838) Die Infusionsthierchen als 16. vollkommene Organismen: ein Blick in das tiefere organische Leben der Natur. Leipzig, Verleg von Leopold Voss, p 547
- Emere MC, Egbe NEL. Protozoan parasites of Synodonits clarias (a fresh water fish in river Kaduna) BEST J. 2006;3(3):58–64. [Google Scholar]
- Gaze WH, Wooten R (1998) Ectoparasitic species of the genus Trichodina (Ciliophora: Peritrichida) parasitising British freshwater fish. Folia Parasitol 45:177–190 [PubMed]
- Hassan MAH. Trichodiniasis in farmed freshwater Tilapia in Eastern Saudi Arabia. J KAU Mar Sci. 1999;10:157–168. doi: 10.4197/mar.10-1.11. [DOI] [Google Scholar]
- Huh MD, Thomas CD, Udomkusonsri P, Noga EJ. Epidemic trichodinosis associated with sever epidermal hyperplasia in largemouth bass, Micropterus salmoides from North Carolina USA. J Wildl Dis. 2005;41(3):647–653. doi: 10.7589/0090-3558-41.3.647. [DOI] [PubMed] [Google Scholar]
- Khan RA. Disease outbreaks and mass mortality in cultured Atlantic cod, Gadus morhua L., associated with Trichodina murmanica (Ciliophora) J Fish Dis. 2004;27:181. doi: 10.1111/j.1365-2761.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- Kinne O. Diseases of marine animals. Hamburg: Biologische Anstalt Helgoland; 1984. pp. 157–161. [Google Scholar]
- Klein BM. The dry silver method and its proper use. J Protozool. 1958;5(9):99–103. doi: 10.1111/j.1550-7408.1958.tb02535.x. [DOI] [Google Scholar]
- Kruger J, Vanas JG, Basson L. Observations on the adhesive disc of Trichodina xenopodos Fantham, 1924 and T. heterodentata Duncan, (1977) (Ciliophora: Peritrichida) during binary fission. Acta Protozooogica. 1995;34(3):203–209. [Google Scholar]
- Lom J. Trichodinid ciliates from fishes of the Rumanian Black Sea coast. Parasitol. 1962;52:49–61. doi: 10.1017/S0031182000023982. [DOI] [Google Scholar]
- Lom J. Observations on Trichodinid ciliates from fresh water fishes. Arch Protistenkd. 1970;112:153–177. [Google Scholar]
- Lom J (1973) The adhesive disc of Trichodina epizootica-ultrastructure and injury to the host tissue. Folia Parasitol 20:193–202
- Lom J (1995) Trichodinidae and other ciliates (phylum Ciliophora). In P.T.K. Woo (ed) Fish diseases and disorders, Vol. Protozoan and metazoan infections. CAB International, Wallingford: 229–262
- Lom J, Dyková I (1992) Protozoan parasites of fishes. Elsevier, Amsterdam
- Lom J, Hoffman GL (1964) Geographic distribution of some species of Trichodinids ( ciliata:peritricha) parasitic on fishes. J parasitol 50:30–35
- Lom J, Stein G. Trichodinids from sticklebacks and a remark on the taxonomic position of Trichodinadomerguei (Wall.) Acta Soc Zool Bohemoslov. 1966;30:30–40. [Google Scholar]
- Madsen HCK, Buchmann K, Mellergaard S (2000) Treatment of trichodiniasis in eel (Anguilla anguilla) reared in recirculation systems in Denmark: alternatives to formaldehyde. Aquaculture 186:221–231
- Martins ML, Ghiraldelli L (2008) Trichodina magna Van As and Basson, 1989 (Ciliophora: Peritrichia) from cultured Nile tilapia in the State of Santa Catarina, Brazil [DOI] [PubMed]
- Martins ML, Onaka EM, Moraes FR, Bozzo FR, Paiva AMFC, Gonçalves A. Recent studies on parasitic infections of freshwater cultivated fish in the State of São Paulo. Brazil Acta Sci Biol Sci. 2002;24(4):981–985. [Google Scholar]
- Omeji S, Solomon SG, Obande RA. A comparative study of the common protozoan parasites of Heterobranchus longifilis from the wild and pond environments in Benue State. Pak J Nut. 2010;9(9):865–872. doi: 10.3923/pjn.2010.865.872. [DOI] [Google Scholar]
- Ozer A (2000) The occurrence of three species of Trichodina (Ciliophora: Peritrichia) on Cyprinus carpio in relation to cultureconditions, seasonality and host characteristics. Acta Protozol 39:61–66
- Ozer A (2003a) The occurrence of Trichodina domerguei Wallengren, 1897 and Trichodina tenuidens Faure-Fremiet, 1944 on three spined stickleback, Gasterosteus aculeatus L, 1758 found in a brackish and freshwater environment. Acta Protozol 42:41–46
- Ozer A ( 2003b) Trichodina domerguei Wallengren, 1897 (Ciliophora: Peritrichia) Infestations on the Round Goby, Neogobius melanostomus Pallas, 1811 in relation to seasonality and host factors. Comp Parasitol 70(2):132–135
- Ozer A, Erdem O. Ectoparasitic protozoa fauna of the common carp (Cyprinus carpio L., 1758) caught in the Sinop region of Turkey. J Nat Hist. 1998;32:441–454. doi: 10.1080/00222939800770231. [DOI] [Google Scholar]
- Ozer A, Erdem O (1999) The relationship between occurrence of ectoparasites, temperature and culture conditions: a comparison of farmed and wild common carp (Cyprinus carpio L. 1758) in the Sinop region of northern Turkey. J Nat Hist 33:483–491
- Padnos M, Nigrelli RF (1942) Trichodina spheroidesi and Trichodina hatti spp. nov. parasitic on the gills and skin of malne fiin, with special reference to the life history of Spheroidesi. Acta Zool 27:65–72
- Pouder RDB, Curtis EW, Yanong RPEdlx (2005) Common freshwater parasite pictorial guide edis
- Sanmartin Duran ML, Ferandez Casal J, Tojo JL, Santamarina MT, Estevez J, Uberia F (1991) Trichodina sp.: effect on growth of farmed turbot (Scophtalmus maximus). Bull Eur Ass Fish Pathol 11:89–91
- Sarig S (1971) Diseases of Fish. Book 3. In Sniezko SF, Axelrod HR (eds) The prevention and treatment of diseases of warm water fish under subtropical conditions with special emphasis on intensive fish farming. TFH publications, Hong Kong
- Tang FH, Zhao YJ (2011) Study of trichodinids (Protozoa, Ciliophora) parasitic on gills of freshwater fishes from Chongqing, China, and identification of a new species, Trichodina cyprinocola sp. nov. Afr J Microbiol Res 5(26):5523–5527
- Tang FH, Zhao YJ (2012) Two trichodinids of Paratrichodina (Ciliophora, Peritrichida, Trichodinidae) infecting gills of Ietalurus punetaus from Chongqing, China. Afr J Microbiol Res 6(9):2145–2149
- Urawa S. Trichodina truttae Mueller, 1937 (Ciliophora: Peritrichida) on juvenile chum salmon (Onchorhynchus keta): pathogenicity and host–parasite interactions. Gyobyo Kenkyu. 1992;27:29–37. doi: 10.3147/jsfp.27.29. [DOI] [Google Scholar]
- Van As J G, Basson L (1987) Host specificity of trichodinid ectoparasites of fresh water fish. Parasitol Today 3:88–90 [DOI] [PubMed]