Abstract
Eliglustat is a recently approved oral therapy in the United States and Europe for adults with Gaucher disease type 1 who are CYP2D6 extensive, intermediate, or poor metabolizers (> 90% of patients) that has been shown to decrease spleen and liver volume and increase hemoglobin concentrations and platelet counts in untreated adults with Gaucher disease type 1 and maintain these parameters in patients previously stabilized on enzyme replacement therapy. In a post-hoc analysis, we compared the results of eliglustat treatment in treatment-naïve patients in two clinical studies with the results of imiglucerase treatment among a cohort of treatment-naïve patients with comparable baseline hematologic and visceral parameters in the International Collaborative Gaucher Group Gaucher Registry. Organ volumes and hematologic parameters improved from baseline in both treatment groups, with a time course and degree of improvement in eliglustat-treated patients similar to imiglucerase-treated patients.
Keywords: Gaucher disease type 1, Eliglustat, Substrate reduction therapy, Acid-β-glucosidase deficiency, Imiglucerase, Enzyme replacement therapy
1. Introduction
Gaucher disease type 1 (GD1) is an inherited lysosomal storage disorder characterized by deficient activity of the enzyme acid β-glucosidase [1]. As a result, glucosylceramide accumulates primarily in lysosomes of tissue macrophages leading to multisystem manifestations, including hepatosplenomegaly, anemia, thrombocytopenia, and bone disease [1], [2].
Two treatment approaches have been used in GD1 to restore the balance between glucosylceramide synthesis and degradation. Enzyme replacement therapy (ERT) with recombinant acid β-glucosidase, the standard of care for more than two decades, augments the patient's residual enzyme activity to break down accumulated glucosylceramide and can improve or reverse hematologic, visceral, and skeletal manifestations [3], [4], [5]. Substrate reduction therapy (SRT) inhibits glucosylceramide synthase, thereby slowing production of the substrate glucosylceramide and decreasing its accumulation [6]. Eliglustat (Cerdelga®, Sanofi Genzyme, Cambridge, MA, USA) [6] is an oral SRT recently approved by the United States (US) Food and Drug Administration and the European Medicines Agency as a first-line treatment for adults with GD1 who are CYP2D6 extensive, intermediate, or poor metabolizers (> 90% of patients [7]). Clinical trials demonstrated that eliglustat reduces spleen and liver volumes and increases hemoglobin levels and platelet counts in treatment-naïve adults with GD1 [8], [9] and maintains stability long-term [10], [14]. The relevant comparison of treatment-naïve GD1 patients treated with eliglustat (SRT) and imiglucerase (ERT) has not been studied. A head-to-head trial comparing eliglustat to ERT in treatment-naïve patients is not feasible due to the difficulty of enrolling the large number of patients such a trial would require, given the rarity and heterogeneity of GD and the availability of effective intravenous treatments. The post-hoc analysis we describe compares clinical response to eliglustat in treatment-naïve patients in the eliglustat clinical trials with clinical response to imiglucerase in selected treatment-naïve patients from an observational database. This evaluation was prepared for European regulatory authorities during their assessment of eliglustat, given that a clinical trial comparing eliglustat to imiglucerase in treatment-naïve patients was not feasible.
2. Materials and methods
We performed a post-hoc analysis of treatment-naïve patients comparing the results of eliglustat treatment in two clinical studies (12-month data from the Phase 2 open-label, single-arm clinical study [NCT00358150] [8] and 9–12-month data from the Phase 3 double-blind, placebo-controlled ENGAGE trial [NCT00891202] [9], [12]) with the results of imiglucerase treatment for up to 12 months in the International Collaborative Gaucher Group (ICGG) Gaucher Registry (NCT00358943), an ongoing Sanofi Genzyme-sponsored, international, observational and voluntary program that, since its establishment in 1991, has tracked demographics and clinical outcomes for approximately 6000 Gaucher patients in a real-world setting regardless of treatment status. No experimental intervention is given; patients in the Gaucher Registry undergo clinical assessments and receive the standard of care as determined appropriate by their treating physicians in accordance with current GD management guidelines [13]. All participants in the eliglustat clinical trials and the Gaucher Registry provided written informed consent allowing post-hoc analysis of anonymous data.
Inclusion and exclusion criteria from the two eliglustat clinical studies [8], [9] were used to select a similar population of imiglucerase-treated patients from the Registry for comparison of organ volume, hematologic, and skeletal outcomes with eliglustat-treated patients. These criteria included known date of GD1 diagnosis; imiglucerase treatment initiation at age 16–65 years; no splenectomy; baseline hemoglobin 8–16 g/dL, platelet count 30–120 × 109/L, spleen volume 5.0–65.0 multiples of normal (MN), and liver volume 0.5–4.0 MN; all baseline values for spleen, hemoglobin, liver, and platelets available and any of the 9-month (8– ≤ 10.5 months after treatment initiation) OR any of the 12-month (> 10.5–13 months after treatment initiation) values available. Baseline was defined as the last assessment prior to the first infusion of ERT. As the Registry is an observational and voluntary database, timing of assessments varies; thus, “and/or” conditions were allowed to ensure that the comparator population would be large enough for comparison. All patients had to have started ERT before June 25, 2007 and all subsequent data points had to be before June 25, 2009 to ensure that their clinical responses were not affected by dose reductions or treatment interruptions that occurred during the imiglucerase supply constraint from 2009 to 2011.
Among the evaluated Registry cohort of patients, sufficient data on bone (i.e., bone mineral density, bone pain, and bone crises) were not available; therefore, bone disease parameters were excluded from this analysis.
3. Results and discussion
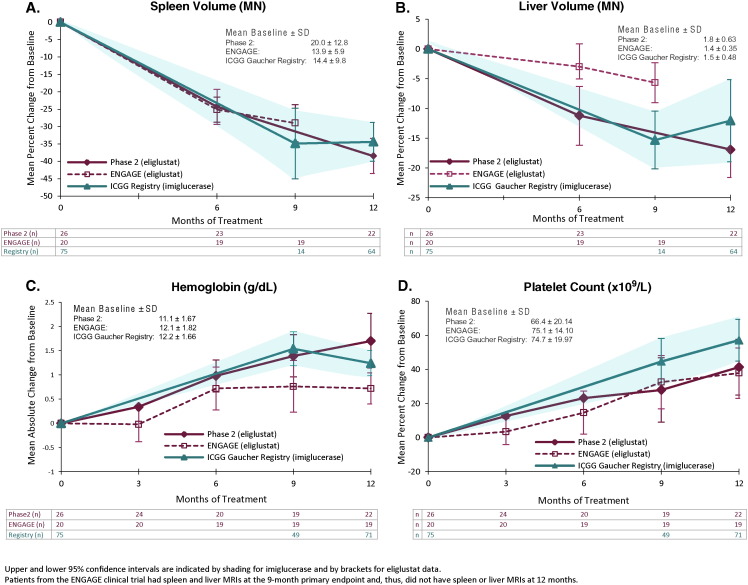
The study population consisted of 46 eliglustat-treated patients (26 from Phase 2, 20 from Phase 3 ENGAGE) and 75 imiglucerase-treated Registry patients who met the inclusion criteria and had an imiglucerase dose ≥ 15 U/kg/2 weeks (mean: 35, range: 15–60). The three groups were similar with respect to percent male (38–49%), mean age at diagnosis (22–25 years), and mean age at first treatment (32–35 years). Registry data were mostly complete through 12 months (n = 64/71 for organ volumes, n = 71/75 for hematologic parameters).
Eliglustat-treated and imiglucerase-treated patients were comparable on baseline hematologic parameters; baseline spleen and liver volumes were higher in the eliglustat Phase 2 study patients than in the ENGAGE and Registry patients (Fig. 1). Mean spleen and liver volumes decreased from baseline with eliglustat treatment, with time courses and degrees of improvement similar to the imiglucerase-treated patients from the Registry (Fig. 1A and 1B). The rate and extent of increase for platelet counts and hemoglobin level were similar across the eliglustat-treated and imiglucerase-treated cohorts (Fig. 1C and 1D).
Fig. 1.
Change from baseline in organ volumes and hematologic parameters.
Limitations of this analysis include the post-hoc design and comparison of eliglustat-treated patients in clinical studies with imiglucerase-treated patients in a real-world setting. Although the imiglucerase-treated patients met the same inclusion and exclusion criteria as the patients in the Phase 2 and Phase 3 ENGAGE studies, baseline spleen and liver volumes differed across the cohorts, and bone data for the Registry patients were too limited to allow for a meaningful comparison. Furthermore, because adverse event data are not recorded in the ICGG Registry, it is not possible to make comparisons or draw conclusions about the safety of eliglustat versus imiglucerase. We could not completely control for baseline characteristics or for imiglucerase dose, both of which can influence treatment response. With regard to eliglustat dosing, the dose-titration scheme utilized in the clinical trials to ensure plasma eliglustat steady-state pre-dose concentrations above 5 ng/mL differs from the approved eliglustat dosing in the US and European product labels, which is determined by the patient's CYP2D6 metabolizer phenotype (i.e., extensive, intermediate, or poor metabolizer). Pharmacokinetic analyses from the clinical trials showed that pre-dose concentrations > 5 ng/mL were not required for therapeutic efficacy and that CYP2D6 phenotype was the most significant determinant of eliglustat exposure.
4. Conclusions
Although lacking the rigor of a head-to-head trial, the findings of this post-hoc analysis in treatment-naïve GD1 patients suggest that, during the initial 9–12 months of treatment, oral eliglustat therapy results in improved organ volumes and hematologic parameters that are comparable to those observed with imiglucerase infusions.
Authorship and conflict-of-interest statements
Contributions: MJP, JA, and JST designed the analysis; JA performed the eliglustat statistical analyses and JST the ICGG Gaucher Registry statistical analyses. JI, JA, LU and MJP analyzed and interpreted the results and wrote the manuscript. All authors reviewed early and final drafts of the manuscript and were fully responsible for the content and editorial decisions related to this manuscript.
Conflict of interest disclosures: All of the authors are employees of Sanofi Genzyme. The study was funded and conducted by Sanofi Genzyme.
Acknowledgments
We thank Marianne B. Zajdel of Sanofi Genzyme for review and contributions to this manuscript and Laurie LaRusso of Chestnut Medical Communications for medical writing support, which was funded by Sanofi-Genzyme.
References
- 1.Grabowski G.A., Petsko G.A., Kolodny E.H. Gaucher disease. In: Valle D., Beaudet A.L., Vogelstein B., Kinzler K.W., Antonarakis S.E., Ballabio A., Gibson K., Mitchell G., editors. OMMBID: The Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York, NY: 2013. http://ommbid.mhmedical.com/content.aspx?bookid=474&Sectionid=45374148 Available at. [Google Scholar]
- 2.Grabowski G.A., Kolodny E.H., Weinreb N.J., Rosenbloom B.E., Prakash-Cheng A., Kaplan P., Charrow J., Pastores G.M., Mistry P.K. Gaucher disease: phenotypic and genetic variation. In: Valle D., Beaudet A.L., Vogelstein B., Kinzler K.W., Antonarakis S.E., Ballabio A., Gibson K., Mitchell G., editors. OMMBID: The Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York, NY: 2011. [Google Scholar]
- 3.Chen M., Wang J. Gaucher disease: review of the literature. Arch. Pathol. Lab. Med. 2008;132(5):851–853. doi: 10.5858/2008-132-851-GDROTL. [DOI] [PubMed] [Google Scholar]
- 4.Grabowski G.A. Phenotype, diagnosis, and treatment of Gaucher's disease. Lancet. 2008;372(9645):1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan P., Baris H., De Meirleir L., Di Rocco M., El-Beshlawy A., Huemer M., Martins A.M., Nascu I., Rohrbach M., Steinbach L., Cohen I.J. Revised recommendations for the management of Gaucher disease in children. Eur. J. Pediatr. 2013;172(4):447–458. doi: 10.1007/s00431-012-1771-z. [DOI] [PubMed] [Google Scholar]
- 6.Shayman J.A. Eliglustat tartrate: glucosylceramide synthase inhibitor treatment of type 1 Gaucher disease. Drugs Future. 2010;35(8):613–620. [PMC free article] [PubMed] [Google Scholar]
- 7.Hicks J.K., Swen J.J., Thorn C.F., Sangkuhl K., Kharasch E.D., Ellingrod V.L., Skaar T.C., Muller D.J., Gaedigk A., Stingl J.C. Clinical pharmacogenetics implementation. clinical pharmacogenetics implementation consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin. Pharmacol. Ther. 2013;93(5):402–408. doi: 10.1038/clpt.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lukina E., Watman N., Arreguin E.A., Banikazemi M., Dragosky M., Iastrebner M., Rosenbaum H., Phillips M., Pastores G.M., Rosenthal D.I., Kaper M., Singh T., Puga A.C., Bonate P.L., Peterschmitt M.J. A phase 2 study of eliglustat tartrate (Genz-112638), an oral substrate reduction therapy for Gaucher disease type 1. Blood. 2010;116(6):893–899. doi: 10.1182/blood-2010-03-273151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mistry P.K., Lukina E., Turkia H. Ben, Amato D., Baris H., Dasouki M., Ghosn M., Mehta A., Packman S., Pastores G., Petakov M., Assouline S., Balwani M., Danda S., Hadjiev E., Ortega A., Shankar S., Solano M.H., Ross L., Angell J., Peterschmitt M.J. Effect of oral eliglustat on splenomegaly in patients with Gaucher disease type 1: the ENGAGE randomized clinical trial. JAMA. 2015;313(7):695–706. doi: 10.1001/jama.2015.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox T.M., Drelichman G., Cravo R., Balwani M., Burrow T.A., Martins A.M., Lukina E., Rosenbloom B., Ross L., Angell J., Puga A.C. Eliglustat compared with imiglucerase in patients with Gaucher's disease type 1 stabilised on enzyme replacement therapy: a phase 3, randomised, open-label, non-inferiority trial. Lancet. 2015;385(9985):2355–2362. doi: 10.1016/S0140-6736(14)61841-9. [DOI] [PubMed] [Google Scholar]
- 12.Mistry P.K., Amato D.J., Dasoukic M., Packman S., Pastores G.M., Assouline S., Balwani M., Ortega A., Shankar S., Solano M.H., Ross L.H., Angell J., Peterschmitt M.J. ENGAGE: a phase 3, randomized, double-blind, placebo-controlled, multi-center study to investigate the efficacy and safety of eliglustat in adults with Gaucher disease type 1: Results after 18 months [abstract] Mol. Genet. Metab. 2015;114(2):S81–S82. [Google Scholar]
- 13.Weinreb N.J., Aggio M.C., Andersson H.C., Andria G., Charrow J., Clarke J.T., Erikson A., Giraldo P., Goldblatt J., Hollak C., Ida H., Kaplan P., Kolodny E.H., Mistry P., Pastores G.M., Pires R., Prakash-Cheng A., Rosenbloom B.E., Scott C.R., Sobreira E., Tylki-Szymanska A., Vellodi A., Dahl S. vom, Wappner R.S., Zimran A. Gaucher disease type 1: revised recommendations on evaluations and monitoring for adult patients. Semin. Hematol. 2004;41(4 Suppl 5):15–22. doi: 10.1053/j.seminhematol.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Cox T.M., Drelichman G.I., Cravo R., Balwani M., Burrow T.A., Martins A.M., Lukina E., Rosenbloom B.E., Ross L.H., Angell J., Peterschmitt M.J. ENCORE, a randomized, controlled, open-label non-inferiority study comparing eliglustat to imiglucerase in Gaucher disease type 1 patients stabilized on enzyme replacement therapy: 24-month results. Mol. Genet. Metab. 2015;114:S11–S130. doi: 10.1016/S0140-6736(14)61841-9. [DOI] [PubMed] [Google Scholar]