Abstract
AML is an aggressive hematological malignancy with highest incidence in the older adults. The adverse features of AML in the elderly, and the frailties and comorbidities frequently present in them, make their management a particularly difficult therapeutic challenge. In this context, it is important to assess carefully patient- as well as disease-associated prognostic features with validated tools. The fittest patients should be considered for curative therapy, such as bone marrow transplantation, whereas low intensity options may be more appropriate for frail patients. Here we review how to assess patients with elderly AML and the treatments options available for them.
Keywords: Acute myeloid leukemia, Elderly, Prognosis, Therapy, Geriatric assessment
Highlights
-
•
Treatment options remain limited for older adults with AML.
-
•
Nowadays only a tiny proportion of elderly patients with AML undergo HCT.
-
•
The unique features of AML in the elderly urge a fresh approach to these patients.
-
•
Low intensity options may be more appropriate for frail patients.
-
•
Geriatric assessment and social support are critical.
1. Introduction
Acute myeloid leukemia (AML) is a rare disease afflicting annually 3–4 persons per 100,000 individuals. With a median age at diagnosis of 67 years, this disease is far more common in the elderly [1]. In this age group, AML has a particularly dismal outcome with less than 5% of the patients being alive 5 years after the diagnosis, as compared to 40% in the young [2], [3]. Considering that individuals aged 65 in the Western world are expected to survive approximately another 20 years, and even those aged 80 three additional years [4], AML has a devastating impact on the survival of this age group.
The reasons for the poor outcomes in the older adults are both patient- and disease-related. Advanced age is often accompanied by frailty and comorbidities, which have an important impact on the tolerance these patients have to intensive treatment modalities [5]. In addition, the lower rates of complete remission (CR) in the elderly (40–50% vs 60–70% in the young) [3] and the short duration seen in those who are eligible for treatment [5], point towards a different disease biology in this age group. From a clinical viewpoint, it is far more frequent for elderly patients to have received previous cytotoxic treatment or radiotherapy or to have antecedent hematologic diseases, such as myelodysplastic (MDS) or myeloproliferative neoplasms [5]. In addition to these clinical features, the cytogenetic profile of elderly patients with AML also differs from that of younger patients, presenting a greater incidence of multiple chromosomal abnormalities [6]. The introduction of new potent sequencing technologies has shed considerable light on the molecular mechanisms of AML pathophysiology, and further reinforced the observation that AML in the elderly is biologically distinct. Thus, mutation screening has identified mutations in genes coding for epigenetic regulators, such as TET2, DMNT3A, IDH1/2, ASXL1 and EZH2, kinases and cell cycle regulators, such as TP53, NPM1 and FLT3 and transcription factors, such as RUNX1 and CEBPA [7], and showed that the cytogenetic and mutational profile of AML in the older adults patients is significantly different from that of younger patients [8]. The methylation patterns seen in them resemble more closely those seen in MDS [9]. These unique features of AML in the elderly population urge a fresh approach to these patients.
2. Geriatric assessment
Older adults frequently have an advanced biological age. This not a constant feature and a number of them keep fit and healthy until late in their lives. Nevertheless, the opportunity to develop several diseases, as well as to acquire a low functional reserve, increases with chronological age and any experienced malignant hematologist recognizes that some elderly AML patients may be unable to withstand intensive chemotherapy and allogeneic hemopoietic cell transplantation. An excruciating clinical problem is how to predict with confidence such a situation when confronting an apparently fit/unfit individual patient. In this situation, a comprehensive geriatric assessment (CGA) is the appropriate answer and the National Comprehensive Cancer Network has issued specific guidelines to help the physician in the care of older adults with cancer [10]. However, CGA is somehow complex and not always readily available. Some screening tools (e.g. VES13 or G8) have been developed to decide which patients deserve a CGA in order to optimize resources [11], [12]. Despite the fact that they predict survival in hematological patients (eg. G8 score up to 14 score points seems a bad prognostic factor), their sensitivity and specificity as compared to the gold standard (i.e. CGA) is not optimal [13], [14] and a simplified geriatric assessment approach is much needed and now actively pursued (see the next section). Our suggestion for this purpose, based on the approach of Klepin et al. [15], [16], is shown in Table 1 and requires that the clinical team becomes familiar with some additional clinical tools beyond performance status and comorbidity scores [18], [17], such us the Short Physical Performance Battery [19], or the more straightforward Gait Speed [20], [21], as well as the Modified Mini-Mental Score [22] or the Short Portable Mental Status Questionnaire [23].
Table 1.
Assessment at diagnosis of an older adult AML patient. Based on Klepin et al. [15], modified.
| Clinical context | Ancillary data | Therapeutic subset | Therapeutic approach |
|---|---|---|---|
| A. Centers with optimal geriatric facilities | |||
| All patients | Perform CGA on time | To be discussed within the geriatric AML team | Clinical trial or Subset-directed AML therapy for adults |
| B. Centers with basic or inadequate geriatric facilities | |||
| ECOG 0–2 | Adequate physical performance | ||
| Lack of major comorbidity | Lack of cognitive dysfunction | ICT/HCT candidate | Clinical trial or Standard AML therapy for adults |
| Preserved ADLs | Low risk for HCT (NRM) | ||
| ECOG 0–2 | Inadequate physical performance | ||
| Lack of major comorbidity | Cognitive dysfunction (even mild) | Vulnerable patients for toxicity under ICT/HCT | Clinical trial or |
| Preserved ADLs | High risk for HCT (NRM) | Alternative AML therapy for adults | |
| ECOG 3–4 not linked to AMLMajor comorbidity (CCI or HCT-CI) | Consider consultation with a geriatrician and social worker | Fragile/very ill patients | Palliative care |
| Dependent for ADL/IADL | |||
| Doubtful cases | Consider consultation with a geriatrician and social worker | Case-by-case discussion | Assign according to above criteria |
ADLs: Activities of daily life. AML: Acute myeloid leukemia. CGA: Comprehensive geriatric assessment. ECOG=Eastern Cooperative Oncology Group. HCT: Hemopoietic cell transplantation. ICT: Intensive chemotherapy. NRM: Non-relapse mortality. Standard AML therapy for adults: the one that would receive a younger AML patient according to risk stratification and predicted HCT-linked NRM. Alternative AML therapy: low-dose cytosine arabinoside, hypomethylating agents, etc.
3. Prognosis
The management of AML in the elderly patient presents a significant challenge for hematologists. Careful evaluation of both patient and disease is required in each individual case to make optimal management decisions, and maximize the benefit to the patient. Apart from the extremely old patients, chronological age per se is particularly unreliable in identifying patients who will not tolerate intensive regimens.
As stated in the introduction, AML in the older adult shows some biological differences with AML in the young. As far as prognostic factors are concerned (see Table 2), the vast majority are the same, but their relative proportion is different. The data from MRC/LRF, based on more than two thousand patients included in clinical trials in UK, showed that age, performance status, white blood cell count, cytogenetics, and type of AML (de novo vs. secondary) are the most relevant prognostic factors [24]. Recent analysis of population-derived data from Sweden [25] included outcomes from over 3000 adult AML patients, of which 26% had secondary AML. Analysis of prognostic factors found that secondary AML significantly impacted survival in younger patients but, in older patients, it did not emerge as an independent poor prognostic factor, suggesting that secondary AML lacks the prognostic impact in this population previously attributed by the MRC/LRF [24].
Table 2.
Prognostic features in elderly AML.
| Study | Rx | Outcomes | Prognosticators |
|---|---|---|---|
| ALFA Malfuson et al. Haematologica [60] | ICT | OS | Poor risk cytogenetics or at least 2 other features (age>74, PS>1, WBC>50.000) |
| MRC/LRF Wheatley et al. BJH [24] | ICT | OS | Age, PS, WBC, cytogenetics, type of AML (de novo vs secondary) |
| MDACC Kantarjian et al. Blood [61] | ICT | ED | Age (>79), PS>1, complex karyotype, creatinine>1.3 mg/dL |
| AMLCG/SAL Krug et al. Lancet [62] | ICT | ED, CR, OS | Age, body temperature, fibrinogen, hemoglobin, platelet count, LDH, type of AML (de novo vs secondary) |
| SAL Röllig et al. Blood [63] | ICT | OS, DFS | Age, cytogenetics, WBC, LDH, CD34, NPM1 |
| Elliot et al. Leuk Res [31] | ICT | ED, CR, OS | Polypharmacy |
| Etienne et al. Cancer [30] | ICT | ED, CR, OS | WBC, cytogenetics, CD34 (+) blasts, CCI |
| Hulegardh et al. Am J Hematol [25] | Any | ED, CR, OS | Secondary AML is not so important in elderly AML |
| Giles et al. BJH [34] | ICT | ED, OS | HCT-CI |
| Klepin et al. Blood [16] | ICT | OS | SPPB<9, 3MS<77 |
| Deschler et al. Haematologica [32] | ICT | OS | PS, IDL, HRQoL-item “fatigue” |
| Lazenby et al. Leukemia [28] | ICT | CR, OS | NPM1, FLT3-ITD |
| Marcucci et al. JCO [29] | ICT | OS, DFS | DNMT3A (R882) |
| Buccisano et al. Ann Oncol [27] | ICT | OS, CIR | MRD, other |
| Khan et al. BJH [26] | Any | CR, OS | CD34(+) CD38(low) blasts |
| E-ALMA Ramos et al. Leuk Res [39] | AZA | CR, OS | Real life conditions 100% Pts: 1st line Rx WBC (10.000) Cyto (abn) & PS [2] |
Abn: Abnormal. AML: Acute myeloid leukemia. AZA: Azacitidine. CCI: Charlson comorbidity index. CIR: Cumulative incidence of relapse. CR: Complete remission. Cyto: Cytogenetics. DFS: Disease-free survival. ECOG: Eastern Cooperative Oncology Group. ED: Early death (first 4-8 weeks). HCT: Hemopoietic cell transplantation. HCT-CI: Hemopoietic cell transplantation specific comorbidity index. HRQoL: Health-related quality-of-life. ICT: Intensive chemotherapy. MRD: Minimal residual disease. OS: Overall survival. PS: Performance status. Rx: Therapy. SPPB: Short physical performance battery. 3MS: Modified mini-mental score.
Flow cytometry has attained a central role in the diagnosis of AML and recent data have shown that the degree of phenotypic maturity determined by this technique can also deliver important prognostic information in the elders [26]. These phenotypes can predict both CR rates and survival, suggesting that they reflect intrinsic resistance to treatment. The prognostic weight of minimal residual disease assessed by flow cytometry seems comparatively higher in the elders than in the young [27].
By contrast, the relative impact of molecular findings in the elderly is poorly defined, probably as a consequence of a less intensive research in this population. At present time, the impact of the associations appears to be in general the same than in the young, despite the fact that their relative weight may be lower [28]. Interestingly, in some particular cases, such as DNMT3A codon R882 mutations [29], the behavior is opposite.
Finally, the best-defined prognostic factors come from selected cohorts of patients participating in clinical trials, something that might have hidden other patient-derived prognostic factors. In this sense, comorbidity [5], [30], polypharmacy [31], physical function and cognition [32], [33], as well as fatigue [32], appear to be also relevant for prognosis, since they impact on patient's ability to withstand the insult of chemotherapy and/or hematopoietic cell transplantation (HCT). Among specific comorbidities, previous cerebrovascular, renal, liver, psychiatric and rheumatological diseases have been particularly associated with increased risk of death [5]. As regards comorbidity scores, the Charlson comorbidity index [17] and the HCT comorbidity index (HCT-CI) [34] have been shown to predict overall survival in elderly AML.
4. Treatment options
Life expectancy in AML not only depends on the disease itself, but also on usual risk factors for mortality in the general population. Nevertheless, the tremendous impact of AML on the short term (conferring a very high relative mortality) overrides any other consideration as regards the eventual need for specific therapy. Thus, if the patient does not receive specific treatment, his/her life expectancy will probably be only a few months since diagnosis, although low-blast count AML might have a slower evolution [35]. Nowadays, only 40% of the elderly population receive specific AML therapy within the first 3 months from diagnosis in the US, according to recent data from SEER-Medicare Database, highlighting that there is much room for improvement in this field [36].
Treatment options for elderly AML must be based on four main factors: patient's clinical condition, disease characteristics, patient wishes and social support. Thus, the most frail and oldest among the oldest will have the least net benefit from chemotherapy or hematopoietic transplantation, even if they receive the best available treatment, while the fittest and youngest patients will benefit most. Having said that, it is obvious that the “relative prognosis” of an elderly AML patient will depend on our capability to administer an effective treatment, and that the risk-benefit of subjecting a patient to intensive chemotherapy must be carefully weighed and based not only on the predicted toxicity of the regimen, but also on the likelihood of response, where cytogenetics play a dominant role. Indeed, the relation between adverse cytogenetics and poor and short-lived responses to intensive chemotherapy have been consistently demonstrated [37], [38]. The most favorable situation would be a patient harboring acute promyelocytic leukemia, who can be treated conventionally in most cases with rather optimistic expectations, followed by core-binding-factor leukemias, where the benefit of conventional chemotherapy is also important. In the remainder of the elderly AML patients, intensive chemotherapy has not consistently shown to improve overall survival within controlled clinical trials (Table 3), and the information that supports its potential benefit comes from registry data. This is the consequence not only of a high early mortality, but also of a short duration of the responses [38]. Thus, the proportion of AML patients aged 65 years and over that nowadays receive intensive chemotherapy in daily practice is much lower than in the young [36]. The role of HCT in the treatment of elderly AML will be discussed later on in this review, but the proportion of older adults that are finally transplanted is also small.
Table 3.
Main therapeutic drug comparisons in elderly AML.
| Trial | Rx | Activity | Obs. |
|---|---|---|---|
| EORTC Lowenberg, JCO [64] | ICT vs. BCT | n=60 CR 58 vs. 0% Longer OS with ICT (+2.5 m), p=0.015 | BCT included HU/LDAC p.r.n. Median OS 21wk vs. 11wk Days at hospital 55% vs 50% |
| Rouen Investigators Tilly, JCO [65] | ICT vs. LDAC | n=87 CR 52 vs. 32% Similar OS, p=NS | ICT: more CRs, more ED, more infections, more transfusions, more days at hospital |
| MRC AML14NI Burnett, Cancer [66] | LDAC vs. BSC | n=212 CR 18 vs. 1% Longer OS with LDAC OR 0.60, p=0.009 | 29 pts with HR-MDS were included BSC included HU p.r.n. No advantage in HR cyto (n=42) Induction MRT 26 vs. 26% 1 yr OS 13% (whole series) |
| AZA-001 Fenaux, JCO [67] | AZA vs. BCT | n=113, BMB 20–30% CR 18% vs.16% Longer OS with AZA (+9.5 m), p=0.015 | BCT included BSC, LDAC and ICT EMA and FDA approved |
| AML-001 Dombret, Blood [40] | AZA vs. BCT | n=488, , BMB>30% CR: 19.5 vs. 21.9% Longer OS with AZA (+5.2 m), p=0.019 | BCT included BSC, LDAC and ICT (n=44). Exclusion: PS >2, WBC >15.000, fit for HSCT CR not needed for OS benefit Median OS: 12.1 vs. 6.9 m. 1y OS: 50.7 vs. 37.7% Same FN, ED and HRQoL EMA: Filed; positive opinion FDA: not filed to date |
| DACO16 Kantarjian, JCO [68] | DAC vs. BCT | n=485 CR+CRp 17.8% Median OS: 8.5 m Longer OS (+2.7 m) | BCT included BSC or LDAC, not ICT Exclusion: PS>2 Exclusion: WBC>40.000 EMA approved (>30% BMB) FDA rejected 1y OS: 30% |
AHD: Antecedent hematological disorder. AZA: Azacitidine. BCT: Best conventional therapy. BMB: Bone marrow blast proportion. BST: Best supportive therapy. CLO: Clofarabine. CR: Complete remission. CRp: Complete remission with incomplete platelet count recovery in peripheral blood. DAC: Decitabine. ED: Early death (first 4-8 weeks). FN: Febrile neutropenia. GO: Gemtuzumab-ozogamicin. HCT: Hemopoietic cell transplantation. HU: Hydroxyurea. HRQoL: Health-related quality-of-life. ICT: Intensive chemotherapy. IDL: impairments in activities of daily living. LDAC: Low-dose cytosine arabinoside. PS: Performance status. OR: Odds-ratio. OS: Overall survival. Rx: Therapy SAE: Severe adverse events.
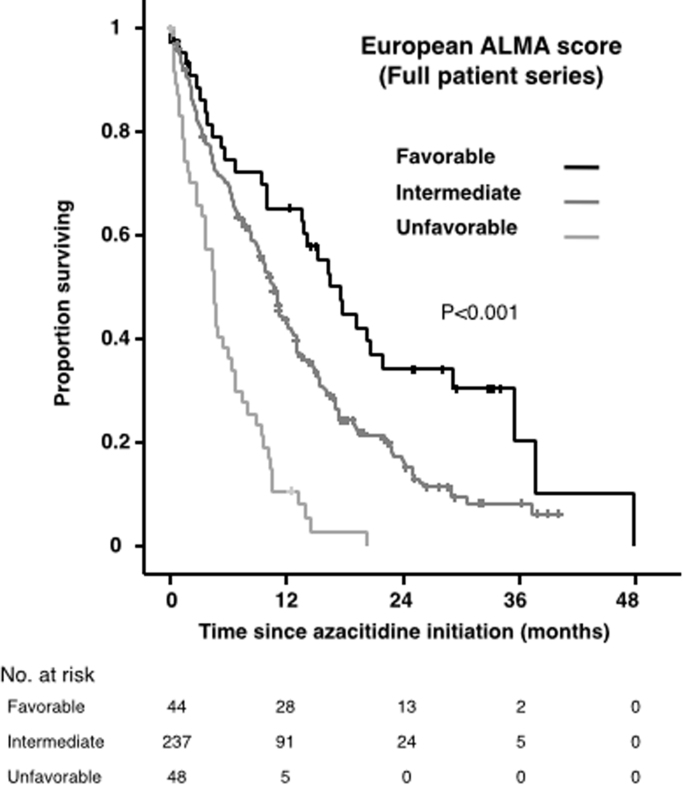
The comparisons of intensive vs. attenuated/supportive therapy are scarce and of low quality. By contrast, higher-level evidence supports the fact that patients treated with low-intensity regimens live longer than those that received only supportive therapy (Table 3). In practice, the physician should aim to proceed to conventional intensive chemotherapy followed by hematopoietic transplantation whenever this is feasible (taking into account disease-related, patient-related and transplantation-related prognostic factors), since this is the best option for curing the leukemia, whereas supportive therapy alone should be avoided as much as possible, and every effort should be made to administer at least low-intensity chemotherapy. Recently, a predictive scoring system for response and survival in elderly patients treated in real world by azacitidine, based exclusively on clinical features, has been developed and validated in Europe [39] (Table 4 and Fig. 1). Furthermore, some data suggest that this drug might even result in superior OS, as compared to intensive chemotherapy or low-dose cytarabine, in patients harboring high-risk cytogenetics, MDS-related AML, or erythroleukemia [40], [41], [42]. This might be improved in the near future by including some molecular predictors such as mutations in TET2 [43] and IDH1/2 [44].
Table 4.
European ALMA score.
| European ALMA score | ||
|---|---|---|
| Applicability | Unfit WHO-defined AML patients (any blast count) treated frontline with azacitidine | |
| Predictors | Strata | Subscore |
| Performance Status (ECOG) | 0 | 0 |
| 1–2 | 1 | |
| 3–4 | 2 | |
| WBC count before AZA onset | Up to 10×109/L | 0 |
| Greater than 10×109/L | 1 | |
| Cytogenetics | Normal | 0 |
| Abnormal | 1 | |
| Patient subsets for OS and response | Score | |
| Favorable | 0 | |
| Intermediate | 1–2 | |
| Unfavorable | 3–4 | |
AML=Acute myeloid leukemia. ECOG: Eastern Cooperative Oncology Group. WBC: White blood cell. OS: Overall survival. AZA: Azacitidine. ALMA stands for “Azacitidina en el tratamiento de la leucemia aguda mieloblástica” (AZA in AML therapy).
Fig. 1.
Overall survival by European ALMA Score. Full patient series. The tick marks on the curves represent censored patients.
Despite promising response rates, none of the various other agents tested, other than azacitidine in low-blast count leukemia, have been able to overcome the 12 months landmark for median overall survival (Table 5) and there is great need for novel agents in this group of patients.
Table 5.
Therapeutic efficacy (complete response rate and median overall survival) of different drugs used for elderly patients with AML.
| Regimen | CR rate (%) | Median OS (months) |
|---|---|---|
| Intensive chemotherapy [64], [65] | 50–60 | 6–12 |
| LDAC [65], [66] | 10–25 | 6 |
| Azacitidine in low-blast count AM [67] | 25 | 24.5 |
| Azacitidine in AML>30% BMB [40] | 25 | 12.1 |
| Decitabine in AML>30% BMB [68] | 18 | 7.7 |
| Clofarabine [69] | 38 | 11.4 |
| Sapacitabine [70] | 37 | 7.9 |
| Barasertib [71] | 35 | 8.2 |
| Volasertib (+ LDAC) [72] | 31 | 8 |
| Tipifarnib [73] | 8 | 3.6 |
| Midostaurin (+AZA) [74] | 2 | NA |
| Quizartinib [75] | 54 | <12 |
| Vorinostat (+LDAC) [76] | 46 | NA |
| Gemtuzumab ozogamicin [77] | 30 | 11 |
CR: Complete remission. LDAC: Low dose cytarabine. BMB: Bone marrow blasts. OS: Overall survival. NA: Not available.
Currently, there are numerous novel agents being tested in clinical trials, many of which target recently described molecular defects. These include modified cytotoxics, epigenetic regulators, cell cycle kinase inhibitors, etc. Many are currently being tested in monotherapy or in combination with already established regimens. According to very preliminary results from Phase I/II trials, several agents hold promise, such as a) The FLT3 inhibitors gilteritinib (ASP2215) [45] and sorafenib [46], which have been tested in monotherapy, and in combination with chemotherapy in the case of sorafenib. Remission rates obtained with monotherapy are as high as 50% in patients with FLT3 mutations. Combination of sorafenib with chemotherapy in adults with mutated FLT3 improved 1 yr survival from 30% to 62% [46]. As regards the multi-kinase inhibitor midostaurin, its efficacy in combination with standard chemotherapy (RATIFY trial) in patients under 60 [47] is yet to be proved in patients over 60, b) The Inhibitors of IDH1 and IDH2, which have obtained overall response rates of about 40% in heavily pre-treated older AML patients harboring IDH-1 or IDH-2 mutations [48], c) The addition of the HDAC inhibitor pracinostat to azacitidine, that achieved 1-year survival rates of 60% with 54% overall response rate [49], d) Monoclonal antibodies, such as vadastuximab talirine (SGN-CD33A), an anti-CD33 antibody conjugated with a pyrrolobenzodiazepine dimer, as well as Durvalumab, an anti-PD-L1 antibody, that have shown promising preliminary response rates and are currently being tested further [50], [51].
The progressive unraveling of the molecular pathophysiology of AML is likely to identify more therapeutic targets, which may widen in the near future the therapeutic armamentarium for elderly AML.
5. Hematopoietic cell transplantation
HCT is generally recommended for AML patients with intermediate or high risk cytogenetics, when the aim of treatment is curative. However, elderly patients are seldom offered a transplant mainly due to concerns regarding toxicity, despite the fact that the main reason for death after HCT in the older adult is not toxicity but relapse [52]. Fortunately, there has been a steady increase in the number of HCTs in this patient group in the past decade [53] as a consequence of the introduction of less intensive conditioning regimes, which have significantly reduced toxicity whilst maintaining successful engraftment. Nevertheless, nowadays only about 8% of elderly patients with AML undergo HCT each year in the US [36], even though they constitute more than 50% of the new AML cases.
Outcome analysis in this population indicates that those who are submitted to HCT have a relatively favorable outcome, with almost 40% 3-year relapse-free and overall survival [54]. It is therefore essential to identify those patients whose fitness and disease characteristics make them the best candidates for transplant. Beyond chronological age [55], factors that predict OS and relapse are high risk AML (cytogenetics, gene mutations), higher HCT-CI scores, patient CMV status and greater HLA disparity, while the impact of donor age is still disputed, especially in the context of reduced-intensity conditioning regimes. In addition, poor performance status and active infections are associated with poorer outcomes and these patients should not be transplanted, unless their condition improves. Similarly, patients who have active disease at the time of HCT are very unlikely to maintain remission long term, and should be rather rescued with chemotherapy and transplanted when in CR. Patients with favorable prognostic disease, especially those with CBF mutations and no additional poor risk cytogenetic or molecular markers, do well with consolidation chemotherapy and do not benefit from transplant in first remission. Therefore all elderly patients with good performance status (ECOG PS 0-1) and with any disease risk other than the good prognostic group should be considered for HCT, depending on their HCT-CI [56] and EBMT [57] risk score (Table 6).
Table 6.
HCT according to patient and AML considerations.
| HCT-CI score[18] | EBMT score[57] | NRM at 4 years |
|---|---|---|
| 0 | 0–3 | 11% |
| 0 | Greater than 3 | 19% |
| 1–2 | 0–3 | 16% |
| 1–2 | Greater than 3 | 28% |
| Greater than 2 | 0–3 | 31% |
| Greater than 2 | Greater than 2 | 41% |
EBMT: European group for blood and marrow transplantation. HCT-CI: Hemopoietic cell transplantation specific comorbidity index. NRM: Non-relapse mortality.
The role of geriatric assessment in identifying patient vulnerabilities before HCT is still in the beginning, but gait speed, instrumental activities of daily living and cognition evaluation, among other clinical parameters, seem to be useful for this purpose [58]. The analysis of the impact of HCT on the quality-of-life of the older adults is also very much in its infancy. Lastly, it should be emphasized that HCT candidates must be identified at diagnosis and this option discussed upfront with the patient as some elderly patients may not agree on an aggressive treatment course.
6. Social issues
Dependency, disability, general functioning, cognition, quality-of-life, caregiver, burden of disease…are recurrent terms when confronting diseases of the elderly. Many older adults, but not most, would keep free of dependency and disability until late in their lives, and the very proportion will depend on their previous life-style and comorbidity. In this population, the optimal target should be to cure the disease without compromising heavily the quality-of-life on the short term, as well maintaining general functioning and cognition on the long term. A systematic evaluation of quality-of-life during treatment and general functioning and cognition of this population, before and after treatment, would be of great help for informing clinical decisions, but for the time being the available information is scarce.
Social and family support is critical in patients dependent for basic activities of daily life, but also for those dependent only for instrumental activities or even in the independent ones (limited budget, frequent hospital visits, unexpected medical complications, less prone to visit the Emergency Department) [59]. Such a support may modulate the type and intensity of the treatment to be given, even in the best developed countries, and becomes critical when welfare state is less than optimal.
Finally, the burden of disease in elderly AML is huge, for the patient, his/her family and the whole society, and specially in this time of targeted therapy. It is the physician's responsibility to try and make an optimal use of scarce resources to achieve the best outcomes while avoiding discrimination purely on grounds of age.
7. Conclusion
Treatment options remain limited for older adults with AML. It is essential to tailor the choice of therapy to patient's condition and disease characteristics, and a few patients benefit from aggressive approaches. Some novel agents hold promise, but it is unlikely that they will achieve much benefit as single agents and, hopefully, combinations may provide better results in the future. HCT is increasingly safer and, therefore, more feasible in this patient population but the toxicities of previous treatments and the logistics of donor availability may delay the transplant and put at risk the fragile remissions which may have been obtained. Whether as a bridge to transplant or on their own, low-intensity approaches may help to improve the outlook of elderly patients with AML.
Finally, additional research on the impact of pre-treatment geriatric assessment and different therapeutic approaches, including HCT, on the quality-of-life of older adults, as well as higher social awareness of the huge resources concerned to improve the outcome of this elderly population in the near future, is much needed.
Disclosures
AA is a consultant for Celgene and Novartis and serves on the speakers bureau of Bristol-Meyers Squibb, Alexion, Shire and Amgen.
FR has received honoraria from Celgene, Janssen-Cilag, Amgen, Novartis, Merck-Sharp & Dohme, Pfizer, Roche and GlaxoSmithKline; research grants from Celgene; has been Consultant for Amgen and Novartis and served as member of Advisory Boards of Celgene and Janssen-Cilag.
References
- 1.Cancer Statistics Review 1975–2003: I Table–11median age of cancer patients at diagnosis, 2000–2003 by primary cancersite, race and sex. [database on the Internet]. National Cancer Institute. (cited 01.02.16), 2003.
- 2.Alibhai S.M., Leach M., Minden M.D., Brandwein J. Outcomes and quality of care in acute myeloid leukemia over 40 years. Cancer. 2009;115(13):2903–2911. doi: 10.1002/cncr.24373. [DOI] [PubMed] [Google Scholar]
- 3.Menzin J., Lang K., Earle C.C., Kerney D., Mallick R. The outcomes and costs of acute myeloid leukemia among the elderly. Arch. Intern Med. 2002;162(14):1597–1603. doi: 10.1001/archinte.162.14.1597. [DOI] [PubMed] [Google Scholar]
- 4.Global Health Observatory Data (database on the Internet). WHO. (cited 01.02.16), 2016. Available from: 〈http://www.who.int/gho/mortality_burden_disease/life_tables/life_tables/en/〉.
- 5.Mohammadi M., Cao Y., Glimelius I., Bottai M., Eloranta S., Smedby K.E. The impact of comorbid disease history on all-cause and cancer-specific mortality in myeloid leukemia and myeloma – a Swedish population-based study. BMC Cancer. 2015;15:850. doi: 10.1186/s12885-015-1857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leith C.P., Kopecky K.J., Godwin J., McConnell T., Slovak M.L., Chen I.M. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89(9):3323–3329. [PubMed] [Google Scholar]
- 7.Patel J.P., Gonen M., Figueroa M.E., Fernandez H., Sun Z., Racevskis J. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. New Engl. J. Med. 2012;366(12):1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao A.V., Valk P.J., Metzeler K.H., Acharya C.R., Tuchman S.A., Stevenson M.M. Age-specific differences in oncogenic pathway dysregulation and anthracycline sensitivity in patients with acute myeloid leukemia. J. Clin. Oncol. 2009;27(33):5580–5586. doi: 10.1200/JCO.2009.22.2547. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa M.E., Skrabanek L., Li Y., Jiemjit A., Fandy T.E., Paietta E. MDS and secondary AML display unique patterns and abundance of aberrant DNA methylation. Blood. 2009;114(16):3448–3458. doi: 10.1182/blood-2009-01-200519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NCCN, National comprehensive cancer network, NCCN clinical practice guidelines in oncology, Older adult oncology. Version 2.2015. Retrieved from 〈http://www.nccn.org/professionals/ physician_gls/PDF/senior.pdf〉. (Accesed 09.01.16), 2016.
- 11.Saliba D., Elliott M., Rubenstein L.Z., Solomon D.H., Young R.T., Kamberg C.J. The vulnerable elders survey: a tool for identifying vulnerable older people in the community. J. Am. Geriatr. Soc. 2001;49(12):1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 12.Hamaker M.E., Mitrovic M., Stauder R. The G8 screening tool detects relevant geriatric impairments and predicts survival in elderly patients with a haematological malignancy. Ann. Hematol. 2014;93(6):1031–1040. doi: 10.1007/s00277-013-2001-0. [DOI] [PubMed] [Google Scholar]
- 13.Hamaker M.E., Prins M.C., Stauder R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy--a systematic review. Leuk. Res. 2014;38(3):275–283. doi: 10.1016/j.leukres.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Hamaker M.E., Schiphorst A.H., ten Bokkel Huinink D., Schaar C., van Munster B.C. The effect of a geriatric evaluation on treatment decisions for older cancer patients--a systematic review. Acta Oncol. 2014;53(3):289–296. doi: 10.3109/0284186X.2013.840741. [DOI] [PubMed] [Google Scholar]
- 15.Klepin H.D. Geriatric perspective: how to assess fitness for chemotherapy in acute myeloid leukemia. Hematol. Am. Soc. Hematol. Educ. Program. 2014;2014(1):8–13. doi: 10.1182/asheducation-2014.1.8. [DOI] [PubMed] [Google Scholar]
- 16.Klepin H.D., Geiger A.M., Tooze J.A., Kritchevsky S.B., Williamson J.D., Pardee T.S. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121(21):4287–4294. doi: 10.1182/blood-2012-12-471680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development andBM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorror M.L., Maris M.B., Storb R., Baron F., Sandmaier B.M., Maloney D.G. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guralnik J.M., Simonsick E.M., Ferrucci L., Glynn R.J., Berkman L.F., Blazer D.G. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik J.M., Ferrucci L., Simonsick E.M., Salive M.E., Wallace R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. New Engl. J. Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Studenski S., Perera S., Patel K., Rosano C., Faulkner K., Inzitari M. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teng E.L., Chui H.C. The modified mini-mental state (3MS) examination. J. Clin. Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 23.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 24.Wheatley K., Brookes C.L., Howman A.J., Goldstone A.H., Milligan D.W., Prentice A.G. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br. J. Haematol. 2009;145(5):598–605. doi: 10.1111/j.1365-2141.2009.07663.x. [DOI] [PubMed] [Google Scholar]
- 25.Hulegardh E., Nilsson C., Lazarevic V., Garelius H., Antunovic P., Rangert Derolf A. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish Acute Leukemia Registry. Am. J. Hematol. 2015;90(3):208–214. doi: 10.1002/ajh.23908. [DOI] [PubMed] [Google Scholar]
- 26.Khan N., Freeman S.D., Virgo P., Couzens S., Richardson P., Thomas I. An immunophenotypic pre-treatment predictor for poor response to induction chemotherapy in older acute myeloid leukaemia patients: blood frequency of CD34+CD38 low blasts. Br. J. Haematol. 2015;170(1):80–84. doi: 10.1111/bjh.13398. [DOI] [PubMed] [Google Scholar]
- 27.Buccisano F., Maurillo L., Piciocchi A., Del Principe M.I., Sarlo C., Cefalo M. Minimal residual disease negativity in elderly patients with acute myeloid leukemia may indicate different postremission strategies than in younger patients. Ann. Hematol. 2015;94(8):1319–1326. doi: 10.1007/s00277-015-2364-5. [DOI] [PubMed] [Google Scholar]
- 28.Lazenby M., Gilkes A.F., Marrin C., Evans A., Hills R.K., Burnett A.K. The prognostic relevance of flt3 and npm1 mutations on older patients treated intensively or non-intensively: a study of 1312 patients in the UK NCRI AML16 trial. Leukemia. 2014;28(10):1953–1959. doi: 10.1038/leu.2014.90. [DOI] [PubMed] [Google Scholar]
- 29.Marcucci G., Metzeler K.H., Schwind S., Becker H., Maharry K., Mrozek K. Age-related prognostic impact of different types of DNMT3A mutations in adults with primary cytogenetically normal acute myeloid leukemia. J. Clin. Oncol. 2012;30(7):742–750. doi: 10.1200/JCO.2011.39.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etienne A., Esterni B., Charbonnier A., Mozziconacci M.J., Arnoulet C., Coso D. Comorbidity is an independent predictor of complete remission in elderly patients receiving induction chemotherapy for acute myeloid leukemia. Cancer. 2007;109(7):1376–1383. doi: 10.1002/cncr.22537. [DOI] [PubMed] [Google Scholar]
- 31.Elliot K., Tooze J.A., Geller R., Powell B.L., Pardee T.S., Ritchie E. The prognostic importance of polypharmacy in older adults treated for acute myelogenous leukemia (AML) Leuk. Res. 2014;38(10):1184–1190. doi: 10.1016/j.leukres.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deschler B., Ihorst G., Platzbecker U., Germing U., Marz E., de Figuerido M. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica. 2013;98(2):208–216. doi: 10.3324/haematol.2012.067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klepin H.D. Predicting treatment outcomes in older patients with acute myelogenous leukemia. Clin. Adv. Hematol. Oncol. 2013;11(10):669–671. [PubMed] [Google Scholar]
- 34.Giles F.J., Borthakur G., Ravandi F., Faderl S., Verstovsek S., Thomas D. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br. J. Haematol. 2007;136(4):624–627. doi: 10.1111/j.1365-2141.2006.06476.x. [DOI] [PubMed] [Google Scholar]
- 35.Hasserjian R.P., Campigotto F., Klepeis V., Fu B., Wang S.A., Bueso-Ramos C. De novo acute myeloid leukemia with 20–29% blasts is less aggressive than acute myeloid leukemia with >/=30% blasts in older adults: a Bone Marrow Pathology Group study. Am. J. Hematol. 2014;89(11) doi: 10.1002/ajh.23808. E193-9. [DOI] [PubMed] [Google Scholar]
- 36.Medeiros B.C., Satram-Hoang S., Hurst D., Hoang K.Q., Momin F., Reyes C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann. Hematol. 2015;94(7):1127–1138. doi: 10.1007/s00277-015-2351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grimwade D., Walker H., Harrison G., Oliver F., Chatters S., Harrison C.J. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98(5):1312–1320. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 38.Kantarjian H., O’Brien S., Cortes J., Giles F., Faderl S., Jabbour E. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106(5):1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 39.Ramos F., Thepot S., Pleyer L., Maurillo L., Itzykson R., Bargay J. Azacitidine frontline therapy for unfit acute myeloid leukemia patients: clinical use and outcome prediction. Leuk. Res. 2015;39(3):296–306. doi: 10.1016/j.leukres.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Dombret H., Seymour J.F., Butrym A., Wierzbowska A., Selleslag D., Jang J.H. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–299. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.J.T.S. Falantes, L. Pleyer, L. Maurillo, V. Martínez-Robles, R. Itzykson, J. Bargay, R. Stauder, A. Venditti, M.P. Martínez, R. Seegers, M.A. Foncillas, S. Burgstaller, C. Gardin, P. Montesinos, P. Musto, R. Greil, M.A. Sanz, P. Fenaux, F. Ramos, For the European ALMA Investigators, Azacitidine in older patients with acute myeloid leukemia (AML), Results from the expanded international E-ALMA series (E-ALMA+) according to the MRC risk index score, in: Proceedings of the Annual Conference of the American Society for Hematology, 2015, pp. 2554.
- 42.Pierdomenico F., Esteves S., Almeida A. Efficacy and tolerability of 5-day azacytidine dose-intensified regimen in higher-risk MDS. Ann. Hematol. 2013;92(9):1201–1206. doi: 10.1007/s00277-013-1762-9. [DOI] [PubMed] [Google Scholar]
- 43.Itzykson R., Kosmider O., Cluzeau T., Mansat-De Mas V., Dreyfus F., Beyne-Rauzy O. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia. 2011;25(7):1147–1152. doi: 10.1038/leu.2011.71. [DOI] [PubMed] [Google Scholar]
- 44.Emadi A., Faramand R., Carter-Cooper B., Tolu S., Ford L.A., Lapidus R.G. Presence of isocitrate dehydrogenase mutations may predict clinical response to hypomethylating agents in patients with acute myeloid leukemia. Am. J. Hematol. 2015;90(5):E77–E79. doi: 10.1002/ajh.23965. [DOI] [PubMed] [Google Scholar]
- 45.Thom C. Preliminary data on ASP2215: tolerability and efficacy in acute myeloid leukemia patients. Future Oncol. 2015;11(18):2499–2501. doi: 10.2217/fon.15.188. [DOI] [PubMed] [Google Scholar]
- 46.Röllig C., Serve H., Hüttmann A., Noppeney R., Müller-Tidow C., Krug U. Study Alliance Leukaemia. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2015;16(16):1691–1699. doi: 10.1016/S1470-2045(15)00362-9. 1. [DOI] [PubMed] [Google Scholar]
- 47.Stone R.M., Mandrekar S., Sanford B.L., Geyer S., Bloomfield C.D., Dohner K. The Multi-Kinase Inhibitor Midostaurin (M) Prolongs Survival Compared with Placebo (P) in Combination with Daunorubicin (D)/Cytarabine (C) Induction (in)d), High-Dose C Consolidation (consol), (and As Maintenance (main)t) (Therapy in Newly Diagnosed Acute Myeloid Leukemia (AML) Patients (pt)s) (Age 18-60 with FLT3 Mutations (mut)s): (An International Prospective Randomized (ran)d) (P-Controlled Double-Blind Trial (CALGB 10603/RATIFY [Allianc)e]) Blood. 2015;126(23) 6-6. [Google Scholar]
- 48.Stein E.M. IDH2 inhibition in AML: finally progress? Best Pract. Res. Clin. Haematol. 2015;28(2–3):112–115. doi: 10.1016/j.beha.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Manero G., Atallah E., Khaled S.K., Arellano M., Patnaik M.M., Butler T. Final Results from a Phase 2 Study of Pracinostat in Combination with Azacitidine in Elderly Patients with Acute Myeloid Leukemia (AML) Blood. 2015;126(23):453. [Google Scholar]
- 50.Fathi A.T., Erba H., Lancet J., Stein E.M., Walter R., De Angelo D.J. SGN-CD33A Plus Hypomethylating Agents: A Novel, Well-Tolerated Regimen with High Remission Rate in Frontline Unfit AML. Blood. 2015;126(23):454. [Google Scholar]
- 51.A. Fandi, D.M. Reiser, D. Barton, D. Begic, Methods for treating a disease or disorder using oral formulations of cytidine analogs in combination with an Anti-PD2 or Anti-PDL1 monoclonal antibody, 2016. Available from: 〈http://www.freepatentsonline.com/y2016/0067336.html〉.
- 52.Aoki J., Kanamori H., Tanaka M., Yamasaki S., Fukuda T., Ogawa H. Impact of age on outcomes of allogeneic hematopoietic stem cell transplantation with reduced intensity conditioning in elderly patients with acute myeloid leukemia. Am. J. Hematol. 2016;91(3):302–307. doi: 10.1002/ajh.24270. [DOI] [PubMed] [Google Scholar]
- 53.Sengsayadeth S., Savani B.N., Blaise D., Malard F., Nagler A., Mohty M. Reduced intensity conditioning allogeneic hematopoietic cell transplantation for adult acute myeloid leukemia in complete remission – a review from the Acute Leukemia Working Party of the EBMT. Haematologica. 2015;100(7):859–869. doi: 10.3324/haematol.2015.123331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aoki J., Kanamori H., Tanaka M., Yamasaki S., Fukuda T., Ogawa H. Impact of age on outcomes of allogeneic hematopoietic stem cell transplantation with reduced intensity conditioning in elderly patients with acute myeloid leukemia. Am. J. Hematol. 2016;91(3):302–307. doi: 10.1002/ajh.24270. [DOI] [PubMed] [Google Scholar]
- 55.Sorror M.L., Storb R.F., Sandmaier B.M., Maziarz R.T., Pulsipher M.A., Maris M.B. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 2014;32(29):3249–3256. doi: 10.1200/JCO.2013.53.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sorror M.L., Estey E. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia in older adults. Hematol. Am. Soc. Hematol. Educ. Program. 2014;2014(1):21–33. doi: 10.1182/asheducation-2014.1.21. [DOI] [PubMed] [Google Scholar]
- 57.Michelis F.V., Messner H.A., Uhm J., Alam N., Lambie A., McGillis L. Modified EBMT pretransplant risk score can identify favorable-risk patients undergoing allogeneic hematopoietic cell transplantation for AML, not Identified by the HCT-CI score. Clin. Lymphoma Myeloma Leuk. 2015;15(5):e73–e81. doi: 10.1016/j.clml.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 58.Muffly L.S., Kocherginsky M., Stock W., Chu Q., Bishop M.R., Godley L.A. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 2014;99(8):1373–1379. doi: 10.3324/haematol.2014.103655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bryant A.L., Deal A.M., Walton A., Wood W.A., Muss H., Mayer D.K. Use of ED and hospital services for patients with acute leukemia after induction therapy: one year follow-up. Leuk. Res. 2015;39(4):406–410. doi: 10.1016/j.leukres.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malfuson J.V., Etienne A., Turlure P., de Revel T., Thomas X., Contentin N. Risk factors and decision criteria for intensive chemotherapy in older patients with acute myeloid leukemia. Haematologica. 2008;93(12):1806–1813. doi: 10.3324/haematol.13309. [DOI] [PubMed] [Google Scholar]
- 61.Kantarjian H., Ravandi F., O’Brien S., Cortes J., Faderl S., Garcia-Manero G. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116(22):4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krug U., Rollig C., Koschmieder A., Heinecke A., Sauerland M.C., Schaich M. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. Lancet. 2010;376(9757):2000–2008. doi: 10.1016/S0140-6736(10)62105-8. [DOI] [PubMed] [Google Scholar]
- 63.Rollig C., Thiede C., Gramatzki M., Aulitzky W., Bodenstein H., Bornhauser M. A novel prognostic model in elderly patients with acute myeloid leukemia: results of 909 patients entered into the prospective AML96 trial. Blood. 2010;116(6):971–978. doi: 10.1182/blood-2010-01-267302. [DOI] [PubMed] [Google Scholar]
- 64.Lowenberg B., Zittoun R., Kerkhofs H., Jehn U., Abels J., Debusscher L. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: a randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J. Clin. Oncol. 1989;7(9):1268–1274. doi: 10.1200/JCO.1989.7.9.1268. [DOI] [PubMed] [Google Scholar]
- 65.Tilly H., Castaigne S., Bordessoule D., Casassus P., Le Prise P.Y., Tertian G. Low-dose cytarabine versus intensive chemotherapy in the treatment of acute nonlymphocytic leukemia in the elderly. J. Clin. Oncol. 1990;8(2):272–279. doi: 10.1200/JCO.1990.8.2.272. [DOI] [PubMed] [Google Scholar]
- 66.Burnett A.K., Milligan D., Prentice A.G., Goldstone A.H., McMullin M.F., Hills R.K. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114–1124. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 67.Fenaux P., Mufti G.J., Hellstrom-Lindberg E., Santini V., Gattermann N., Germing U. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J. Clin. Oncol. 2010;28(4):562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 68.Kantarjian H.M., Thomas X.G., Dmoszynska A., Wierzbowska A., Mazur G., Mayer J. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012;30(21):2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burnett A.K., Russell N.H., Hunter A.E., Milligan D., Knapper S., Wheatley K. Clofarabine doubles the response rate in older patients with acute myeloid leukemia but does not improve survival. Blood. 2013;122(8):1384–1394. doi: 10.1182/blood-2013-04-496596. [DOI] [PubMed] [Google Scholar]
- 70.Burnett A.K., Russell N., Hills R.K., Panoskaltsis N., Khwaja A., Hemmaway C. A randomised comparison of the novel nucleoside analogue sapacitabine with low-dose cytarabine in older patients with acute myeloid leukaemia. Leukemia. 2015;29(6):1312–1319. doi: 10.1038/leu.2015.38. [DOI] [PubMed] [Google Scholar]
- 71.Kantarjian H.M., Martinelli G., Jabbour E.J., Quintas-Cardama A., Ando K., Bay J.O. Stage I of a phase 2 study assessing the efficacy, safety, and tolerability of barasertib (AZD1152) versus low-dose cytosine arabinoside in elderly patients with acute myeloid leukemia. Cancer. 2013;119(14):2611–2619. doi: 10.1002/cncr.28113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dohner H., Lubbert M., Fiedler W., Fouillard L., Haaland A., Brandwein J.M. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood. 2014;124(9):1426–1433. doi: 10.1182/blood-2014-03-560557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harousseau J.L., Martinelli G., Jedrzejczak W.W., Brandwein J.M., Bordessoule D., Masszi T. A randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood. 2009;114(6):1166–1173. doi: 10.1182/blood-2009-01-198093. [DOI] [PubMed] [Google Scholar]
- 74.Strati P., Kantarjian H., Ravandi F., Nazha A., Borthakur G., Daver N. Phase I/II trial of the combination of midostaurin (PKC412) and 5-azacytidine for patients with acute myeloid leukemia and myelodysplastic syndrome. Am. J. Hematol. 2015;90(4):276–281. doi: 10.1002/ajh.23924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cortes J.E., Kantarjian H., Foran J.M., Ghirdaladze D., Zodelava M., Borthakur G. Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3-internal tandem duplication status. J. Clin. Oncol. 2013;31(29):3681–3687. doi: 10.1200/JCO.2013.48.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prebet T., Braun T., Beyne-Rauzy O., Dreyfus F., Stammatoullas A., Wattel E. Combination of vorinostat and low dose cytarabine for patients with azacitidine-refractory/relapsed high risk myelodysplastic syndromes. Leuk. Res. 2014;38(1):29–33. doi: 10.1016/j.leukres.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 77.Amadori S., Suciu S., Selleslag D., Aversa F., Gaidano G., Musso M. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J. Clin. Oncol. 2016;34(9):972–979. doi: 10.1200/JCO.2015.64.0060. [DOI] [PubMed] [Google Scholar]