Abstract
Differences in the activity of neurotransmitters and neuromodulators, and consequently different neural responses, can be found between anesthetized and awake animals. Therefore, methods allowing the manipulation of synaptic systems in awake animals are required in order to determine the contribution of synaptic inputs to neuronal processing unaffected by anesthetics. Here, we present methodology for the construction of electrodes to simultaneously record extracellular neural activity and release multiple neuroactive substances at the vicinity of the recording sites in awake mice. By combining these procedures, we performed microiontophoretic injections of gabazine to selectively block GABAA receptors in neurons of the inferior colliculus of head-restrained mice. Gabazine successfully modified neural response properties such as the frequency response area and stimulus-specific adaptation. Thus, we demonstrate that our methods are suitable for recording single-unit activity and for dissecting the role of specific neurotransmitter receptors in auditory processing.
The main limitation of the described procedure is the relatively short recording time (~3 hr), which is determined by the level of habituation of the animal to the recording sessions. On the other hand, multiple recording sessions can be performed in the same animal. The advantage of this technique over other experimental procedures used to manipulate the level of neurotransmission or neuromodulation (such as systemic injections or the use of optogenetic models), is that the drug effect is confined to the local synaptic inputs to the target neuron. In addition, the custom-manufacture of electrodes allows adjustment of specific parameters according to the neural structure and type of neuron of interest (such as the tip resistance for improving the signal-to-noise ratio of the recordings).
Keywords: Neuroscience, Issue 111, auditory, stimulus-specific adaptation, inferior colliculus, gabazine, inhibition, frequency response area, multibarrels, tungsten electrode, synaptic inputs
Introduction
The interplay of neural excitation and inhibition is fundamental for the processing of sensory information1. It is also known that anesthesia has a strong impact on the dynamics of cortical activation and the temporal pattern of synaptic inputs2,3. For example, it has been observed that anesthetics alter the duration of visually-evoked responses in cortical neurons3,4. Moreover, the ratio between excitatory and inhibitory synaptic inputs is different in anesthetized and awake animals4,5, altering both evoked and spontaneous activity rates6,7. By measuring the synaptic conductances, Haider and colleagues4 found that inhibition matched excitation in amplitude under anesthesia whereas during wakefulness, inhibition was stronger than excitation. These findings prompt the development of experimental procedures to study the impact of specific synaptic inputs on sensory processing in awake animals.
The controlled ejection of charged neuroactive substances by applying small current injections (on the order of nA) has been extensively used to study the contribution of synaptic inputs and the role of putative cell receptors in sensory processing8-13. This technique, known as microiontophoresis, allows the application of drugs in the vicinity of the recorded neuron, which contributes to a rapid and confined effect. This procedure is more suitable for studying local effects of neuroactive substances, compared to the widespread effect elicited by other experimental manipulations such as systemic injections, microdialysis or the use of optogenetic techniques. Usually, a piggy-back electrode configuration14,15 is used to simultaneously record the target neuron and deliver the neuroactive substances of interest. It consists of a recording electrode attached to a multibarrel pipette that carries the neuroactive substances. Modifications of the original procedure described by Havey and Caspary14 have been implemented. For example, a tungsten electrode, instead of a glass one, can be used to record the neural activity16. Previously published methods for the manufacture of tungsten electrodes17,18 involve three general steps: electrolytic etching of tungsten wire tips, glass insulation, and adjustment of the tip exposure to meet recording requirements.
An interesting and emergent field in auditory neuroscience is the study of stimulus-specific adaptation (SSA19). SSA is a specific decrease in the neural response to repetitive sounds that does not generalize to other, rarely presented sounds. The importance of SSA resides in its potential role as a neural mechanism underlying deviance detection in the auditory brain, as well as a possible neuronal correlate for the late mismatch negativity component of the auditory evoked potential20,21. SSA occurs from the IC up to the auditory cortex19,22-24. GABAA-mediated inhibition has been demonstrated to act as a gain control mechanism on SSA7,16,25, which has also been shown to be affected by anesthesia26. Here we present a protocol that combines previously described methods for recording the single-unit activity of IC neurons before and during the application of a selective antagonist of the GABAA-receptors in awake mice. First, we describe the manufacture of piggy-back electrodes and next, the surgical and recording methods. To test for the efficacy of drug release, we compared the receptive field as well as the level of SSA of IC neurons before and during the microiontophoretic ejection of gabazine.
Protocol
All experimental procedures were carried out at the University of Salamanca with the approval of, and using methods conforming to the standards of, the University of Salamanca Animal Care Committee as well as the standards of the European Union (Directive 2010/63/EU) for the use of animals in neuroscience research.
1. Tungsten Electrodes
Note: The manufacture of tungsten electrodes is based on the original technique described in Merrill and Ainsworth27 and Ainsworth et al.28 and performed using the workstation setup described in Bullock et al.17.
To record single-unit extracellular activity of IC neurons, use electrodes with a tip impedance of 1.5 - 2.5 MΩ. Here, the steps involved in the tungsten electrode manufacture are described only briefly, since a detailed description is found in the above-mentioned references.
Place tungsten wires in a custom-built alignment tool (Figure 1A) to assist cutting them to the desired length. The alignment tool consists of 30 needles (25 G) glued in parallel at 2 mm intervals, with a stop at one end to limit the length of the wires to ~40 mm. Carefully attach the loose ends of the wires with adhesive tape, and cut the rest of the wire with strong scissors.
Pull gently from the tape in order to remove the tungsten wires from the alignment tool, making sure that all the wires remain attached to the tape. Roll the tape onto a polished brass spindle (Figure 1B,C), holding the wires firmly against the spindle so there is a good electrical connection.
Connect the spindle to a 5 rpm motor in the workstation, and immerse the protruding wire tips into an etching solution (KNO2, 90 g per 80 ml). Angle the spindle axis 45° relative to the surface of the etching solution. Immerse the wire tips so that ~1/3 of the wires contact the solution.
Immerse a carbon rod electrode into the etching bath to close the circuit. Use a spring copper braid brush to apply the etching supply to the spindle shaft while the spindle is rotating. Adjust the current that is passed through the electrodes and the etching solution to 250 mA. Stop the tip etching when the current drops to 200 mA. The wire tips will be etched into very fine points.
Pass one sharpened wire through a flame to remove any grease and adhesive residue. Place the wire (blunt end first) into a borosilicate glass capillary (1.5 mm O.D., 0.86 mm I.D)with its lower end blocked with modelling clay. Clamp the pipette vertically at the top and bottom. Pull the pipette using a heating coil (diameter 5 mm, depth 5 mm) and a suspended plunger. As the wire is pulled down by the weight of the plunger, the molten glass forms a very fine coat around it.
Remove the glass covering the tip at the sharp end of the electrode with the help of a molten bead of sodium tetraborate. To form the bead, mix the sodium tetraborate (~0.25 g) with a small amount of water (~0.5 ml) and slowly place it on a heating element, until it melts into a bead ~2 mm in diameter. The temperature of the heating element is controlled by a potentiometer. Place the bead and heating element on a manipulator arm fixed to the table, and move it until the bead is focused under the microscope at low magnification.
Secure the glass-covered wire to a slide and place it under the microscope. Move the slide with the stage knobs until the tip and tetraborate bead are in focus. Heat the bead until it melts slightly, and insert the wire tip ~10 - 15 µm. Turn the heating potentiometer off. As the bead cools down, it contracts and will remove the glass from the tip of the electrode, exposing the underlying tungsten. The tungsten electrode is ready to use.
2. Multibarrel Glass Pipette Manufacturing
Protect the ends of the multibarrel glass capillaries (five barrels in H-configuration) with heat-shrink tubing, to allow a better grip to the puller clamps and prevent breakage, and place one in the vertical puller.
To obtain tip lengths of ~15 mm, set the puller to the following parameters: Heat: 84, Sub magnet force: 49, Main magnet force: 38. Fine-tune these parameters according to the particular setup, as well as after changing the heating element.
Pull the capillary to obtain two pipettes with the tips occluded. Mount a pipette on a slide using modelling clay and break the pipette tip under the microscope until the outer diameter is ~20 - 30 µm, using fine scissors or forceps, or the flat side of a scalpel. If the broken edge is too rough, refine it using the tetraborate bead technique described in the step 1.7.
Place a tungsten electrode (from section 1) in a holder made with a 20 G needle at the end of a 3-axis miniature micropositioner mounted on the microscope stage. Using forceps, bend the end of the electrode 5 - 10°, ~30 mm from the tip.
Under the microscope, carefully align the tungsten electrode over the multibarrel tip. Once aligned, lower the electrode until it contacts the multibarrel, fitting in the groove between the two upper barrels. Slide the tip of the electrode until it protrudes ~15 - 20 µm away from the tip of the multibarrel. Make the angle between the electrode and the multibarrel as small as possible to obtain a better bonding and a thinner ensemble.
Apply a small drop of light-curable adhesive ~ 5 mm away from the tips. Be careful the glue does not reach the tip to avoid blocking the multibarrel channels.
Cure the glue using a blue light LED lamp. Repeat the glue application/curing if needed, for better bonding.
Move the microscope stage gently until the end of the tungsten electrode is released from its holder. Remove the piggy-back ensemble from the slide.
Wrap the middle section of the piggy-back electrode in a short length of heat-shrink tube and apply rapid curing epoxy appropriate for glass to further secure the tungsten electrode to the multibarrel.
3. Drugs for Microiontophoresis
Note: The drugs used for microiontophoresis must have an electrical charge when dissolved in water. Check the literature to see if the drug of interest is appropriate for this procedure. Here, the procedure for gabazine, an antagonist of the GABAA receptor, is described.
Filter distilled water using a 0.2 µm syringe filter to sterilize the water and secure containing no solutes. Prepare ~1,000 µl of 20 mM gabazine using this distilled water.
Adjust the pH of the dilution to 4 with 0.2 µm filtered NaOH using a fine-tip pH electrode.
Store 100 - 200 µl aliquots at -20 ºC. Defrost on the day of the recording. That day, before the animal is restrained, fill the barrels (3 - 5 µl) of the multibarrel electrode with the drugs, using a 100 µl microsyringe fitted with a flexible plastic needle.
4. Surgery and Headpost Implantation
Perform experiments in two male CBA/J mice (Mus musculus) with weights of 27 and 30 g. Follow surgical and recording procedures of Bryant and colleagues29, Portfors and collaborators30-32, and Duque and Malmierca26.
In the days prior to the surgery, handle the animals and habituate them to the recording chamber by allowing them to explore freely. Perform 3 sessions per day, each one lasting at least 30 min. This way the animals will be calmer and comfortable during the recordings.
Anesthetize the mouse using a mix of ketamine (50 mg/kg) and xylazine (10 mg/kg) injected intramuscularly. With this dose the animal is deeply anesthetized for around 1 hr. Give supplementary doses of one third of the initial dose as needed.
Place the animal on a heating blanket set to maintain a temperature of 38 ± 1 ºC and stabilize the animal's head in a stereotaxic frame by using two ear bars and a bite bar. Be careful not to pierce the tympanic membrane with the ear bars.
Protect the eyes by applying a drop of ophthalmic gel.
Shave the scalp using scissors and apply povidone-iodine to disinfect the skin.
Using a scalpel, make an incision along the midline to expose the skull and retract the periosteum covering the parietal and the more rostral part of the occipital bones.
Glue a lightweight duralumin headpost (weight ~0.65 g, length 30 mm, Figure 2A) with a round and flat base (5 mm diameter) on the skull. Glue a silver wire to the middle of the headpost leaving its ends free. The silver wire will be used as the reference electrode for the electrophysiological recording.
Apply a thin layer of light-curable adhesive on the skull and the base of the headpost. Wait 10 sec and place the headpost over the rostral area of the parietal bones, along the midline.
For an effective bonding strength, light cure the adhesive using a blue light source for 20 sec.
Drill three holes around and behind the base of the headpost using an electric drill and a small drill bit.
Place two watch screws (~2 mm length) in the drilled holes to serve as additional contact points between the skull and the headstage. The screws require 1½ turns to achieve a snug fit. Test the screws with forceps to make sure they are not loose.
Insert the tip of the silver ground wire into the third hole making sure it contacts the dura. Apply a small drop of water resistant cyanoacrylate glue to bind the wire to the skull.
Apply light-curing composite to reinforce the bond of the headpost with the skull. Cover the base of the headpost, the low part of the silver wire, and the screws, being careful not to extend behind lambda, to allow access during recording. Cure using a blue light source.
Retract the muscle at the back of the neck near its insertion on the skull. Using a small trephine (2.35 mm diameter), drill a round window just below lambda suture and lateral to the midline, to expose the inferior colliculus (IC). When the borders are loose, pull up the covering bone with fine tweezers. If bleeding occurs, rinse with cold sterile saline to stop it.
Make a small (~1 mm wide and ~1 mm high) wall around the window with composite and light-cure it.
Cover the exposed skull and muscle with antibiotic ointment. Then, wet the exposed surface of the IC with sterile saline and cover it with petroleum jelly, filling the well generated after making the wall around the recording window.
Inject buprenorphine subcutaneously (0.03 mg/kg, diluted 1:10 in sterile saline) as an analgesic.
Keep the animal on the heating blanket until it awakes and return it to its housing cage to recover from surgery for three days prior to recording sessions. House animals in individual rat´s housing cage to prevent cage mates cleaning out the jelly and the headpost touch the metal-grid cover. Place some enrichment in the cage when the animal is single housed. Also, change daily the sawdust to prevent infection and carefully check that the animal recovers properly and does not show any signs of discomfort. Also check if petroleum jelly is over the recording window protecting the exposed brain.
5. Electrophysiological Recording and Microiontophoresis
After recovery, give the animal time to acclimate to the recording environment and having its head restrained. Acclimate the animal by fixing the headpost to the holder while the mouse is sitting on a custom-made cushioned foam restrainer carved to its body size (Figure 2B). This will limit the movements of the mouse and will contribute to its calmness.
Start with 10 min sessions and progressively increase the duration up to 120 min. During the session, reward the animals with sweet liquids (condensed milk, diluted 30:70 in water) at given intervals33. Later, during the recording session, give the same reward at the start and at the end of the session or while no neuron is isolated.
If animal is very excited prior to the recording, inject the mild sedative acepromazine (2 mg/kg, intraperitoneal). According to the neural system of interest, the sedative can affect your results.
Let the animal freely explore the recording chamber for 5 - 10 min.
Place the animal's body into the foam custom-made cushioned restrainer (Figure 2B).
Secure the headpost to a holder attached to the stereotactic frame (Figure 2C). This is a good time to give the animal a liquid reward.
Cover the animal's body with a cotton blanket and loosely fasten it using a plastic hemicylinder and adhesive tape.
Remove the petroleum jelly from the recording window and rinse with warm sterile saline.
- Record neural activity and iontophoretic application of gabazine.
- Place the multibarrel electrode on its holder, controlled by a microdrive and attached to a micromanipulator.
- Connect the wires for recording, using small crocodile clips. Connect the positive terminal to the end of the tungsten electrode, and the ground terminal to the silver wire that contacts the dura.
- Place an uncoated silver wire inside each barrel, so one end contacts the solution and the other end is accessible. Connect those ends to the microiontophoresis device, following the instructions of the manufacturer.
- Move the multibarrel electrode until the tip contacts the surface of the brain. Apply warm sterile saline to prevent the desiccation of the brain tissue. At that point apply a retention current (-10 nA for gabazine, check the literature for the particular drug) to avoid leakage of the drug from the barrel tip while looking for single-unit activity.
- Use standard techniques for isolating single neuron extracellular activity. Obtain the frequency response area (FRA) of the neuron, i.e., the combination of frequencies and intensities capable of evoking a response because the neuron is sensitive to these sounds34-36.
- Choose two frequencies (f1 and f2) that evoke a similar firing rate and pattern. Present each of those frequencies as rare and repetitive stimulus under the oddball paradigm. Note: Briefly, in the oddball paradigm, one frequency (f1) is presented as the repetitive stimulus with a high probability of occurrence, while the second (f2) is the rarely occurring deviant stimulus, interspersed randomly among the repetitive ones. In a second oddball sequence, the relative probabilities of the two stimuli are reversed. Details of the stimulation paradigm have been described previously22,37.
- Release the gabazine by shifting the current to positive values (10 - 20 nA). Obtain again the FRA and repeat the oddball paradigm under the gabazine application. Maintain the ejection until a visible effect in the firing rate is observed; it usually takes 5 - 20 min, depending on the particular drug, its concentration, and the magnitude of the ejection current. Repeat the recording protocol to obtain the values under the effect of the drug.
- Stop the injection by returning the current to retention values. Wait for the effect of the drug to wash away by monitoring that the FRA or response to the oddball paradigm returns to control values. Note: The recording sessions can last 2 - 3 hr per day, and should be ended earlier if the animal shows any sign of discomfort or struggling. Give reward liquid and cover the recording chamber with petroleum jelly.
6. Data Analysis
Measure the FRA before and during the gabazine injection as well as the level of SSA.
Quantify the strength of SSA response by the common-SSA index (CSI) and the frequency-specific SSA index (SI) as described previously19,22,23,38,39. The CSI and SI reflect the normalized difference between the neuronal response to the rare stimulus and the response to the repetitive one. Positive CSI and SI values indicate the neural response (spikes per stimulus) was higher to rare than to repetitive tones.
Compare data from the control and drug ejection condition.
Representative Results
We recorded the single-unit activity of 4 well-isolated neurons of the IC. Typical signal-to-noise ratios obtained during extracellular recordings in awake mice are shown in Figure 3B. Figure 4A shows the frequency response area (FRA) of each neuron before and during the blockade of the GABAA-receptors with gabazine. An increment in the response strength (spikes/stimulus) as well as a broadening of the spectral tuning was observed. The increment in the evoked response was also evident in the accumulated peri-stimulus time histograms obtained from the neural response to all the frequencies and intensities presented (Figure 4B).
One or two pairs of frequencies within the neuron's FRA (crosses in Figure 4A) were chosen to be presented as repetitive or rare sounds in an oddball paradigm, to study the level of SSA in their responses. For two example neurons, the sound-evoked responses to each frequency (f1 and f2) before and during the gabazine injection are shown as dot rasters in Figure 5A,B. For the two example neurons, there was a clear change in the sound-evoked response as observed in the dot raster and PSTHs.
Likewise, the local blockade of the GABAA-receptors increased the neuronal response to the great majority of rare and repetitive tones (Figure 5C) in agreement with our previous study16. Different levels of SSA were observed in the neural responses to f1 and f2, which was reflected in positive CSI (CSI interval: -0.26 to 0.43, 0.25 ± 0.23) and SI values (SIs interval: -0.41 to 0.83, 0.25 ± 0.23). For most of the cases, the increased response to the repetitive tone caused a drop in those indices as observed in Figure 5D.
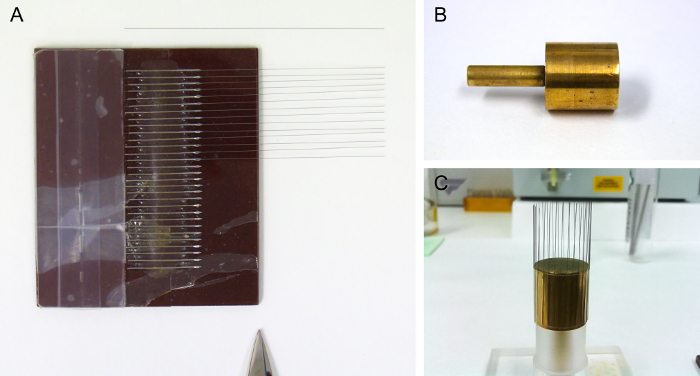 Figure 1. Material for the Manufacture of Tungsten Electrodes. (A) Electrode alignment tool, with some tungsten wires in place. The lighter piece on the left is a stop for the wires. The scissor tip indicates the side used as a guide for cutting the wires to the desired length. (B) Empty spindle. (C) Spindle with a batch of electrodes attached, and resting on a holder. Please click here to view a larger version of this figure.
Figure 1. Material for the Manufacture of Tungsten Electrodes. (A) Electrode alignment tool, with some tungsten wires in place. The lighter piece on the left is a stop for the wires. The scissor tip indicates the side used as a guide for cutting the wires to the desired length. (B) Empty spindle. (C) Spindle with a batch of electrodes attached, and resting on a holder. Please click here to view a larger version of this figure.
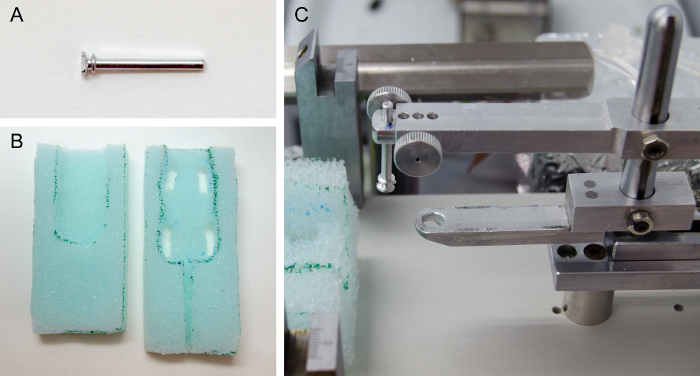 Figure 2. Accessories for Awake Mice Recordings. (A) Headpost. (B) Custom-made cushioned foam restrainer. Place the mouse in between both pieces, leaving the head outside. (C) Modifications to the stereotaxic frame. The headpost holder (top bar) assists during headpost implantation and restrains the head during the recordings. The bite piece (lower bar) is only used during the headpost implantation. Please click here to view a larger version of this figure.
Figure 2. Accessories for Awake Mice Recordings. (A) Headpost. (B) Custom-made cushioned foam restrainer. Place the mouse in between both pieces, leaving the head outside. (C) Modifications to the stereotaxic frame. The headpost holder (top bar) assists during headpost implantation and restrains the head during the recordings. The bite piece (lower bar) is only used during the headpost implantation. Please click here to view a larger version of this figure.
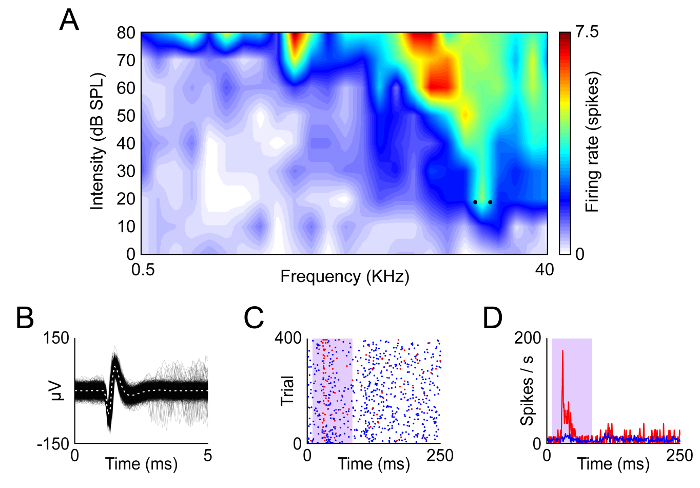 Figure 3. Example of Recording in the Awake Mouse. (A) Frequency response area of a neuron from the mouse IC. (B) Waveforms of the spikes recorded from this neuron. (C, D) Dot raster and peristumulus-time histogram recorded from this neuron using an oddball paradigm at the frequencies indicated by the dots in (A). Redrawn from data published in Duque and Malmierca26. Please click here to view a larger version of this figure.
Figure 3. Example of Recording in the Awake Mouse. (A) Frequency response area of a neuron from the mouse IC. (B) Waveforms of the spikes recorded from this neuron. (C, D) Dot raster and peristumulus-time histogram recorded from this neuron using an oddball paradigm at the frequencies indicated by the dots in (A). Redrawn from data published in Duque and Malmierca26. Please click here to view a larger version of this figure.
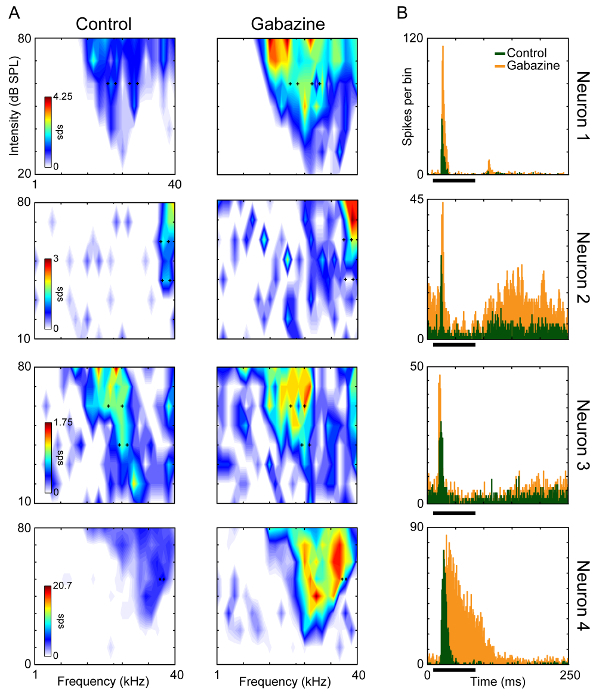 Figure 4. Effect of the Blockade of the GABAA-receptors on the Spectral and Temporal Response Properties. (A) Frequency response area of the four IC recorded neurons before and during the microiontophoretic injection of gabazine. The black crosses in (A) indicate the frequencies chosen to be presented as rare and repetitive sounds. (B) Accumulated peri-stimulus time histograms of the neural response to all the frequencies and intensities presented to construct the response area before and during the injection of gabazine. The black bars indicate the sound duration. Please click here to view a larger version of this figure.
Figure 4. Effect of the Blockade of the GABAA-receptors on the Spectral and Temporal Response Properties. (A) Frequency response area of the four IC recorded neurons before and during the microiontophoretic injection of gabazine. The black crosses in (A) indicate the frequencies chosen to be presented as rare and repetitive sounds. (B) Accumulated peri-stimulus time histograms of the neural response to all the frequencies and intensities presented to construct the response area before and during the injection of gabazine. The black bars indicate the sound duration. Please click here to view a larger version of this figure.
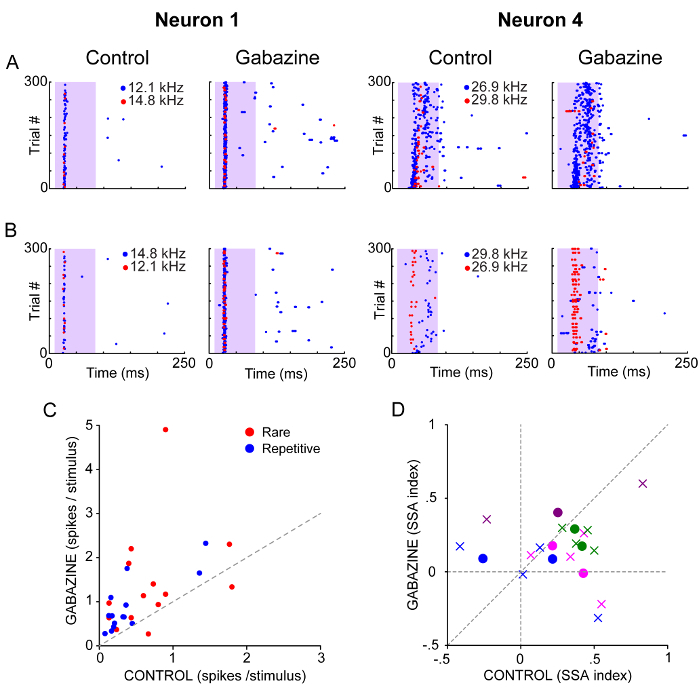 Figure 5. Effect of the Blockade of GABAA receptors on the Response to Repetitive and Rare Sounds. (A, B) Dot rasters of the spiking response to a pair of frequencies presented as rare (red, 10%) and repetitive stimulus (blue, 90%) before and during the application of gabazine. Each frequency is played in two sequences such that each one was presented as rare- and repetitive. The shaded background indicates the duration of the stimulus. (C). Single-neuron responses to seven pairs of frequencies presented as the rare and as the repetitive sound before and during the application of gabazine. (D) SSA indices (circles: CSI, crosses: SI) of the neural response to rare and repetitive sounds measured before and during the application of gabazine. The responses of the same neuron are indicated with the same color. Please click here to view a larger version of this figure.
Figure 5. Effect of the Blockade of GABAA receptors on the Response to Repetitive and Rare Sounds. (A, B) Dot rasters of the spiking response to a pair of frequencies presented as rare (red, 10%) and repetitive stimulus (blue, 90%) before and during the application of gabazine. Each frequency is played in two sequences such that each one was presented as rare- and repetitive. The shaded background indicates the duration of the stimulus. (C). Single-neuron responses to seven pairs of frequencies presented as the rare and as the repetitive sound before and during the application of gabazine. (D) SSA indices (circles: CSI, crosses: SI) of the neural response to rare and repetitive sounds measured before and during the application of gabazine. The responses of the same neuron are indicated with the same color. Please click here to view a larger version of this figure.
Discussion
The microiontophoresis of neuroactive substances in awake animals is a powerful technique to probe and dissect the role of specific synaptic inputs on the activity of single neurons40,41. More importantly, this procedure allows the determination of the impact of the neurotransmitters and neuromodulators on neural circuits without the potential interference of anesthetics. Here, we demonstrate that the application of gabazine in the IC of awake mice robustly changed the frequency tuning (Figure 4A), the temporal response patterns (Figure 4B) and the SSA (Figure 5).
The main limitation of the described procedure is the relatively short recording time (~3 hr), which is determined by the level of habituation of the animal to the recording sessions. On the other hand, multiple recording sessions can be performed on the same animal. Using microiontophoresis it is unclear how far the applied neuroactive substances diffuse into the surrounding tissue. Therefore, a possible effect on neuronal network activity can be elicited by affecting not only the recorded neuron but also the surrounding glial and neuronal cells. For example, Candy and colleagues42 have shown that certain iontophoretically delivered molecules, which are not rapidly removed, can diffuse up to 600 µm. In the mice IC this range would cover most of the extent of the dendritic arbors but also affect the neuronal response of adjacent cells. In summary, the drug released by iontophoresis is not as spatially restricted as intracellular drug dialysis, which allows the dissection in finer detail of the synaptic inputs to the target neuron and test of local network processes16,43, it is more specific than other experimental procedures used to manipulate the level of neurotransmission or neuromodulation, such as systemic injections, the use of optogenetic techniques, or push-pull dialysis.
The tip diameter of the glass pipettes and the magnitude of the retention and injection currents are key factors to monitor in order to avoid nonspecific drug leakage into the tissue. The low injection currents (of the order of tens of nA) limit the spread of the drug allowing recording of neurons close to one another. For example, three of the four neurons used as examples (neurons 1 - 3) were recorded along the same track with distances of 16 and 800 µm between them. Despite the short distance of 16 µm between neuron 1 and 2, a clearly different response of neuron 2 was observed (Figure 4).
Some alternatives to the proposed piggy-back configuration have been used previously. One of them consists of the use of one of the multibarrel pipettes for recording, instead of a separate electrode. While the construction of the ensembles is less complicated in this case, there are drawbacks. First, all the channels will have the same opening diameter at the tip, which may not be optimal for both recording and drug delivery. Also, the protruding electrode tip in the piggy-back configuration prevents damage to the target cell likely caused by the wider tip of the multibarrel. The second common alternative is the use of single-barrel glass pipettes for recording15,44 instead of tungsten electrodes. Glass electrodes are easy to manufacture to the required dimensions, but tend to clog during long recordings or when travelling deep into the brain, so in our experience tungsten electrodes provide more reliable recordings. Moreover, by using tungsten electrodes it is possible to produce electrolytic lesion to locate the recording sites, without the further elaborate histological procedures required when a neural tracer injection is used45. The use of multibarrel glass pipettes allows the release of multiple agonists and/or antagonists very close to the recording site, which is a tremendous advantage when the interaction between neurotransmitter systems on sensory processing is under study.
The procedures described in the present protocol for the manufacturing of the piggy-back electrodes make it possible to produce recording electrodes according to the specific requirements of the experiment and of the characteristics of the target area of interest in a systematic, yet customized manner. In addition, the materials for the headpost fixation are conventionally used in dentistry so they are readily available. Thus, the critical steps in the protocol are the animal habituation and the etching of the tungsten electrodes, which are the main factors contributing to well-isolated neurons and stable electrophysiological recordings. Overall, this technique is relatively simple, economical and very reliable as a means to study the role of multiple neuroactive substances on single or multi-unit neuronal activity in awake, head-restrained mice.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This project was funded by the MINECO grants BFU201343608-P and PSI2013-49348-EXP, and the JCYL grant SA343U14 to MSM and MRC core funding to ARP. YAA held a CONACyT (216106) and a SEP fellowship.
References
- Harris KD, Thiele A. Cortical state and attention. Nat Rev Neurosci. 2011;12(9):509–523. doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron. 2011;69(6):1061–1068. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers KK, Bennett DV, Hutt A, Williams JH, Frohlich F. Awake vs. anesthetized: layer-specific sensory processing in visual cortex and functional connectivity between cortical areas. J Neurophysiol. 2015;113(10):3798–3815. doi: 10.1152/jn.00923.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, Hausser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature. 2013;493(7430):97–100. doi: 10.1038/nature11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph M, Pospischil M, Timofeev I, Destexhe A. Inhibition determines membrane potential dynamics and controls action potential generation in awake and sleeping cat cortex. J Neurosci. 2007;27(20):5280–5290. doi: 10.1523/JNEUROSCI.4652-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buran BN, von Trapp G, Sanes DH. Behaviorally gated reduction of spontaneous discharge can improve detection thresholds in auditory cortex. J Neurosci. 2014;34(11):4076–4081. doi: 10.1523/JNEUROSCI.4825-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque D, Malmierca MS, Caspary DM. Modulation of stimulus-specific adaptation by GABA(A) receptor activation or blockade in the medial geniculate body of the anaesthetized rat. J Physiol. 2014;592:729–743. doi: 10.1113/jphysiol.2013.261941. (Pt 4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW. Microiontophoresis and Pressure Ejection. Wiley; 1985. [Google Scholar]
- Lalley PM. In: Modern techniques in neuroscience research) Windhorst U, Johansson H, editors. Springer; 1999. pp. 193–212. [Google Scholar]
- Foeller E, Celikel T, Feldman DE. Inhibitory sharpening of receptive fields contributes to whisker map plasticity in rat somatosensory cortex. J Neurophysiol. 2005;94(6):4387–4400. doi: 10.1152/jn.00553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foeller E, Vater M, Kossl M. Laminar analysis of inhibition in the gerbil primary auditory cortex. J Assoc Res Otolaryngol. 2001;2(3):279–296. doi: 10.1007/s101620010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt S, Crook JM, Ohl FW, Scheich H, Schulze H. Differential effects of iontophoretic in vivo application of the GABA(A)-antagonists bicuculline and gabazine in sensory cortex. Hear Res. 2006;212(1-2):224–235. doi: 10.1016/j.heares.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Sivaramakrishnan S, et al. GABA(A) synapses shape neuronal responses to sound intensity in the inferior colliculus. J Neurosci. 2004;24(21):5031–5043. doi: 10.1523/JNEUROSCI.0357-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havey DC, Caspary DM. A simple technique for constructing 'piggy-back' multibarrel microelectrodes. Electroencephalogr Clin Neurophysiol. 1980;48(2):249–251. doi: 10.1016/0013-4694(80)90313-2. [DOI] [PubMed] [Google Scholar]
- Dondzillo A, Thornton JL, Tollin DJ, Klug A. Manufacturing and using piggy-back multibarrel electrodes for in vivo pharmacological manipulations of neural responses. J Vis Exp. 2013. p. e4358. [DOI] [PMC free article] [PubMed]
- Perez-Gonzalez D, Hernandez O, Covey E, Malmierca MS. GABA(A)-Mediated Inhibition Modulates Stimulus-Specific Adaptation in the Inferior Colliculus. PLoS ONE. 2012;7(3):e34297. doi: 10.1371/journal.pone.0034297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock DC, Palmer AR, Rees A. Compact and easy-to-use tungsten-in-glass microelectrode manufacturing workstation. Med Biol Eng Comput. 1988;26(6):669–672. doi: 10.1007/BF02447511. [DOI] [PubMed] [Google Scholar]
- Sugiyama K, Dong WK, Chudler EH. A simplified method for manufacturing glass-insulated metal microelectrodes. J Neurosci Methods. 1994;53(1):73–80. doi: 10.1016/0165-0270(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, Nelken I. Processing of low-probability sounds by cortical neurons. Nat Neurosci. 2003;6(4):391–398. doi: 10.1038/nn1032. [DOI] [PubMed] [Google Scholar]
- Escera C, Malmierca MS. The auditory novelty system: An attempt to integrate human and animal research. Psychophysiology. 2014;51(2):111–123. doi: 10.1111/psyp.12156. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Sanchez-Vives MV, Escera C, Bendixen A. Neuronal adaptation, novelty detection and regularity encoding in audition. Front Syst Neurosci. 2014;8:111. doi: 10.3389/fnsys.2014.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS, Cristaudo S, Perez-Gonzalez D, Covey E. Stimulus-specific adaptation in the inferior colliculus of the anesthetized rat. J Neurosci. 2009;29(17):5483–5493. doi: 10.1523/JNEUROSCI.4153-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes FM, Nelken I, Covey E, Malmierca MS. Stimulus-specific adaptation in the auditory thalamus of the anesthetized rat. PLoS ONE. 2010;5(11):14071. doi: 10.1371/journal.pone.0014071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Behrens W, Bauerle P, Kossl M, Gaese BH. Correlating stimulus-specific adaptation of cortical neurons and local field potentials in the awake rat. J Neurosci. 2009;29(44):13837–13849. doi: 10.1523/JNEUROSCI.3475-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gonzalez D, Malmierca MS. Variability of the time course of stimulus-specific adaptation in the inferior colliculus. Front Neural Circuits. 2012;6:107. doi: 10.3389/fncir.2012.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque D, Malmierca MS. Stimulus-specific adaptation in the inferior colliculus of the mouse: anesthesia and spontaneous activity effects. Brain Struct Funct. 2014. [DOI] [PubMed]
- Merrill EG, Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Ainsworth A, Dostrovsky JO, Merrill EG, Millar J. An improved method for insulating tungsten micro-electrodes with glass [proceedings] J Physiol. 1977;269(1):4–5. [PubMed] [Google Scholar]
- Bryant JL, Roy S, Heck DH. A technique for stereotaxic recordings of neuronal activity in awake, head-restrained mice. J Neurosci Methods. 2009;178(1):75–79. doi: 10.1016/j.jneumeth.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV, Roberts PD, Jonson K. Over-representation of species-specific vocalizations in the awake mouse inferior colliculus. Neuroscience. 2009;162(2):486–500. doi: 10.1016/j.neuroscience.2009.04.056. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Mayko ZM, Jonson K, Cha GF, Roberts PD. Spatial organization of receptive fields in the auditory midbrain of awake mouse. Neuroscience. 2011;193:429–439. doi: 10.1016/j.neuroscience.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Muniak MA, Mayko ZM, Ryugo DK, Portfors CV. Preparation of an awake mouse for recording neural responses and injecting tracers. J Vis Exp. 2012. [DOI] [PMC free article] [PubMed]
- Deacon RM. Housing, husbandry and handling of rodents for behavioral experiments. Nat Protoc. 2006;1(2):936–946. doi: 10.1038/nprot.2006.120. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, et al. A discontinuous tonotopic organization in the inferior colliculus of the rat. J Neurosci. 2008;28(18):4767–4776. doi: 10.1523/JNEUROSCI.0238-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo MA, Gutierrez-Conde PM, Merchan MA, Malmierca MS. Non-plastic reorganization of frequency coding in the inferior colliculus of the rat following noise-induced hearing loss. Neuroscience. 2008;154(1):355–369. doi: 10.1016/j.neuroscience.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Palmer AR, Shackleton TM, Sumner CJ, Zobay O, Rees A. Classification of frequency response areas in the inferior colliculus reveals continua not discrete classes. J Physiol. 2013;591(16):4003–4025. doi: 10.1113/jphysiol.2013.255943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala YA, Malmierca MS. Stimulus-specific adaptation and deviance detection in the inferior colliculus. Front Neural Circuits. 2013;6:89. doi: 10.3389/fncir.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque D, Perez-Gonzalez D, Ayala YA, Palmer AR, Malmierca MS. Topographic distribution, frequency, and intensity dependence of stimulus-specific adaptation in the inferior colliculus of the rat. J Neurosci. 2012;32(49):17762–17774. doi: 10.1523/JNEUROSCI.3190-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala YA, Perez-Gonzalez D, Duque D, Nelken I, Malmierca MS. Frequency discrimination and stimulus deviance in the inferior colliculus and cochlear nucleus. Front Neural Circuits. 2013;6:119. doi: 10.3389/fncir.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins MN, Stone TW. In vivo release of [3H]-purines by quinolinic acid and related compounds. Br J Pharmacol. 1983;80(2):263–267. doi: 10.1111/j.1476-5381.1983.tb10029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM. In: Modern Techniques in Neuroscience Research. Windhorst U, Johansson H, editors. Berlin Heiderlberg: Springer; 1999. pp. 193–212. Ch. 7. [Google Scholar]
- Candy JM, Boakes RJ, Key BJ, Worton E. Correlation of the release of amines and antagonists with their effects. Neuropharmacology. 1974;13(6):423–430. doi: 10.1016/0028-3908(74)90130-0. [DOI] [PubMed] [Google Scholar]
- Martins AR, Froemke RC. Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat Neurosci. 2015. [DOI] [PMC free article] [PubMed]
- LeBeau FE, Rees A, Malmierca MS. Contribution of GABA- and glycine-mediated inhibition to the monaural temporal response properties of neurons in the inferior colliculus. Journal of Neurophysiology. 1996;75(2):902–919. doi: 10.1152/jn.1996.75.2.902. [DOI] [PubMed] [Google Scholar]
- Ayala YA, Malmierca MS. Cholinergic modulation of stimulus-specific adaptation in the inferior colliculus. The Journal of Neuroscience. 2015;35(35):12261–12272. doi: 10.1523/JNEUROSCI.0909-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


