Abstract
While the isolation and culture of vascular smooth muscle cells (VSMCs) from large vessels is well established, we sought to isolate and culture VSMCs from the coronary circulation. Hearts with intact aortic arches were removed and perfused via retrograde Langendorff with digestion solution containing 300 Units/ml of collagenase type II, 0.1 mg/ml soybean trypsin inhibitor and 1 M CaCl2. The perfusates were collected at 15 min intervals for 90 min, pelleted by centrifugation, resuspended in plating media, and plated on tissue culture dishes. VSMCs were characterized by presence of SM22α, α-SMA, and vimentin. One of the main advantages of using this technique is the ability to isolate VSMCs from the coronary circulation of mice. Although the small number of cells obtained can limit some of the applications for which the cells can be utilized, isolated coronary VSMCs can be used in a variety of well-established cell culture techniques and assays. Studies investigating VSMCs from genetically modified mice can provide further information about structure-function and signaling processes associated with vascular pathologies.
Keywords: Basic Protocol, Issue 111, coronary circulation, vascular, smooth muscle cell, isolation, culture, mouse
Introduction
The goal of this method is to isolate vascular smooth muscle cells (VSMCs) from the murine coronary circulation for use in cell culture and standard cell culture assays. We developed this technique to assess the molecular mechanisms of vascular remodeling in diabetes. We have previously reported inward hypertrophic remodeling in the septal coronary arterioles in the db/db mouse model of diabetes1. Due to the limited amount of tissue found in the murine septal coronaries, standard experimental techniques investigating protein changes (e.g. western blot) in db/db and control mice are difficult at best. In addition, we have previously shown that the angiotensin receptor blocker (ARB) losartan reduces the remodeling observed in db/db mice2. Therefore, isolation of primary VSMCs from the coronary circulation allows us to further investigate changes in VSMC phenotype or activated signaling pathways in diabetic mice, which may be contributing to adverse coronary arteriole remodeling.
Numerous studies have elucidated canonical signaling pathways using VSMCs isolated from rodent aorta, rather than in each specific vascular bed. However, we have demonstrated vascular-bed specific remodeling in coronary, aortic, and mesenteric circulations of db/db mice1, suggesting the VSMCs in each vascular bed may be different. Therefore, it is necessary to isolate VSMCs from each vascular bed in order to better understand the pathological changes occurring in each set of VSMCs. There are a plethora of different methods for isolating and culturing aortic VSMCs. However, currently, there is only one study that has been published on the isolation of VSMCs from the mouse coronary circulation3. Teng et al. was the first to report a method for isolating VSMCs from the mouse coronary circulation; however, we have amended the protocol significantly as they also isolated endothelial cells. Other labs have also used the protocol from Teng et al. to isolate coronary arterial myocytes and airway smooth muscle cells4,5. The alterations we have incorporated will yield a population of cells highly enriched for VSMCs from the coronary circulation.
The retrograde perfusion of the isolated mammalian heart, or Langendorff technique, was established in 18976 by Oscar Langendorff and is still widely used today for the isolation of cardiovascular cells. The technique presented here, coupled with the advancement of modern murine genetic modifications, provides a valuable tool for closer investigation of the molecular behavior of VSMCs from the coronary circulation.
Protocol
Ethics Statement: This study was conducted in accordance with the National Institutes of Health Guidelines, and it was approved by the Institution Animal Care and Use Committee at Nationwide Children's Hospital.
1. Preparation/Set up
Note: This isolation technique requires two Langendorff heating coils positioned side-by-side on a ring stand, and connected in parallel to a circulating water bath.
- Turn on circulating water bath and adjust temperature to achieve a final temperature of 33-34 °C at the tip of the Langendorff cannula (the water bath used in this set up is set to 40 °C due to a 6-7 °C loss in temperature across tubing). Turn on the perfusion pumps and set perfusion rate to 0.5 ml/min.
- Clean both of the water-jacketed heating coils, attached tubing and 25 gauge cannula by flushing each with 50 ml of 70% ethanol, followed by 100 ml of autoclaved ultrapure water.
- Clear the heating coils by pushing air with a syringe and wash with 10 ml of Hank's Balanced Salt Solution (HBSS) without phenol red. Leave HBSS in the heating coils and tubing.
- Attach a sterile 10 ml luer-lock syringe filled with room temperature HBSS without phenol red to the tubing. Ensure that no air bubbles are trapped inside the tubing and heating coils when attaching the HBSS-filled syringe as this could cause air emboli during the digestion to negatively impact the VSMC isolation.
Remove the 25 gauge cannula from the heating coils and place on another sterile 10 ml syringe filled with ice-cold HBSS without phenol red. Keep this on ice until ready to use. Repeat for the other cannula.
- Prepare Enzyme Digestion Solution.
- Weigh collagenase type 2 according to specific lot number for 75 ml of digestion solution to achieve a final concentration of 300 Units/ml.
- Dissolve collagenase in 75 ml of HBSS with phenol red. Next, add 1.5 ml of 0.1 mg/ml soybean trypsin inhibitor and 75 µl of 1 M CaCl2 to the mixture.
If using the digestion solution immediately, warm it to 37 °C. If not, place it at 4 °C for up to 6 hr. This solution must be made fresh daily.
- Prepare the stop solution/plating media.
- Add 100 ml heat-inactivated FBS, 5 ml of L-glutamine, 5 ml of non-essential amino acids, 5 ml of HEPES, and 1 ml of primocin to 500 ml of high glucose (4.5 g/L) DMEM. Filter sterilization is recommended.
- Aliquot 6 ml of the stop solution into each of twelve 15 ml conical tubes, numbered A1-A6 and B1-B6, and place at 4 °C. Aliquot 50 ml of stop solution into a 50 ml conical and place in 37 °C incubator. Note: This step can also be prepared the day prior to isolation if necessary. This stop solution will be used as the plating media following cell isolation.
Prepare two 1 ml syringes equipped with a 30 G needle with 100 µl of heparin (1,000 units/ml).
Cut a 5 cm piece of 5-0 surgical silk. Tie a loose overhand knot in the surgical silk with one end twice as long as the other. Place onto the 25-gauge cannula, but do not tighten. Repeat for the other 25 G cannula.
Fill four 35 mm sterile Petri dishes (#1-4) with 2 ml of sterile, ice-cold, HBSS without phenol red. Keep on ice.
2. Euthanasia, Heart Isolation and Cannulation
Before heart isolation, ensure that the digestion solution and 50 ml conical of stop solution are warm. Remove the HBSS-filled syringe with attached 25 G cannula from the ice and place in a burette clamp (or ring stand clamp) on a ring stand.
Anesthetize mice using 3% isofluorane. Confirm the mouse is anesthetized by a toe touch on each hind limb. If the leg twitches, the mouse is not yet anesthetized so wait 1 min and repeat the toe touch.
Expose the jugular vein by removing the fur and skin on the anterior of the neck with scissors. Next, use forceps to carefully remove any fat surrounding the jugular vein. After removing fat, slowly inject 100 µl of heparin (1,000 units/ml). Place a surgical sponge over the vein to avoid excessive bleeding.
After 1 min, open chest to expose heart, lift heart with curved forceps and excise it using scissors, making sure to keep the ascending aorta intact through the first brachiocephalic branch. Immediately place heart into a 35 mm dish (#1) with ice-cold HBSS without phenol red and rinse. Note: Perform the following 4 steps using a dissecting scope.
Position the 25 G cannula that is mounted on the ring stand at a 45° angle so that the tip is located just under the surface of ice-cold HBSS in dish #2 and is clearly visible through the dissecting microscope. Transfer the heart to dish #2.
Using Dumont forceps, expose the aorta by gently removing excess adipose via blunt dissection to prevent puncturing or tearing of the aorta. Next, use forceps to slide the aorta over the 25 G cannula.
Guide the cannula downward through the aorta, close to, but not beyond the aortic valve, which will compromise the integrity of the valve and therefore, the retrograde perfusion. Once the cannula is in place, slide the pre-tied silk over the aorta, and tighten. Tie a second knot to form a square knot around the aorta to secure the heart.
Once the aorta is securely tied, gently flush the remaining blood from the coronary circulation with the ice-cold HBSS in the syringe attached to the 25 G cannula. Take care to not push too much to avoid excessive coronary pressure. Only a very slow and gentle flush is required to complete the flush. Leak-free aortic cannulation can also be observed during this gentle flush.
Turn on the perfusion pump to begin the flow of HBSS. Remove the 25 G cannula (with the heart attached) from the HBSS-filled syringe, taking care to keep the cannula hub filled with HBSS, and connect it to the warm HBSS-filled heating coils. Begin perfusion of the heart with the perfusion pump at a rate of 0.5 ml/min for 8 min.
After the first heart is cannulated and is being perfused on the system, repeat steps 2.1-2.8 for the second heart. These isolations will be staggered for each heart therefore careful planning and timing are required.
3. Digestion
After flushing with HBSS, change the 10 ml HBSS syringe currently on the pump to one filled with 10 ml of pre-warmed digestion solution. Perfuse each heart with digestion solution for 12 min at a rate of 0.5 ml/min. Do not collect fractions stemming from this first digestion as this will contain any remnant blood and endothelial cells.
After 12 min, change the perfusion rate to 0.4 ml/min, place a 15 ml conical (tube A1 or B1, corresponding to the first or second heart for each digestion) filled with cold stop solution into an ice bucket beneath the heart in order to begin VSMC-enriched perfusate collection.
After 15 min, change the collection conical to tube A2 or B2 and spin conical tube A1 and B1 at 89 x g for 10 min immediately after collection of both fractions. When the syringe filled with the digestion solution on the pump has less than 1 ml of solution, replace with a freshly-filled 10 ml syringe of digestion solution.
After centrifugation, aspirate supernatant from each collection tube, leaving approximately 0.5 ml. Resuspend pellet from A1 with 2 mL of warm stop solution (or plating media). Add resuspension from A1 tube to B1 tube and place tube in 37 °C incubator with the cap loose.
Repeat steps 3.3 and 3.4 until all the tubes (through A6 and B6) are used for a total collection time of 90 min. At 80 min of digestion, cut open the heart apex to flush out any cells that may be trapped inside the ventricle and collect these cells in tube A6 and B6, respectively. Note: Occasionally the heart will fall off the cannula prior to the 90 min time point. If this occurs, retrieve the heart and place into a sterile 35 mm Petri dish filled with 2 ml of digestion solution. Cut the apex of the heart and gently rinse the heart with digestion solution. Next, filter the 2 ml of digestion solution through a sterile 100 µm cell strainer into a 50 ml conical and add to tube A6 or B6.
After each spin of subsequent tubes (A2 and B2), add the resuspended VSMCs to the previous collection tube (combined A1 and B1). Also, take care not to let the digestion solution in the pump run out and switch the 10 ml syringes as necessary throughout the digestion.
After all the tubes are combined, centrifuge the VSMC-rich suspension at 89 x g for 10 min.
Aspirate as much supernatant from collection tubes as possible. Resuspend the remaining pellet in 2 ml of plating media and plate on one 35 mm culture dish. Place culture dish in 37 °C incubator for 24 hr.
4. Cell Culture
Note: Complete the remaining steps in the biosafety cabinet.
After 24 hr, gently aspirate the plating media. The culture will normally have a large amount of debris. To wash the cells, add 2 ml of warm, sterile PBS and gently tap the dish against two fingers while rotating in a clockwise motion.
After rotating 360°, aspirate the PBS and repeat a second time. Aspirate the PBS and add another 2 ml of sterile, warm PBS. Check the culture dish under the microscope to make sure there is little to no debris on the plate. Cell confluence can also be determined at this time.
If debris remains in the culture dish, repeat step 4.2. If the culture dish is clear of debris, aspirate the PBS and add 2 ml of warm plating media.
After 24 hr, wash the cells again with warm, sterile PBS to remove any final debris or dead cells from the culture. Aspirate the PBS and place another 2 ml of warm plating media on the cells.
Check on the cells every day and feed every 48 hr with fresh warmed plating media. At this point, cells can be used for standard cell culture assays. VSMCs should be approximately 80% confluent 4-5 days after plating, although may take up to 7 days until the procedure is perfected. If splitting to subsequent passages, use a maximum of 1:4 35 mm dishes (or to a comparable area of culture dishes).
Representative Results
Due to the novel aspect of our coronary VSMC isolation technique, we sought to determine the purity of the cell isolation. Mouse coronary VSMCs were identified based on their morphology and immunofluorescence staining up to passage 2. Based on morphology of the cells in culture after the first wash, the isolation procedure effectively removes cardiac myocytes and endothelial cells. The VSMCs retain their morphology up to passage 2 (Figure 1). However, there is a possibility of contamination by adventitial and interstitial fibroblasts. To rule out this potential confound, we stained isolated cells for SM22α, α-smooth muscle actin (α-SMA), both VSMC markers7,8, and vimentin, a fibroblast marker9, at passages 1-2 (Figure 2). We observed no change of these markers through passage 2. We also show that our cell isolation is devoid of CD31/PECAM staining (human coronary microvascular endothelial cells used as a positive control), indicating the lack of endothelial cell contamination using this isolation protocol. Collectively, our cell isolation procedure yields a nearly pure population of VSMCs from the coronary circulation and can now be used for further experimentation.
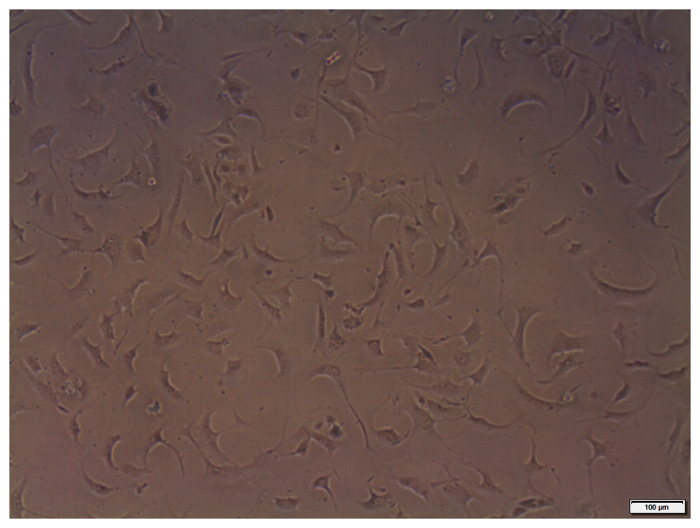 Figure 1: Representative cultured coronary VSMCs. Cultured coronary VSMCs (passage 2) isolated from heterozygous Db/db mice imaged at 10X magnification under bright field phase contrast conditions. Scale bars = 100 µm. Please click here to view a larger version of this figure.
Figure 1: Representative cultured coronary VSMCs. Cultured coronary VSMCs (passage 2) isolated from heterozygous Db/db mice imaged at 10X magnification under bright field phase contrast conditions. Scale bars = 100 µm. Please click here to view a larger version of this figure.
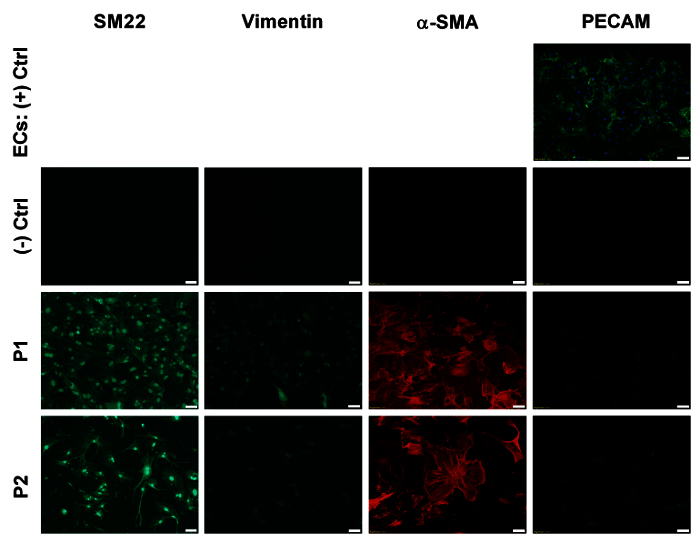 Figure 2: Immunofluorescence staining of cultured coronary VSMCs. Plated coronary VSMCs from heterozygous (Db/db) mice at passages 1-2 labeled with antibodies for SM22α, α-SMA, vimentin, and CD31/PECAM. Images taken at 10X magnification. No significant change was observed with passage. Scale bars = 100 µm. Please click here to view a larger version of this figure.
Figure 2: Immunofluorescence staining of cultured coronary VSMCs. Plated coronary VSMCs from heterozygous (Db/db) mice at passages 1-2 labeled with antibodies for SM22α, α-SMA, vimentin, and CD31/PECAM. Images taken at 10X magnification. No significant change was observed with passage. Scale bars = 100 µm. Please click here to view a larger version of this figure.
Discussion
The purpose of this study was to adapt existing cell isolation protocols to increase the yield of coronary vascular smooth from murine hearts. Most of the pioneering work in vascular smooth muscle biology was performed with cultured rat aortic smooth muscle cells. These studies provided fundamental knowledge of molecular mechanisms that control VSMC growth, migration and hypertrophy7. However, as the field progressed, it became apparent that VSMC phenotype and function was controlled by a number of vascular bed specific factors. For example, compared to peripheral vessels, coronary arteries display different functional responses, exhibit distinct patterns of growth in responses to injury and display a unique array of channels, receptors and signaling molecules10,11. The coronary bed is also exposed to unique hemodynamic forces, since the vessels are compressed during systole by the contracting myocardium. Our recent work suggests that coronary microvascular remodeling in type 2 diabetic db/db mice differed from aortic and mesenteric resistance vessels1,12. To understand molecular mechanisms that account for these differences, in vitro studies using low passage VSMCs are necessary. Although there are a variety of different and well-established methods for isolating and culturing murine aortic or mesenteric artery VSMCs, currently, there is only one study that has been published on the isolation of VSMCs from the mouse coronary circulation3.
In this protocol, we demonstrate an improved method for isolation of primary murine VSMCs from the coronary circulation. Our protocol is loosely based on one published by Teng et al.3. The modifications we have applied to the original technique provide an enriched population and higher yield of VSMCs. The critical steps necessary to achieve these results include correct placement of the cannula, gentle flushing of the heart to remove excess blood, and cutting the apex of the heart at the end of digestion. Of the three, placement of the cannula is of the utmost importance. The coronary circulation is perfused from the coronary ostia just distal to the aortic valve. Therefore disruption of valve integrity via cannula insertion into the left ventricular chamber will lead to poor coronary perfusion and a dramatic reduction in the number of viable VSMCs. Gentle flushing of the coronary circulation will not only remove excess blood from the final pellet used for culture, but will also determine whether correct placement of the cannula is achieved. Finally, during digestion, some VSMCs may become trapped inside of the ventricles, thus cutting the apex at the conclusion of digestion will release the cells and yield a greater confluence at plating.
An important modification that can be utilized is the use of a 25 G gavage needle or a Harvard Apparatus mouse aorta cannula in place of the needle cannula. However, when using the gavage needle, one must take extra care not to block the coronary ostia with the ball at the end of the needle. The gentle flushing step will reveal if adjustment of the needle is necessary prior to perfusion. In addition, the type of collagenase used in the digestion solution is important for optimal VSMC isolation. Type II collagenase is not pure – it contains other enzymes such as trypsin, clostripain, and caseinase. Therefore, the amount of soybean trypsin inhibitor added to the digestion solution may need to be adjusted based on the specific lot of collagenase utilized in digestion.
One limitation of the technique is that VSMCs from the entire coronary circulation are isolated. Our previous studies focused on the coronary microvessels (80-120 μm in diameter)1,2, however the main coronary arteries in mice are larger (>160 μm)13. In addition, this technique also isolates cells from the coronary venous circulation, not just the arterial circulation.
While the Langendorff technique has been used for over 100 years, the more recent advancements of mouse genome manipulation (e.g. knock-in, knock-out, mutations) provide a unique opportunity to utilize our updated technique for a wide variety of applications. After isolation and culture, the cells can be used for additional experiments including viral infection and treatment with pharmacological agents. The joint usage of genetically modified mice and standard cell culture will allow for deeper investigation of VSMCs from the murine coronary circulation.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This work was supported by the National Institutes of Health (R01HL056046 to PAL and K99HL116769 to AJT), and The Research Institute at Nationwide Children's Hospital (to PAL and AJT).
References
- Katz PS, et al. Coronary arterioles in type 2 diabetic (db/db) mice undergo a distinct pattern of remodeling associated with decreased vessel stiffness. Basic Res Cardiol. 2011;106(6):1123–1134. doi: 10.1007/s00395-011-0201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husarek KE, et al. The angiotensin receptor blocker losartan reduces coronary arteriole remodeling in type 2 diabetic mice. Vascul Pharmacol. 2015. [DOI] [PMC free article] [PubMed]
- Teng B, Ansari HR, Oldenburg PJ, Schnermann J, Mustafa SJ. Isolation and characterization of coronary endothelial and smooth muscle cells from A1 adenosine receptor-knockout mice. Am J Physiol Heart Circ Physiol. 2006;290(4):H1713–H1720. doi: 10.1152/ajpheart.00826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg PJ, Wyatt TA, Sisson JH. Ethanol attenuates contraction of primary cultured rat airway smooth muscle cells. Am J Respir Cell Mol Biol. 2010;43(5):539–545. doi: 10.1165/rcmb.2009-0252OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, et al. Intracellular two-phase Ca2+ release and apoptosis controlled by TRP-ML1 channel activity in coronary arterial myocytes. Am J Physiol Cell Physiol. 2013;304(5):C458–C466. doi: 10.1152/ajpcell.00342.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypiec-Spring M, Grotthus B, Szelag A, Schulz R. Isolated heart perfusion according to Langendorff---still viable in the new millennium. J Pharmacol Toxicol Methods. 2007;55(2):113–126. doi: 10.1016/j.vascn.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Li L, Miano JM, Cserjesi P, Olson EN. SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res. 1996;78(2):188–195. doi: 10.1161/01.res.78.2.188. [DOI] [PubMed] [Google Scholar]
- Santiago JJ, et al. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn. 2010;239(6):1573–1584. doi: 10.1002/dvdy.22280. [DOI] [PubMed] [Google Scholar]
- Fisher SA. Vascular smooth muscle phenotypic diversity and function. Physiol Genomics. 2010;42A(3):169–187. doi: 10.1152/physiolgenomics.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SL. Diversity of phenotype and function of vascular smooth muscle cells. J Lab Clin Med. 1996;127(6):524–529. doi: 10.1016/s0022-2143(96)90142-0. [DOI] [PubMed] [Google Scholar]
- Souza-Smith FM, et al. Mesenteric resistance arteries in type 2 diabetic db/db mice undergo outward remodeling. PLoS One. 2011;6(8):e23337. doi: 10.1371/journal.pone.0023337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuroff JW, Hort W, Lichti H. Diameter of coronary arteries in 36 species of mammalian from mouse to giraffe. Basic Res Cardiol. 1984;79(2):199–206. doi: 10.1007/BF01908306. [DOI] [PubMed] [Google Scholar]


