Abstract
In reconstructive surgery, there is a clinical need for an alternative to the current methods of autologous reconstruction which are complex, costly and trade one defect for another. Tissue engineering holds the promise to address this increasing demand. However, most tissue engineering strategies fail to generate stable and functional tissue substitutes because of poor vascularization. This paper focuses on an in vivo tissue engineering chamber model of intrinsic vascularization where a perfused artery and a vein either as an arteriovenous loop or a flow-through pedicle configuration is directed inside a protected hollow chamber. In this chamber-based system angiogenic sprouting occurs from the arteriovenous vessels and this system attracts ischemic and inflammatory driven endogenous cell migration which gradually fills the chamber space with fibro-vascular tissue. Exogenous cell/matrix implantation at the time of chamber construction enhances cell survival and determines specificity of the engineered tissues which develop. Our studies have shown that this chamber model can successfully generate different tissues such as fat, cardiac muscle, liver and others. However, modifications and refinements are required to ensure target tissue formation is consistent and reproducible. This article describes a standardized protocol for the fabrication of two different vascularized tissue engineering chamber models in vivo.
Keywords: Bioengineering, Issue 111, Tissue engineering, vascular pedicle, arteriovenous loop, vascularization, microsurgery, femoral vessel, angiogenesis, capillaries
Introduction
Fabricating functional vascularized tissue using a tissue engineering approach is an emerging paradigm in regenerative medicine.1,2 Many approaches to engineer new and healthy tissue for the replacement of injured tissue or defective organs have been developed,3-6 experimentally in small animal models with promising clinical potential.7,8 However, vascularization remains one of the great challenges for tissue engineering limiting its potential to grow tissues of clinically relevant size.9
Current approaches to vascularize tissue follow either an extrinsic pathway where new vessels grow from the recipient vascular bed and invade throughout the implanted tissue constructs10 or an intrinsic vascularization pathway where the vasculature grows and expands in unison with the newly developing tissue.11 The extrinsic approach traditionally involves seeding cells onto a scaffold in vitro and implanting the complete construct into the living animal with the expectation that nutrients, previously supplied by culture media, will be sourced from the circulation.12,13 The concept is simplistic as vascular ingrowth is too slow and only very thin implants (<1-2 mm thick) will remain viable. Providing nutrients and oxygen by means of a sufficient and rapid vascularization is at the heart of any successful attempts to grow more complex and larger tissue-engineered substitutes such as bone, muscle, fat and solid organs.14,15 Intrinsic vascularization offers the potential for larger constructs to develop by progressive tissue growth commensurate with its expanding blood supply. One design is the in vivo implantation into a chamber of a vascular pedicle with or without a cell seeded scaffold.5,6 This has paved the way to new procedures for the generation of thicker intrinsically vascularized tissues.16,17
More recently, strategies have been developed to pre-vascularize tissue grafts, prior to implantation. These incorporated blood vessel networks are aimed to inosculate with host vessels at implantation allowing for the rapid provision of a complete blood supply to improve the survival of all parts of a transplanted thick tissue graft.18
We pioneered an in vivo vascularized tissue engineering model in small animals that involves a subcutaneously implanted semi-rigid enclosed chamber containing a perfused vascular pedicle and cell-containing biomaterials. The chamber creates an ischemic environment that stimulates angiogenic sprouting from the implanted vessels.3 The vascular pedicle can either be a reconstructed arteriovenous loop or an intact flow-through artery and vein.3-6,19 This vascular pedicle sprouts a functioning and extensive arterio-capillary-venous network that links at both arteriole and venous ends with the vascular pedicle.3,20 Furthermore, the surrounding hollow support chamber protects the developing tissue from potentially deforming mechanical forces and prolongs the ischaemic drive to enhance vascularization.3,21,22 If the vessel pedicle is simply implanted into normal tissue and not inside the protected space of the chamber, angiogenic sprouting ceases along the same timeline as a normal wound and no new tissue will accumulate around the pedicle. Investigators have used this in vivo configuration to produce three-dimensional functional vascularized tissue constructs with supportive vasculature and of clinically relevant size.4,23 Furthermore, the engineered vascularized tissue constructs with its intact vascular pedicle can be harvested for subsequent transplantation at the injury site.24,25 A more clinically feasible scenario would be creating the chamber at the definitive site for reconstruction such as the breast. Thus, this de novo tissue engineering approach could have clinical potential to provide a new source of functional target tissue for reconstructive surgery.26-28
The following protocol will provide a general guide to construct an in vivo vascularized tissue engineering chamber in the rat, which could be adapted in different animal models and employed to examine the intricate processes of angiogenesis, matrix production, and cellular migration and differentiation.
Protocol
The protocols described here have been approved by the Animal Ethics Committee of St. Vincent's Hospital Melbourne, Australia, and were conducted under strict adherence to the Australian National Health and Medical Research Council Guidelines.
NOTE: Two chamber protocols are described below. The two different models and their specific chamber designs are illustrated in Figure 1. Chamber (1) is made of polycarbonate (for rat arteriovenous loop chamber model). It is cylindrical with an internal diameter 13 mm and height 4 mm. A window at one point in the wall allows unimpeded access for the pedicle. In the second model (for rat flow-through pedicle chamber model), the chamber is made of polyacrylic and is rectangular (10 x 8 x 4 mm3 internal dimensions). It has two 1.5 mm openings on opposite sides to accommodate the femoral artery and vein as they transgress the chamber.
1. Rat Arteriovenous Loop Chamber Model (One Chamber Per Animal)
NOTE: Prior to starting surgery, make sure all the instruments have been properly sterilized. Likewise, ensure the instruments rest on sterile towels and are at a reasonable distance from the surgical field to avoid contamination during the procedure.
- Preparation of the Animal for Surgery
- Use rats weighing at least 250 g for their large size of vessels for creation of the arteriovenous loop.
- Anaesthetize the animal with 4% isoflurane inhalation. Corroborate adequate depth of anesthesia by assessing unresponsiveness to toe-pinch. After anesthesia, keep the animal adequately anesthetized throughout the procedure with 2% isoflurane.
- Place the animal in a supine position on a warming pad and apply sterile lubricant to the eyes to prevent desiccation during surgery.
- Using an electric razor, shave both groins and remove hair with a piece of moist gauze.
- Prep the surgical sites with chlorhexidine/70% ethanol solution and drape the animal with sterile towels. Administer a single dose of Carprofen (5 mg/kg, subcutaneously) as analgesic.
- Harvest of Femoral Vein Graft
- Using a #15 blade, make a 4 cm long skin incision on the left groin parallel to the inguinal ligament. This exposes the inguinal fat pad.
- Cut through the fat pad circumferentially with scissors leaving it attached to its vascular pedicle based on the epigastric vessels.
- Using micro scissors, free the filmy connective tissue adhesions between the abdominal wall and underlying femoral vessels.
- Place a retractor on the abdominal wall and pull medially. This exposes the inguinal ligament and the whole length of the femoral vessels.
- Using micro forceps and curved scissors dissect the epigastric vein and isolate it from its surrounding fat by gently pulling and cutting. This vein acts as a tether when constructing the loop.
- Using micro forceps and curved micro scissors open the perivascular sheath containing the femoral vessels and nerve all the way from the inguinal ligament to its bifurcation distal to the epigastric branch.
- Using micro forceps, pick up the femoral vein by its adventitia and gently separate it from the surrounding tissues and accompanying artery. Do this with micro forceps and curved round-pointed micro scissors by pulling the tissue apart and cutting through it. NOTE: Never grab the whole thickness of the vein wall as this might cause trauma to the intima making it prone to thrombosis.
- Ligate side branches found during the dissection with 10/0 nylon suture or coagulate them with a bipolar coagulator.
- With the femoral vein completely free, ligate its proximal and distal ends with 4/0 silk sutures. Make sure to obtain a vein graft of at least 10 mm length and include approximately 0.5 cm length of the epigastric branch to be used as a guy rope tether to hold the loop open in the chamber.
- Using micro forceps and straight micro scissors, trim the adventitia from the graft's ends by gently pulling and cutting. This can also be done later, before microsurgical anastomoses.
- Flush the vein graft with heparinized saline solution (10 U/ml of heparin) and leave it to rest in the solution. Close the wound using continuous running 4/0 silk suture plus two or three additional simple interrupted stitches.
- Creation of Arteriovenous Loop and Implantation of Chamber
- Repeat steps 1.2.1 to 1.2.4 in the exact same way on the contralateral limb.
- Using micro forceps, dissect and isolate both the epigastric artery and vein form the surrounding fat pad. Do this by gently pulling the tissue away from the vessels
- Using micro forceps, pick up the femoral artery by its adventitia and free it from the surrounding tissues. Do this with micro forceps and curved round-pointed micro scissors by pulling the tissue apart and cutting through it. Ligate or coagulate its side branches.
- Ligate the femoral artery and vein distal to the emergence of the epigastric vessels using 4/0 silk suture.
- Place a single clamp proximally on each of the femoral artery and vein. Using a sharp straight micro scissor, make a clean transverse cut in each vessel distal to the emergence of the epigastric branches. Place a sterile plastic contrast background under the vessels.
- Flush the vessels vigorously with generous amounts of heparinized saline until all the blood is removed from the lumen.
- Bring the vein graft into the operative field and remove any redundant adventitia from the vessels' ends as per step 1.2.10, if needed.
- Perform both microsurgical anastomoses with 10/0 nylon suture. Anastomose the proximal end of the vein graft to the femoral vein and the distal end to the femoral artery. This will allow the blood to flow from the arterial to the venous side without resistance from valves inside the vein graft. NOTE: Make sure the femoral vessels and the vein graft rest in their natural position without any twists.
- Check for leaks at both anastomotic sites. Resolve small leaks, which look like non-pulsating blood coming out of the anastomotic site, by placing a small piece of fat on top and gently compressing for 5-10 min. Larger pulsating leaks that rapidly flood the entire field will need additional stitches.
- Check patency of the arteriovenous loop. Gentle occlusion of the femoral artery should make it shrink while the same in the femoral vein should engorge it.
- Place the base of the tissue engineering chamber under the arteriovenous loop with the latter resting in its natural position without twists or kinks.
- Secure the base of the chamber to the inguinal ligament and underlying muscle fascia with 6/0 nylon sutures.
- Place the lid over the base so that the femoral vessels enter the chamber through a notch (window in the side of the chamber). When closing the lid, make sure it catches the epigastric branches, between the chamber base and lid, which act as tethers to hold the arteriovenous loop into position.
- Close the wound using continuous running 4/0 silk suture plus two or three additional simple interrupted stitches.
- Allow the animal to recover from anesthesia on a warming pad.
- Do not leave the animal unattended until it has regained sufficient consciousness to maintain sternal recumbency. Likewise, do not return an animal that has undergone surgery to the company of other animals until fully recovered. 24 hr later, administer another single dose of Carprofen (5 mg/kg, subcutaneously) as analgesic.
- Treat the wound with topical antibiotic ointment for 5 days. If the wound is opened, anesthetize the animal as in step 1.1.2 through 1.1.5 and close the wound as in 1.3.14. Monitor the health of animal daily. Euthanize the animal using a lethal dose of intraperitoneal lethabarb injection (163 mg/kg in 0.25 ml by 23 G needle) if the animal shows more than one moderate signs of inacitivity, poor appetite, weight loss and loss of color.
2. Flow-through Pedicle Chamber (Two Chambers per Animal)
- Preparation of the Animal for Surgery
- Repeat steps 1.1.1 through 1.1.4. Two chambers can be implanted into both groin regions of a single rat.
- Isolation of Femoral Vessels and Insertion of the Chamber
- Repeat steps 1.2.1 through 1.2.8.
- With both artery and vein completely freed of surrounding tissues and their branches ligated, bring the chamber into the operative field.
- Place each of the intact femoral vessels on the corresponding slit of the chamber base making sure there are no twists or kinks.
- Close the chamber by attaching the lid to the base. Close the wound using continuous running 4/0 silk suture plus two or three additional simple interrupted stitches and allow the animal to recover as previously described.
3. Harvest of Chambers and Tissue Processing
Once the experiment's time points (4-6 weeks post-implantation) are reached, anesthetize the animal as in step 1.1.2 and repeat steps 1.1.3 through 1.1.5.
Open the wound using a #15 blade and cut through the tissues with scissors until the chamber is completely exposed.
Expose the femoral vessels proximal to the construct and test for vascular patency: gently occlude the vessel with two microforceps, then milk the blood in a distal direction and finally release the proximal forceps. If the vessel fills with blood again, this confirms patency. Ligate the femoral vessels proximally in the case of the arteriovenous loop and both proximally and distally in the case of the flow-through configuration, and remove the chambers with the containing tissue en bloc.
At the end of the experiment, euthanize the animal using a lethal dose of intraperitoneal lethobarb injection (163 mg/kg in 0.25 ml by 23 G needle).
Fix tissues in 4% paraformaldehyde at room temperature for 24 hr. Divide tissues into multiple transverse sections (1-2 mm thick) and embed in paraffin wax or optimal cutting temperature compound for paraffin sections (5 µm) or frozen sections (10 µm), respectively.3,4,8,17,22,24
Perform routine histological staining such as hematoxylin and eosin to examine the general morphology of tissues. Perform immunohistochemical staining with specific antibody to identify cell type of interest,3,4,8,17,22,24,29 for example cardiac troponin T immunostaining for cardiomyocytes.
Representative Results
The microsurgical creation of tissue engineering chambers was performed as described in the protocol above. Tissues generated inside the chambers can be examined histologically as describe in protocol step 3. Various tissue types have been successfully engineered using the in vivo vascularized chamber (Figure 2). These include cardiac tissue with neonatal rat cardiomyocytes (Figure 2A), muscle tissue with rat skeletal myoblasts (Figure 2B), and adipose tissue with a hydrogel derived from adipose tissue extracellular matrix (Figure 2C).
Morphometric evaluation of the tissues can be performed with either a stereo investigator system or ImageJ software.3,4,8,17,22,29 In addition to qualitative assessment of various tissue components, the stereology system also allows for unbiased quantification of specific tissue volume. For example, lectin (a marker for rodent endothelial cells) stained transverse sections (Figure 3) can be used to estimate the vascular volume of the harvested tissue constructs using video microscopy with stereology system. Similar quantification methods can be applied to assess the volume of other tissue types.
The tissue engineering chambers can also be employed to track cell fate following in vivo implantation. Cells can be pre-labelled with fluorescent dyes such as DiI, PKH26 or quantum dots before implantation. For example, neonatal rat cardiomyocytes pre-labelled with DiI can be detected in the tissue constructs harvested at 3 day post-implantation (Figure 4A). We have also successfully tracked implanted cells that have been pre-labelled with DiI for up to 4 weeks post-implantation. Alternatively, species-specific antibodies can be used to identify implanted cells in xenotransplantation studies. For example, human induced pluripotent stem cells implanted inside tissue engineering chambers in immunocompromized rats can be identified in the harvested tissue constructs by immunostaining with human-specific Ku80 antibody (Figure 4B).
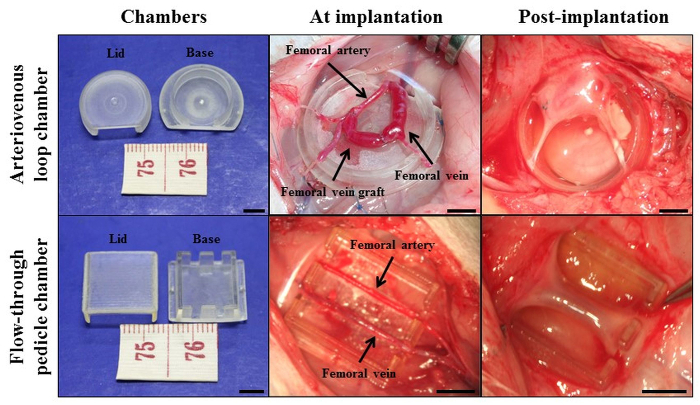 Figure 1: Cardiac tissue engineering with the in vivo vascularized chamber. Intrinsic vascularization approach with arteriovenous loop chamber model and flow-through pedicle chamber model. The chambers were made from either polycarbonate or polyacrylic, these materials were tested to be non-inflammatory and non-toxic in vivo. Scale bar = 5 mm. Re-printed with permission from30. Please click here to view a larger version of this figure.
Figure 1: Cardiac tissue engineering with the in vivo vascularized chamber. Intrinsic vascularization approach with arteriovenous loop chamber model and flow-through pedicle chamber model. The chambers were made from either polycarbonate or polyacrylic, these materials were tested to be non-inflammatory and non-toxic in vivo. Scale bar = 5 mm. Re-printed with permission from30. Please click here to view a larger version of this figure.
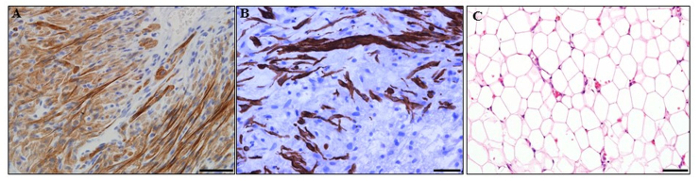 Figure 2: Tissues engineered from the in vivo vascularized chambers. (A) Cardiac tissue with neonatal rat cardiomyocytes. Cardiomyocytes were immunostained with cardiac troponin T antibody (brown). (B) Muscle tissue with rat skeletal myoblasts. Muscle cells were immunostained with desmin antibody (brown). (C) Fat tissue with a hydrogel derived from adipose tissue extracellular matrix.31 Haematoxylin and eosin staining. Scale bar = 50 µm. Please click here to view a larger version of this figure.
Figure 2: Tissues engineered from the in vivo vascularized chambers. (A) Cardiac tissue with neonatal rat cardiomyocytes. Cardiomyocytes were immunostained with cardiac troponin T antibody (brown). (B) Muscle tissue with rat skeletal myoblasts. Muscle cells were immunostained with desmin antibody (brown). (C) Fat tissue with a hydrogel derived from adipose tissue extracellular matrix.31 Haematoxylin and eosin staining. Scale bar = 50 µm. Please click here to view a larger version of this figure.
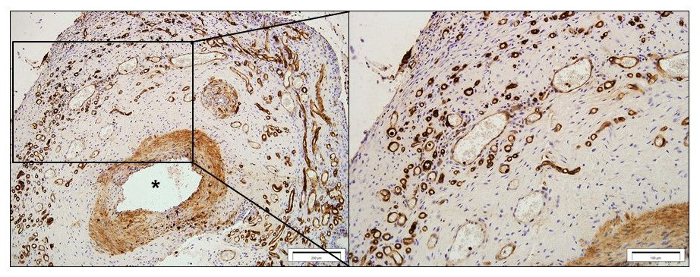 Figure 3: Vascularity of a tissue constructs harvested at 4 weeks post-implantation. Representative images of lectin-stained sections. The blood vessels sprouting from the femoral artery (*) were labelled with lectin (brown). Scale bar = 200 µm (left) and 100 µm (right). Please click here to view a larger version of this figure.
Figure 3: Vascularity of a tissue constructs harvested at 4 weeks post-implantation. Representative images of lectin-stained sections. The blood vessels sprouting from the femoral artery (*) were labelled with lectin (brown). Scale bar = 200 µm (left) and 100 µm (right). Please click here to view a larger version of this figure.
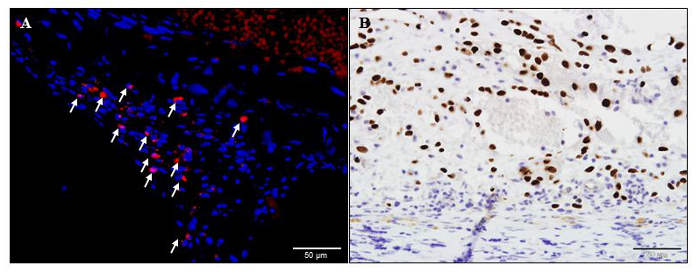 Figure 4: Identification of transplanted cells in the tissue constructs. (A) DiI-label neonatal rat cardiomyocytes (red and white arrows) in a rat tissue construct harvested at 3 days post-implantation. (B) A representative human-specific Ku80 stained histology image of a tissue construct harvested from a rat tissue engineering chamber implanted with human induced pluripotent stem cells at 28 days post-implantation. Human nuclei were immuno-labelled brown and immunocompromized rat was used to prevent rejection of human cells. Scale bar = 50 µm. Please click here to view a larger version of this figure.
Figure 4: Identification of transplanted cells in the tissue constructs. (A) DiI-label neonatal rat cardiomyocytes (red and white arrows) in a rat tissue construct harvested at 3 days post-implantation. (B) A representative human-specific Ku80 stained histology image of a tissue construct harvested from a rat tissue engineering chamber implanted with human induced pluripotent stem cells at 28 days post-implantation. Human nuclei were immuno-labelled brown and immunocompromized rat was used to prevent rejection of human cells. Scale bar = 50 µm. Please click here to view a larger version of this figure.
Discussion
Engineering of the microcirculation is currently being investigated essentially through two approaches. The first involves developing a highly interconnected vascular network within the construct in vitro so that when implanted, capillaries from the host vascular bed connect with those of the transplanted construct through a process called inosculation, thus ensuring the delivery of nutrients not only to the periphery but also to the core.21,32,33 This is called pre-vascularization. The second approach attempts to enhance the host's own vasculature directly in vivo, so that capillary sprouting occurs either before or concomitantly with implanted cell differentiation and tissue growth.17,34
The in vivo tissue engineering chamber protocol presented here exploits the latter concept by placing an artery and a vein, either as an arteriovenous loop or a flow-through pedicle configuration, inside a protected empty space, allowing significant sprouting and formation of new capillaries over time.3 Advantages of the chamber include (1) the absence of in vitro manipulations; (2) the generation of a completely autologous vascular network which will not be rejected by the host; and (3) the fact that it does not need an inosculation period which can take between 1 to 7 days rendering the tissue construct prone to ischemia.35,36
Nevertheless, it must be noted that it takes around 3-7 days for significant capillary sprouting to occur, a period during which the implanted tissue is also poorly supplied.3 Delayed cell implantation once the chamber is adequately vascularized has in fact shown improved survival.17 A further advantage includes the biocompatibility and non-immunogenicity of the chambers' material (i.e. polycarbonate and polyacrylic). In addition, the rigid non-collapsible chamber provides a protected space for the tissue and vasculature to grow without merging and integrating with the surrounding environment, which would otherwise hamper expansion and make harvest of the newly formed tissue difficult. Contrastingly, the fact that chambers are closed may limit tissue growth. This, however, has been addressed in the arteriovenous loop model, which now uses a perforated chamber that has been shown to grow tissue more effectively than the closed one.37
The tissue-engineering chamber protocols presented here are highly reproducible but it should be stressed that the chambers rarely fill to completion, usually to about 70% capacity. Consistent results are achieved provided that some critical steps and technical issues are taken into consideration. Pedicle patency is the ultimate aim when working with blood vessels, particularly if microsurgical anastomoses are performed. We have consistently found no tissue grows if the vascular pedicle thromboses early post-surgery. Factors affecting patency can be broadly grouped into four categories, namely surgical technical, blood flow, thrombosis and spasm.
First, a delicate surgical technique is the key to success. In this sense, it should be noted that these procedures, especially the one involving the creation of an arteriovenous loop require a certain level of surgical skill which can be readily acquired by practicing first in non-living models and subsequently in the rat's femoral vessels following the principles and techniques described here and elsewhere.38 Damage to the vessel wall, especially the intima, must be avoided at all times by proper handling of the vessels, which includes never grabbing the full thickness of the vessel wall, preventing excessive stretching, judicious use of the bipolar coagulator, and in the case of the arteriovenous loop, performance of meticulous and atraumatic microsurgical anastomoses. Although flushing with heparinized saline helps prevent clotting, it will never replace a fine surgical technique. Second, blood flow factors relate mainly to turbulence and stasis. Turbulent flow secondary to twists, bends or kinks of vessels promotes thrombus formation. Therefore a streamline unrestricted flow must be ensured in both the arteriovenous loop and the flow-through models. In this sense, the tethering effect of the epigastric branches in the arteriovenous loop model is essential to prevent bending; if for any reason these branches cannot be used, simple 10/0 nylon stitches from the vessel wall to the surrounding tissues should be carefully placed instead. Static blood at the anastomotic site during the arteriovenous loop procedure is also highly thrombogenic and must be prevented by flushing the vessel vigorously with heparinized saline prior to and throughout the anastomosis. Thirdly, pro-thrombotic factors such as contaminants from the operative field and most importantly the presence of pieces of adventitia inside the anastomosis are to be avoided. Preparing the vessel ends correctly prior to microsurgical suturing and keeping the field and anastomosis clean by flushing regularly are important aspects to consider when constructing the arteriovenous loop. Last but not least, there is the issue of vessel spasm. Even though the pathophysiology of vessel spasm has not been completely elucidated, it is likely to be due to both local and systemic factors. Local factors include vessel trauma, presence of blood in the operative field and tissue desiccation. Systemic factors, on the other hand, comprise low core temperature, hypotension and sympathetic response to pain.38
Thus, in both arteriovenous loop and flow-through models, proper handling and preparation of the animal together with a delicate surgical technique cannot be overemphasized. Strategies to resolve spasm include irrigation with warm saline or undiluted 1% xylocaine and having a rest period of 5-10 min. Vessel dilation and stripping of the adventitia can also help to relieve spasm due to its local sympathectomy effect.38 To circumvent the need to perform microsurgical anastomoses and the technical challenge it implies, the cuff technique can be employed as described elsewhere.39 This technique consists of inserting one of the vessel ends into a cuff, evert it and secure it with a circumferential 6/0 nylon suture. Next, the other vessel end is slid over the cuff and secured in a similar way.
The tissue-engineering chamber has opened a new window of possibilities in experimental research and is steadily advancing towards a potential clinical purpose. Until now, the models presented here have been used mainly for the generation of tissues of different lineages.4,7,8,17,22,,24,25,27-29,37,40 Nevertheless, they do have a number of other potential applications. For example, we have used the flow-through chamber as an effective and comparatively fast model for teratoma formation after implantation of human induced pluripotent stem cells.41,42 Thus, this approach may be used as a "quality control" tool to achieve tumor-free tissue engineering with pluripotent stem cells. Also, drug toxicology studies could be explored in human tissues grown inside the chamber. Likewise, generation of pathologic tissue could yield an interesting approach towards disease modeling and drug testing. Finally, the tissue-engineering chamber might also become a potential model to study growth of tissue and tracking of cell fate in vivo.
In conclusion, we have described a protocol involving the placement of a subcutaneous chamber in animals through two different approaches: using a microsurgical arteriovenous loop, or a flow-through pedicle configuration. The technique is highly reproducible and yields consistent results. Its use has been exploited mainly in the field of tissue engineering so far, however, there are other potential research fields for which these chambers might be of great application.
Disclosures
The authors declare no competing interests.
Acknowledgments
This work was supported by grant funding from NHMRC and Stafford Fox Medical Foundation. The authors acknowledge the surgical assistance of Sue McKay, Liliana Pepe, Anna Deftereos and Amanda Rixon of the Experimental Medical and Surgical Unit, St. Vincent's Hospital, Melbourne. Support is also provided by the Victorian State Government's Department of Innovation, Industry and Regional Development's Operational Infrastructure Support Program.
References
- Spiliopoulos K, et al. Current status of mechanical circulatory support: A systematic review. Cardiol Res Pract. 2012. p. 574198. [DOI] [PMC free article] [PubMed]
- Hsu PL, Parker J, Egger C, Autschbach R, Schmitz-Rode T, Steinseifer U. Mechanical circulatory support for right heart failure: Current technology and future outlook. Artif Organs. 2012;36(4):332–347. doi: 10.1111/j.1525-1594.2011.01366.x. [DOI] [PubMed] [Google Scholar]
- Lokmic Z, Stillaert F, Morrison WA, Thompson EW, Mitchell GM. An arteriovenous loop in a protected space generates a permanent, highly vascular, tissue-engineered construct. FASEB J. 2007;21(2):511–522. doi: 10.1096/fj.06-6614com. [DOI] [PubMed] [Google Scholar]
- Morritt AN, et al. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115(3):353–360. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Tsutsumi A, Crowe DM, Tajima S, Morrison WA. Generation of an autologous tissue (matrix) flap by combining an arteriovenous shunt loop with artificial skin in rats: preliminary report. B J Plast Surg. 2000;53(1):51–57. doi: 10.1054/bjps.1999.3186. [DOI] [PubMed] [Google Scholar]
- Cronin KJ, et al. New murine model of spontaneous autologous tissue engineering, combining an arteriovenous pedicle with matrix materials. Plast Reconstr Surg. 2004;113(1):260–269. doi: 10.1097/01.PRS.0000095942.71618.9D. [DOI] [PubMed] [Google Scholar]
- Forster NA, et al. A prevascularized tissue engineering chamber supports growth and function of islets and progenitor cells in diabetic mice. Islets. 2011;3(5):271–283. doi: 10.4161/isl.3.5.15942. [DOI] [PubMed] [Google Scholar]
- Choi YS, Matsuda K, Dusting GJ, Morrison WA, Dilley RJ. Engineering cardiac tissue in vivo from human adipose-derived stem cells. Biomaterials. 2010;31(8):2236–2242. doi: 10.1016/j.biomaterials.2009.11.097. [DOI] [PubMed] [Google Scholar]
- Jeyaraj R, G N, Kirby G, Rajadas J, Mosahebi A, Seifalian AM, Tan A. Vascularisation in regenerative therapeutics and surgery. Mater Sci Eng C Mater Biol Appl. 2015;54:225–238. doi: 10.1016/j.msec.2015.05.045. [DOI] [PubMed] [Google Scholar]
- Dew L, Macneil S, Chong CK. Vascularization strategies for tissue engineers. Regen Med. 2015;10(2):211–224. doi: 10.2217/rme.14.83. [DOI] [PubMed] [Google Scholar]
- Weigand A, et al. Acceleration of vascularized bone tissue-engineered constructs in a large animal model combining intrinsic and extrinsic vascularization. Tissue Eng Part A. 2015;21(9-10):1680–1694. doi: 10.1089/ten.TEA.2014.0568. [DOI] [PubMed] [Google Scholar]
- Vacanti JP, Langer R, Upton J, Marler JJ. Transplantation of cells in matrices for tissue regeneration. Adv Drug Deliv Rev. 1998;33(1-2):165–182. doi: 10.1016/s0169-409x(98)00025-8. [DOI] [PubMed] [Google Scholar]
- Beahm EK, Walton RL, Patrick CW. Progress in adipose tissue construct development. Clin Plast Surg. 2003;30(4):547–558. doi: 10.1016/s0094-1298(03)00072-5. [DOI] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, et al. Challenges in cardiac tissue engineering. Tissue Eng Part B Rev. 2010;16(2):169–187. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JR, Garcia AJ. Biomaterial-mediated strategies targeting vascularization for bone repair. Drug Deliv Transl Res. 2015. [DOI] [PMC free article] [PubMed]
- Forster N, et al. Expansion and hepatocytic differentiation of liver progenitor cells in vivo using a vascularized tissue engineering chamber in mice. Tissue Eng Part C Methods. 2011;17(3):359–366. doi: 10.1089/ten.TEC.2009.0519. [DOI] [PubMed] [Google Scholar]
- Tilkorn DJ, et al. Implanted myoblast survival is dependent on the degree of vascularization in a novel delayed implantation/prevascularization tissue engineering model. Tissue Eng Part A. 2010;16(1):165–178. doi: 10.1089/ten.TEA.2009.0075. [DOI] [PubMed] [Google Scholar]
- Chang Q, Lu F. A novel strategy for creating a large amount of engineered fat tissue with an axial vascular pedicle and a prefabricated scaffold. Med hypotheses. 2012;79(2):267–270. doi: 10.1016/j.mehy.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Walton RL, Beahm EK, Wu L. De novo adipose formation in a vascularized engineered construct. Microsurg. 2004;24(5):378–384. doi: 10.1002/micr.20056. [DOI] [PubMed] [Google Scholar]
- Debels H, Gerrand YW, Poon CJ, Abberton KM, Morrison WA, Mitchell GM. An adipogenic gel for surgical reconstruction of the subcutaneous fat layer in a rat model. J Tissue Eng Regen Med. 2015. [DOI] [PubMed]
- Lokmic Z, Mitchell GM. Engineering the microcirculation. Tissue Eng Part B Rev. 2008;14(1):87–103. doi: 10.1089/teb.2007.0299. [DOI] [PubMed] [Google Scholar]
- Yap KK, et al. Enhanced liver progenitor cell survival and differentiation in vivo by spheroid implantation in a vascularized tissue engineering chamber. Biomaterials. 2013;34(16):3992–4001. doi: 10.1016/j.biomaterials.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Findlay MW, et al. Tissue-engineered breast reconstruction: Bridging the gap toward large-volume tissue engineering in humans. Plast Reconstr Surg. 2011;128(6):1206–1215. doi: 10.1097/PRS.0b013e318230c5b2. [DOI] [PubMed] [Google Scholar]
- Tee R, Morrison WA, Dusting GJ, Liu GS, Choi YS, Hsiao ST, Dilley RJ. Transplantation of engineered cardiac muscle flaps in syngeneic rats. Tissue Eng Part A. 2012;18(19-20):1992–1999. doi: 10.1089/ten.tea.2012.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolderer JH, et al. Long-term stability of adipose tissue generated from a vascularized pedicled fat flap inside a chamber. Plast Reconstr Surg. 2011;127(6):2283–2292. doi: 10.1097/PRS.0b013e3182131c3e. [DOI] [PubMed] [Google Scholar]
- Sekine H, et al. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118:145–152. doi: 10.1161/CIRCULATIONAHA.107.757286. (14 Suppl) [DOI] [PubMed] [Google Scholar]
- Ting AC, et al. The adipogenic potential of various extracellular matrices under the influence of an angiogenic growth factor combination in a mouse tissue engineering chamber. Acta Biomater. 2014;10(5):1907–1918. doi: 10.1016/j.actbio.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Zhan W, et al. Self-synthesized extracellular matrix contributes to mature adipose tissue regeneration in a tissue engineering chamber. Wound Repair Regen. 2015;23(3):443–452. doi: 10.1111/wrr.12292. [DOI] [PubMed] [Google Scholar]
- Messina A, Bortolotto SK, Cassell OC, Kelly J, Abberton KM, Morrison WA. Generation of a vascularized organoid using skeletal muscle as the inductive source. FASEB J. 2005;19(11):1570–1572. doi: 10.1096/fj.04-3241fje. [DOI] [PubMed] [Google Scholar]
- Lim SY, Hernández D, Dusting GJ. Growing vascularized heart tissue from stem cells. J Cardiovasc Pharmacol. 2013;62(2):122–129. doi: 10.1097/FJC.0b013e31829372fc. [DOI] [PubMed] [Google Scholar]
- Poon CJ, et al. Preparation of an adipogenic hydrogel from subcutaneous adipose tissue. Acta Biomater. 2013;9(3):5609–5620. doi: 10.1016/j.actbio.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Dilley RJ, Morrison WA. Vascularisation to improve translational potential of tissue engineering systems for cardiac repair. Int J Biochem Cell Biol. 2014;56:38–46. doi: 10.1016/j.biocel.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Lesman A, Koffler J, Atlas R, Blinder YJ, Kam Z, Levenberg S. Engineering vessel-like networks within multicellular fibrin-based constructs. Biomaterials. 2011;32(31):7856–7869. doi: 10.1016/j.biomaterials.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Hussey AJ, et al. Seeding of pancreatic islets into prevascularized tissue engineering chambers. Tissue Eng Part A. 2009;15(12):3823–3833. doi: 10.1089/ten.TEA.2008.0682. [DOI] [PubMed] [Google Scholar]
- Chen X, Aledia AS, Popson SA, Him L, Hughes CC, George SC. Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng Part A. 2010;16(2):585–594. doi: 10.1089/ten.tea.2009.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RZ, Melero-Martin JM. Fibroblast growth factor-2 facilitates rapid anastomosis formation between bioengineered human vascular networks and living vasculature. Methods. 2012;56(3):440–451. doi: 10.1016/j.ymeth.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolderer JH, et al. Spontaneous large volume adipose tissue generation from a vascularized pedicled fat flap inside a chamber space. Tissue Eng. 2007;13(4):673–681. doi: 10.1089/ten.2006.0212. [DOI] [PubMed] [Google Scholar]
- Wei FC, Lin Tay SK. Principles and techniques of microvascular surgery. In: Neligan PC, Gurtner GC, editors. Plastic Surgery. Volume 1. Elsevier; 2013. pp. 587–620. Chapter 26. [Google Scholar]
- Sekine H, et al. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat.Comm. 2013;4(1399):1–10. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Sivakumaran P, Crombie DE, Dusting GJ, Pébay A, Dilley RJ. Trichostatin A enhances differentiation of human induced pluripotent stem cells to cardiogenic cells for cardiac tissue engineering. Stem Cells Transl Med. 2013;2(9):715–725. doi: 10.5966/sctm.2012-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, et al. In vivo tissue engineering chamber supports human induced pluripotent stem cell survival and rapid differentiation. Biochem Biophys Res Commun. 2012;422(1):75–79. doi: 10.1016/j.bbrc.2012.04.108. [DOI] [PubMed] [Google Scholar]
- Piao Y, Hung SS, Lim SY, Wong RC, Ko MS. Efficient generation of integration-free human induced pluripotent stem cells from keratinocytes by simple transfection of episomal vectors. Stem Cells Transl Med. 2014;3(7):787–791. doi: 10.5966/sctm.2013-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]


