Abstract
Fatigue is a prominent symptom in many diseases and disorders and reduces quality of life for many people. The lack of clear pathogenesis and failure of current interventions to adequately treat fatigue in all patients leaves a need for new treatment options. Despite the therapeutic need and importance of preclinical research in helping identify promising novel treatments, few preclinical assays of fatigue are available. Moreover, the most common preclinical assay used to assess fatigue-like behavior, voluntary wheel running, is not suitable for use with some strains of mice, may not be sensitive to drugs that reduce fatigue, and has relatively low throughput. The current protocol describes a novel, non-voluntary preclinical assay of fatigue-like behavior, the treadmill fatigue test, and provides evidence of its efficacy in detecting fatigue-like behavior in mice treated with a chemotherapy drug known to cause fatigue in humans and fatigue-like behavior in animals. This assay may be a beneficial alternative to wheel running, as fatigue-like behavior and potential interventions can be assessed in a greater number of mice over a shorter time frame, thus permitting faster discovery of new therapeutic options.
Keywords: Behavior, Issue 111, Mouse, Treadmill, Fatigue, Chemotherapy, Cancer-related fatigue, Chemotherapy-induced fatigue
Introduction
Fatigue affects a wide range of people, can markedly reduce quality of life, and frequently has an unclear or unknown pathogenesis. Cancer-related fatigue (CRF), for example, is experienced by the majority of cancer patients undergoing treatment and can persist long after cancer treatment has been completed and in the absence of detectable cancer1. Moreover, fatigue is also a prominent symptom in numerous other diseases and disorders, including chronic fatigue syndrome, depression, diabetes, and fibromyalgia. Fortunately, there are non-pharmacological interventions that are capable of helping some people experiencing fatigue (e.g., exercise can reduce CRF for some breast cancer patients2,3), but many individuals still lack effective treatment. Furthermore, existing drug treatments for CRF have not been found to be broadly, if at all, efficacious4-7.
Despite the therapeutic need and lack of drug treatment options, preclinical assays of fatigue to aid in the discovery and development of novel fatigue treatments are lacking, especially in animal models. One of the only preclinical assays of fatigue for rodent studies is voluntary wheel running activity (VWRA)9-15, in which mice or other rodents are given free access to a running wheel and their daily running activity is recorded. In many studies, VWRA is the only measure of fatigue-like behavior, with fatigue-like behavior defined (in either VWRA or the current protocol) as a decrease in the measured physical activity in the experimental group. Although VWRA can provide a useful longitudinal measure of fatigue-like behavior, it is a relatively low-throughput assay, running varies considerably between inbred mouse strains16, and it requires subjects to be individually housed, which may cause changes in behavior and test performance17-19. Other assays, such as home cage behavioral monitoring and analysis, can also provide continuous data collection and some systems may allow for subjects to be housed in pairs20. These assays have utility, but may be less sensitive as a means of detecting fatigue-like behavior and, like wheel running, are also low-throughput.
In contrast to VWRA, mouse treadmill tests do not rely upon voluntary activity and can be completed in a short time frame, allowing for higher throughput. In comparison to VWRA, these tests employ external motivators. Specifically, there is usually an electrified metal grid located to the rear of the moving belt to provide mice with an electric shock should they cease to run. In addition to this shock grid, mice may be motivated to run on the treadmill via several other methods, including prodding, poking, or touching them with a hand, brush, or other tool and directing short puffs of air at them. Instead of fatigue, mouse treadmill tests are often used to measure aerobic and/or anaerobic exercise capacity21-25. Mice are motivated to run until they are incapable of or unwilling to continue running on the treadmill as a means of escaping further electric shocks. Testing then ends when mice meet the criterion for exhaustion. In these protocols, to ensure that mice reach true physiologic exhaustion, the criterion for exhaustion is often defined as spending five continuous seconds laying on top of the shock grid and failing to continue running in the face of repeated aversive stimuli. Thus, fatigue-like behavior may be masked in typical treadmill tests due to the strong aversive nature of the external motivation and criterion for ending the test. Interestingly, and in contrast to many other studies using rodent treadmills, a recent publication describes another version of a treadmill fatigue test, which was used as part of an examination of the effects of social stress in mice26. Although the method used by this group markedly differed from the current protocol (i.e., they employed a single-lane treadmill and required 10 sec of electric shock as the criterion for ending their test), their study highlights the utility of and interest in developing a quick, simple fatigue test using the mouse treadmill.
Fatigue is likely to be detectable by means other than wheel running and alterations in routine behaviors. CRF makes patients feel exhausted by a lesser amount of muscle fatigue, as determined by electromyographic analysis, than people without CRF27. Additionally, reduced motivation has been noted in and is measured by several scales measuring human fatigue28,29. Thus, a useful preclinical assay of fatigue-like behavior should distinguish between healthy and fatigued mice on the basis of a measure other than physiological capability and should not obscure decreases in motivation. To achieve that end while avoiding limitations of VWRA and other assays, the current method was developed by adapting the mouse treadmill test. This method uses a shock grid as the sole external motivator to make mice run on the treadmill. Mice quickly learn that the grid provides an aversive stimulus and will promptly move away from it when placed on the treadmill and maintain some distance from it when running.
When mice fatigue, they spend progressively more time toward the back of the treadmill instead of maintaining speed toward the front end. Therefore, the criterion for test completion in this protocol is spending five continuous seconds in the designated fatigue zone (i.e., the rear of the treadmill, ranging from approximately one body length from the shock grid to, and including, the shock grid). This takes advantage of the aversive nature of the grid without requiring mice to receive many or any actual shocks after training. By allowing mice to complete testing using the current criterion rather than exhaustion (as defined above), this method provides a means of using the treadmill to measure fatigue-like behavior rather than its maximal (or near-maximal) physiological capability. Thus, this method can provide a simple, high-throughput assay of fatigue-like behavior in mice and can serve either as an independent or complementary measure to other assays of fatigue-like behavior.
Protocol
This procedure was approved by the National Institute of Diabetes and Digestive and Kidney Diseases Animal Care and Use Committee.
1. Preparation
To allow for rapid identification of each mouse prior to testing, tattoo the tails of all mice to be trained and tested with identifying marks. NOTE: This step is optional. Permanent marker or other methods of identification can be used as an alternative to tattooing.
Prior to training and testing mice, ensure that the treadmill is on a flat surface and set to the treadmill to desired angle of inclination (recommended angle of inclination: 10°, to be kept consistent throughout training and testing) and set the electric shock frequency and intensity appropriately (recommended: 2 Hz, 1.22 mA). NOTE: The electric shock used should produce no more than a mild tingling sensation when touched by an ungloved finger and should be delivered in a pulsatile fashion (with each shock lasting 200 msec).
Place a clean sheet of butcher's paper or an absorbent pad under the treadmill to collect fecal boli and urine during training and testing.
Place a sheet of paper or an absorbent pad over the third of the treadmill housing (i.e., the clear plastic lid that covers the treadmill lanes) furthest from the shock grid. NOTE: This step is optional, but will create a darker space and may provide additional encouragement to avoid the lower portion of the treadmill.
If planning to use a wire brush to provide additional motivation during training, ensure that one is readily available prior to beginning training sessions.
Ensure that any drug or method for inducing and/or alleviating fatigue is available and can be prepared or performed during Step 2.14.
2. Training Mice to Use the Treadmill
NOTE: Training is necessary to ensure that mice are familiar with the treadmill and task and can perform appropriately when tested. If the majority of mice being trained are receiving frequent shocks or otherwise performing poorly during any training session, additional training sessions should be performed. On the first day, most mice will be shocked several times. By the second day of training, mice should be rarely making contact with the grid. If a mouse displays consistently poor training performance, it should be removed from the study. For female C57BL/6NCr mice, this is a rare occurrence (less than 1% have been removed from studies due to poor training performance), but it should be noted that other strains may perform differently during training.
With the treadmill off (and speed set to 0 m/min), individually lift the mice by the tail and place mice into separate lanes of a mouse treadmill. Promptly turn on the corresponding grid after placing each mouse on the treadmill. Ensure that mice are placed directly on the treadmill belt. NOTE: The amount of time and distance each mouse is held by its tail should be minimized by placing the cage near the treadmill prior to transferring mice to the treadmill and/or allowing mice to stand on a solid platform (e.g., a wire cage lid) until they are near the treadmill and the experimenter is ready to place them in the treadmill.
Allow mice to freely explore the treadmill for 1-3 min or until each mouse has explored its lane and/or received at least one shock from the grid.
Turn on the treadmill and slowly increase the speed until it begins moving (approximately 1.5 to 3.0 m/min). Monitor all mice to ensure that they begin walking. If a mouse does not begin walking or walks toward the shock grid, be prepared to intervene by tapping the mouse with a wire brush or tail tickling.
Slowly increase the treadmill speed to 8 m/min. Start a timer and continue monitoring behavior.
Increase treadmill speed to 9 m/min at 5 min, 10 m/min at 7 min, and stop the treadmill at 10 min.
Allow the mice to briefly explore the treadmill, then remove and return each to its cage.
Clean the treadmill and grid with alcohol and replace the paper or absorbent pad beneath the treadmill.
To train additional mice, repeat Steps 2.1 through 2.7. NOTE: Allow alcohol to dry prior to placing new mice on the treadmill.
On the second day of training, repeat Step 2.1. Turn on the treadmill and increase the speed to 10 m/min. Start a timer. NOTE: Treadmill speed can be increased more rapidly than on the first day of training.
Increase treadmill speed to 11 m/min at 5 min, 12 m/min at 10 min, and stop the treadmill at 15 min.
Remove mice and return them to their cages.
Clean the treadmill and grid with alcohol and replace the paper or absorbent pad beneath the treadmill. To train additional mice, repeat Steps 2.9 through 2.12.
Perform additional days (3 days) of training in the same manner as the second day. NOTE: This step is optional, but is strongly recommended if most or all mice (of the same sex and strain) being trained display difficulty with the task. Mice can generally perform well in Step 3 when they have been trained for 3 days (i.e., with one additional day of training), although additional or fewer days of training may be appropriate depending on their performance during the second training day and the duration of Step 2.14.
Allow at least one full day to pass in which the mice have no exposure to the treadmill before proceeding to Step 3. NOTE: Any drug(s) used to induce and/or alleviate fatigue should be administered during this step. NOTE: This time period can be varied in length and used to induce fatigue and/or test interventions to reduce or eliminate fatigue. If testing mice more than 7 days after completing training, a pilot study is recommended to verify that the mice used will perform during testing.
3. Treadmill Fatigue Test
NOTE: In this test, fatigue-like behavior is defined as spending 5 consecutive seconds in the "fatigue zone". The fatigue zone is defined as the region encompassing the portion of the treadmill belt within approximately 1 body length of the shock grid as well as the grid, itself. Prior to testing, ensure that the point delineating this zone is clear to the experimenter, such as by applying a mark to the top or side of the treadmill lanes.
Set the treadmill speed to 12 m/min. Do not start the treadmill. Ensure that shock grids are turned off.
Individually place mice into separate lanes of the treadmill. Turn on the corresponding grid immediately after placing each mouse on the treadmill.
Simultaneously start the treadmill and a stopwatch. NOTE: Do not intervene during testing except to remove mice that meet the criterion for removal (see Step 3.5).
Increase treadmill speed as indicated in Table 1. Carefully observe all mice throughout the test. NOTE: The treadmill speeds listed in Table 1 were selected based on observations from adult female C57BL/6NCr mice. Higher treadmill speeds may be appropriate for larger (e.g., outbred CD-1 mice) or more athletic mice.
If a mouse remains in the fatigue zone for 5 continuous sec, promptly remove the mouse from the treadmill and record the duration and distance it ran.
When no mice remain on the treadmill, stop the treadmill. Clean the treadmill and grid with alcohol and replace the paper or absorbent pad beneath the treadmill.
To test additional mice, repeat Steps 3.1 through 3.6. NOTE: This step is optional.
Representative Results
This protocol allows fatigue-like behavior to be measured in mice using a treadmill. The data presented in this section was obtained by training and testing 3 separate groups of mice using the current protocol (excluding Figure 1A and 1C). To induce fatigue, 5-fluorouracil (5-FU), a cytotoxic chemotherapy drug known to cause fatigue in humans30 and fatigue-like behavior in mice10,13, was administered. All data presented are from adult female C57BL/6NCr mice. Mice were 9-10 (Figure 1 and 2) or 9-13 (Figure 3) weeks of age at time of testing.
Figure 1 shows data from mice that were trained for 5 days, then treated with 5-FU (60 mg/kg/day for 5 days), as in a previously published model10, to induce fatigue. After completing treatment, they were tested using an exercise capacity test (Figure 1A), which used treadmill speeds listed in Table 2 and a wire brush, tail tickling, and air puffs to motivate mice to run until incapable of running. The test ended when a mouse spent 5 continuous seconds on the shock grid. On the following day, mice were tested using the treadmill fatigue test (Figure 1B). This protocol can detect a significant difference in the distance run during testing between chemotherapy-treated and control mice (Figure 1B), whereas a treadmill exercise capacity test did not (Figure 1A). To validate that the difference found in the treadmill fatigue test was measuring fatigue-like behavior, mouse VWRA was measured in a separate experiment. Following acclimation and collection of baseline wheel running activity, VWRA was measured during the dark cycle ("night", when wheel running primarily occurs) during the 5 days of 5-FU treatment and for an additional night beyond completion of 5-FU treatment. Mice undergoing 5-FU treatment displayed fatigue-like behavior by the second night of treatment (Figure 1C). This effect increased over the course of the experiment and persisted beyond the end of treatment, indicating that fatigue-like behavior should have been detectable in the mice from Figures 1A and 1B. As the treadmill fatigue test was capable of detecting differences in the distance run by control and 5-FU-treated mice, this supports the conclusion that the treadmill fatigue test is capable of measuring fatigue-like behavior.
The treadmill fatigue test can also detect fatigue-like behavior in mice receiving chemotherapy at different doses and treatment schedules. Mice receiving one 80 mg/kg dose of 5-FU per week for two weeks (for a cumulative dose of approximately half of what mice received in Figure 1) displayed fatigue-like behavior, as demonstrated by a decrease in distance run (Figure 2).
As the number of training sessions and/or length of time between training and testing may vary depending upon the mice used and the method used to induce fatigue, it is important that changes in these variables do not prevent the detection of fatigue-like behavior. The experiments shown in Figures 1A and 1B (in which mice received 5 days of training) and Figure 2 (in which mice received 3 days of training) illustrate that fatigue-like behavior is detectable when the number of training sessions and time between training and testing are changed.
In Figure 3, no chemotherapy drugs were administered, but mice were tested using the treadmill fatigue test weekly. Although mice can be tested repeatedly using this protocol, but they may become less willing to run upon repeated testing (Figure 3). The percentage of mice that would not run during weekly tests increased with every test and, after the second test, at least half of the mice tested would not run on the treadmill. This data suggests that testing with this protocol should be limited to one or two tests to avoid a high rate of non-compliant mice.
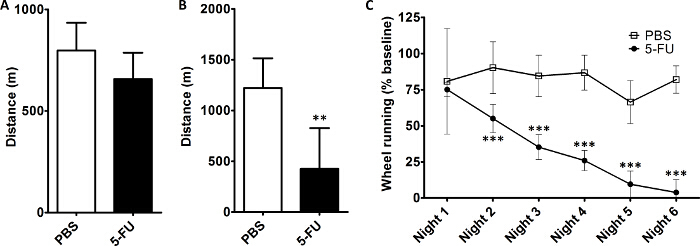 Figure 1: The Treadmill Fatigue Test, like Voluntary Wheel Running and in Contrast to the Treadmill Exercise Capacity Test, Detects Fatigue-like Behavior in Mice Receiving Daily Chemotherapy. On days 1-5, mice were trained daily on the treadmill. On days 6-10, mice underwent treatment with 5-FU (60 mg/kg/day) to induce fatigue or PBS. (A) On day 11, mice were tested using a standard treadmill exercise capacity test. (B) On day 12, mice underwent the treadmill fatigue test. (C) Wheel running activity (shown as a percentage of untreated baseline running). Mice were acclimated to running wheel cages for 7 days and baseline wheel running was collected over 4 additional nights and averaged to determine baseline wheel running for each mouse. On days 1-5, mice were treated with 5-FU (60 mg/kg/day) or PBS. Night 1 is the night after the first dose of 5-FU. For panels A and B, data are mean + SD from 5-6 mice per treatment group. For panel C, data are mean ± SD from 6 mice per treatment group. **p <0.01, Student's t-test; ***p <0.001, two-way repeated measures analysis of variance with Bonferroni correction Please click here to view a larger version of this figure.
Figure 1: The Treadmill Fatigue Test, like Voluntary Wheel Running and in Contrast to the Treadmill Exercise Capacity Test, Detects Fatigue-like Behavior in Mice Receiving Daily Chemotherapy. On days 1-5, mice were trained daily on the treadmill. On days 6-10, mice underwent treatment with 5-FU (60 mg/kg/day) to induce fatigue or PBS. (A) On day 11, mice were tested using a standard treadmill exercise capacity test. (B) On day 12, mice underwent the treadmill fatigue test. (C) Wheel running activity (shown as a percentage of untreated baseline running). Mice were acclimated to running wheel cages for 7 days and baseline wheel running was collected over 4 additional nights and averaged to determine baseline wheel running for each mouse. On days 1-5, mice were treated with 5-FU (60 mg/kg/day) or PBS. Night 1 is the night after the first dose of 5-FU. For panels A and B, data are mean + SD from 5-6 mice per treatment group. For panel C, data are mean ± SD from 6 mice per treatment group. **p <0.01, Student's t-test; ***p <0.001, two-way repeated measures analysis of variance with Bonferroni correction Please click here to view a larger version of this figure.
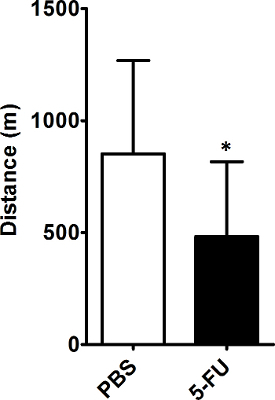 Figure 2: Weekly Treatment with 5-FU Induces Fatigue-like Behavior in Mice. On days 1 through 3, mice were trained daily on the treadmill. On days 4 and 11, mice received injections of 5-FU (80 mg/kg) or PBS. On day 12, mice underwent the treadmill fatigue test. Data are mean + SD from 12 mice per treatment group. *p <0.05, Student's t-test Please click here to view a larger version of this figure.
Figure 2: Weekly Treatment with 5-FU Induces Fatigue-like Behavior in Mice. On days 1 through 3, mice were trained daily on the treadmill. On days 4 and 11, mice received injections of 5-FU (80 mg/kg) or PBS. On day 12, mice underwent the treadmill fatigue test. Data are mean + SD from 12 mice per treatment group. *p <0.05, Student's t-test Please click here to view a larger version of this figure.
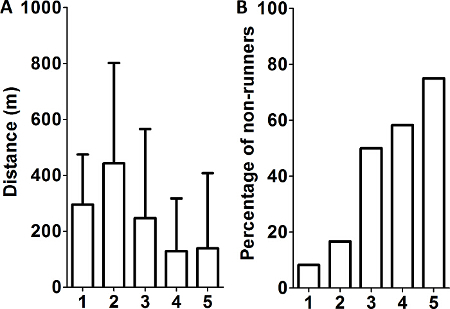 Figure 3: Distance Run and Task Compliance of Mice During Repeated Treadmill Fatigue Tests. On days 1 through 3, mice were trained daily on the treadmill. On days 5, 12, 19, 26, and 33, mice underwent treadmill fatigue testing. Mice received two injections of PBS the day before testing and a single injection 30 min prior to testing. (A) Distance run by mice during each week of testing. Data are mean + SD from 12 mice. (B) The percentage of non-runner mice during each week of testing. Non-runner mice were arbitrarily defined as mice that did not run for at least 6 min. Please click here to view a larger version of this figure.
Figure 3: Distance Run and Task Compliance of Mice During Repeated Treadmill Fatigue Tests. On days 1 through 3, mice were trained daily on the treadmill. On days 5, 12, 19, 26, and 33, mice underwent treadmill fatigue testing. Mice received two injections of PBS the day before testing and a single injection 30 min prior to testing. (A) Distance run by mice during each week of testing. Data are mean + SD from 12 mice. (B) The percentage of non-runner mice during each week of testing. Non-runner mice were arbitrarily defined as mice that did not run for at least 6 min. Please click here to view a larger version of this figure.
| Time (min) | Speed (m/min) |
| 0 | 12 |
| 0.5 | 14 |
| 1 | 16 |
| 6 | 18 |
| 30 | 20 |
| 45 | 22 |
| 60 | 24 |
| 75 | 26 |
Table 1: Treadmill Speed During Fatigue Testing.
| Time (min) | Speed (m/min) |
| 0 | 10 |
| 10 | 15 |
| 15 | 16.8 |
| 18 | 18.6 |
| 21 | 20.4 |
| 24 | 22.2 |
| 27 | 24 |
| 30 | 25.8 |
| 33 | 27.6 |
| 36 | 29.4 |
| 39 | 31.2 |
| 42 | 33 |
| 45 | 34.8 |
| 48 | 36.6 |
Table 2: Treadmill Speed During Exercise Capacity Testing.
Discussion
The current protocol describes how to use a mouse treadmill to measure fatigue-like behavior. This method has several advantages over VWRA, a common preclinical assay of fatigue-like behavior. VWRA requires that mice choose to interact with the test apparatus. As a result, some inbred strains of mice rarely interact with the wheel16 and run so little that it may be difficult or impossible to identify a fatigue-induced decrease in activity. In contrast, the treadmill fatigue test eliminates that choice and therefore provides a viable alternative assay of fatigue-like behavior for mice that do not run on running wheels. This protocol could be used as a replacement or complement to VWRA and other measures of fatigue-like behavior and may be particularly useful in testing potential drug therapies to reduce fatigue in mouse models. After establishing via a pilot study that fatigue-like behavior is observable in a particular mouse model, potential treatments could be administered to alleviate fatigue and reduce fatigue-like behavior. If a drug treatment attenuates fatigue-like behavior when tested using this protocol, it (or a similar drug) may be of therapeutic value for treating some forms of human fatigue. Moreover, although there are still many necessary steps in transitioning from preclinical studies to clinical trials, this protocol permits a greater number of mice to be tested in a much shorter time frame than VWRA so that fatigue-like effects and potential treatments can be studied and understood faster.
There are several important limitations and considerations to be aware of when using this protocol. First, it should be noted that, as this test requires physical activity to measure fatigue-like behavior, it may not be suitable for testing conditions that induce cachexia or muscle atrophy (e.g., advanced cancer). We have also observed that, if the same mice are tested repeatedly, there may be a decrease in overall compliance (Figure 3B). This effect may not be observed under all testing schedules or in all types of mice, and drug treatment or other interventions might alter this effect, but it is an important consideration when planning studies using this method. Additionally, there is a risk of injury if a mouse falls into the gap between the treadmill belt and the shock grid while the treadmill is running. To minimize this risk, mice should be carefully observed throughout training and testing to ensure their safety and the use of very young or small (<15 g) mice should be avoided. Lastly, although pilot data collected suggests that female CD-1 and male and female transgenic mice on a 129S1/SvImJ background will perform this task (data not shown), to date, this protocol has primarily been used to test female C57BL/6NCr mice. As such, it should be noted that other sexes and mouse strains may differ in training and test performance. Lastly, although pilot data collected suggests that female CD-1 and male and female transgenic mice on a 129S1/SvImJ background will perform this task (data not shown), to date, this protocol has primarily been used to test 9-10 week-old female C57BL/6NCr mice. As such, it should be noted that mice of different ages, sex, or strains may differ in training and test performance.
During testing, it is crucial that mice meeting the fatigue criteria are efficiently and quickly removed, as poor removal technique may provide additional motivation for a mouse to continue running, causing something other than fatigue-like behavior to be measured. Although the particular method of removal will depend upon experimenter comfort, a simple method of removal involves using the index and middle fingers of one hand. Each finger should be held straight and slightly apart from each other prior to entering the treadmill lane and promptly closed around the tail, near the base, or over the scruff of the mouse. Once securely grasped, the mouse can be readily removed.
It is important for mice to be familiar with the shock grid to provide motivation to run during testing, but frequent shocks during training may be detrimental to test performance. After the first day of training, most mice will walk on the treadmill successfully and respond to a shock by running or hopping away on the treadmill, then resume walking to avoid drifting back toward the grid. Some mice, however, may react strongly to shocks and/or find ways of not performing the task without receiving any. Mice that react strongly to the shock grid may receive more frequent shocks, spend less time walking on the treadmill, and may attempt to escape from the treadmill. With these mice, the experimenter can place a gloved hand at the rear of the lane to gently encourage the mouse to continue running. To avoid walking on the treadmill, some mice may exploit a limitation of the shock grid. The grid requires at least two points of direct skin contact (i.e., two or more paws must be touching the grid) to shock an animal. Thus, if a mouse sits on it without allowing two feet to touch the grid, it will not be shocked. If this behavior is observed, the experimenter can gently nudge the mouse to cause it to move its feet and receive a shock or lift the mouse to replace it on the treadmill. If these interventions are successful, the mouse should begin walking on the treadmill more consistently within several minutes and in future training sessions. If this intervention is not successful, the mouse should be removed from the study.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Grant 1Z01 DK011006. We wish to thank Michele Allen for providing technical assistance, Eleni Solomos for editorial assistance, and the NIH veterinary and animal care staff for providing care for the mice used in developing this method.
References
- Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12 Suppl 1:4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- Schwartz AL. Daily fatigue patterns and effect of exercise in women with breast cancer. Cancer Pract. 2000;8(1):16–24. doi: 10.1046/j.1523-5394.2000.81003.x. [DOI] [PubMed] [Google Scholar]
- Schwartz AL, Mori M, Gao R, Nail LM, King ME. Exercise reduces daily fatigue in women with breast cancer receiving chemotherapy. Med. Sci. Sports Exerc. 2001;33(5):718–723. doi: 10.1097/00005768-200105000-00006. [DOI] [PubMed] [Google Scholar]
- Butler JM, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2007;69(5):1496–1501. doi: 10.1016/j.ijrobp.2007.05.076. [DOI] [PubMed] [Google Scholar]
- Jean-Pierre P, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer. 2010;116(14):3513–3520. doi: 10.1002/cncr.25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar Fan HG, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support. Care Cancer. 2008;16(6):577–583. doi: 10.1007/s00520-007-0341-9. [DOI] [PubMed] [Google Scholar]
- Moraska AR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J. Clin. Oncol. 2010;28(23):3673–3679. doi: 10.1200/JCO.2010.28.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AL, Thompson JA, Masood N. Interferon-induced fatigue in patients with melanoma: a pilot study of exercise and methylphenidate. Oncol. Nurs. Forum. 2002;29(7):E85–E90. doi: 10.1188/02.ONF.E85-E90. [DOI] [PubMed] [Google Scholar]
- Coletti D, et al. Substrains of inbred mice differ in their physical activity as a behavior. Sci. World J. 2013. p. 237260. [DOI] [PMC free article] [PubMed]
- Mahoney SE, Davis JM, Murphy EA, McClellan JL, Gordon B, Pena MM. Effects of 5-fluorouracil chemotherapy on fatigue: role of MCP-1. Brain Behav. Immun. 2013;27(1):155–161. doi: 10.1016/j.bbi.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya J, Chen R, Yamakawa J, Sasaki K, Ishigaki Y, Takahashi T. Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biol. Pharm. Bull. 2011;34(3):354–359. doi: 10.1248/bpb.34.354. [DOI] [PubMed] [Google Scholar]
- Sheng WS, Hu S, Lamkin A, Peterson PK, Chao CC. Susceptibility to immunologically mediated fatigue in C57BL/6 versus Balb/c mice. Clin. Immunol. Immunopathol. 1996;81(2):161–167. doi: 10.1006/clin.1996.0172. [DOI] [PubMed] [Google Scholar]
- Weymann KB, Wood LJ, Zhu X, Marks DL. A role for orexin in cytotoxic chemotherapy-induced fatigue. Brain. Behav. Immun. 2014;37:84–94. doi: 10.1016/j.bbi.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LJ, Nail LM, Perrin NA, Elsea CR, Fischer A, Druker BJ. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biol. Res. Nurs. 2006;8(2):157–169. doi: 10.1177/1099800406290932. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Fey EG, Lyng GD, Sonis ST. A clinically translatable mouse model for chemotherapy-related fatigue. Comp. Med. 2013;63(6):491–497. [PMC free article] [PubMed] [Google Scholar]
- Lightfoot JT, et al. Strain screen and haplotype association mapping of wheel running in inbred mouse strains. J. Appl. Physiol. 2010;109(3):623–634. doi: 10.1152/japplphysiol.00525.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomucci A, et al. Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Psychoneuroendocrinology. 2003;28(4):540–558. doi: 10.1016/s0306-4530(02)00039-2. [DOI] [PubMed] [Google Scholar]
- Martin AL, Brown RE. The lonely mouse: verification of a separation-induced model of depression in female mice. Behav. Brain Res. 2010;207(1):196–207. doi: 10.1016/j.bbr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Võikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4(4):240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Salem GH, et al. SCORHE: a novel and practical approach to video monitoring of laboratory mice housed in vivarium cage racks. Behav. Res. Methods. 2015;47(1):235–250. doi: 10.3758/s13428-014-0451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney SM, Massett MP. Identification of exercise capacity QTL using association mapping in inbred mice. Physiol. Genomics. 2012;44(19):948–955. doi: 10.1152/physiolgenomics.00051.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen SB, et al. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005;19(9):1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- Knab AM, Bowen RS, Moore-Harrison T, Hamilton AT, Turner MJ, Lightfoot JT. Repeatability of exercise behaviors in mice. Physiol. Behav. 2009;98(4):433–440. doi: 10.1016/j.physbeh.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot JT, Turner MJ, Debate KA, Kleeberger SR. Interstrain variation in murine aerobic capacity. Med. Sci. Sports Exerc. 2001;33(12):2053–2057. doi: 10.1097/00005768-200112000-00012. [DOI] [PubMed] [Google Scholar]
- Lightfoot JT, et al. Quantitative trait loci associated with maximal exercise endurance in mice. J. Appl. Physiol. 2007;103(1):105–110. doi: 10.1152/japplphysiol.01328.2006. [DOI] [PubMed] [Google Scholar]
- Azzinnari D, et al. Mouse social stress induces increased fear conditioning, helplessness and fatigue to physical challenge together with markers of altered immune and dopamine function. Neuropharmacology. 2014;85:328–341. doi: 10.1016/j.neuropharm.2014.05.039. [DOI] [PubMed] [Google Scholar]
- Kisiel-Sajewicz K, et al. Myoelectrical manifestation of fatigue less prominent in patients with cancer related fatigue. PloS One. 2013;8(12):e83636. doi: 10.1371/journal.pone.0083636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets EMA, Garssen B, Bonke B, De Haes JCJM. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- Vercoulen JHMM, Swanink CMA, Fennis JFM, Galama JMD, van der Meer JWM, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J. Psychosom. Res. 1994;38(5):383–392. doi: 10.1016/0022-3999(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Tsujimoto H, et al. Tolerability of adjuvant chemotherapy with S-1 after curative resection in patients with stage II/III gastric cancer. Oncol. Lett. 2012;4(5):1135–1139. doi: 10.3892/ol.2012.882. [DOI] [PMC free article] [PubMed] [Google Scholar]


