Abstract
Mice emit ultrasonic vocalizations in different contexts throughout development and in adulthood. These vocal signals are now currently used as proxies for modeling the genetic bases of vocal communication deficits. Characterizing the vocal behavior of mouse models carrying mutations in genes associated with neuropsychiatric disorders such as autism spectrum disorders will help to understand the mechanisms leading to social communication deficits. We provide here protocols to reliably elicit ultrasonic vocalizations in pups and in adult mice. This standardization will help reduce inter-study variability due to the experimental settings. Pup isolation calls are recorded throughout development from individual pups isolated from dam and littermates. In adulthood, vocalizations are recorded during same-sex interactions (without a sexual component) by exposing socially motivated males or females to an unknown same-sex conspecific. We also provide a protocol to record vocalizations from adult males exposed to an estrus female. In this context, there is a sexual component in the interaction. These protocols are established to elicit a large amount of ultrasonic vocalizations in laboratory mice. However, we point out the important inter-individual variability in the vocal behavior of mice, which should be taken into account by recording a minimal number of individuals (at least 12 in each condition). These recordings of ultrasonic vocalizations are used to evaluate the call rate, the vocal repertoire and the acoustic structure of the calls. Data are combined with the analysis of synchronous video recordings to provide a more complete view on social communication in mice. These protocols are used to characterize the vocal communication deficits in mice lacking ProSAP1/Shank2, a gene associated with autism spectrum disorders. More ultrasonic vocalizations recordings can also be found on the mouseTube database, developed to favor the exchange of such data.
Keywords: Behavior, Issue 112, neuroscience, mouse, social communication, ultrasonic vocalization, pup isolation calls, male vocalizations, female-female interactions, neuropsychiatric disorders, mouse models
Introduction
Patients with neuropsychiatric disorders usually display deficits in social communication (e.g., patients with autism spectrum disorders, schizophrenia, or Alzheimer disease)1. Genetically engineered mice are more and more frequently used to model genetic causes of these disorders2. Studying social communication in these mouse models is of high interest for understanding the mechanisms of genetic mutations leading to atypical social dysfunctions and for testing new therapies. Since mice are social animals and communicate with each others using olfactory, tactile, visual and acoustic signals, they are suitable models to evaluate social communication.
Mouse ultrasonic vocalizations are now currently used as a proxy for modeling the genetic bases of vocal communication deficits3,4 (but the existence of vocal learning in this species is still debated5,6, even if most recent studies argue for the absence of vocal learning7). Laboratory mice have been found to emit ultrasonic vocalizations in mother-infant relationships, in male-female socio-sexual interactions, in same-sex social interactions (reviewed in reference8) and in juvenile-juvenile social interactions9. Mouse pups emit isolation calls during their first two weeks of life when isolated from dam and littermates10. Males emit ultrasonic vocalizations when in presence of an estrus female (or urinary cues from her)11,12. Males and females emit ultrasonic vocalization when interacting with an unknown conspecific of the same sex13,14. The organization and functions of these vocalizations are not completely clear and need further investigations. Current knowledge on the functional aspect is limited to the elicitation of retrieval behavior in mothers hearing pup isolation calls, the facilitation of proximity of adult females toward adult male vocalizations15 and the increased exploratory behavior of adult males hearing adult female vocalizations16.
Characterizing the abnormalities in vocal communication in mouse models of neuropsychiatric disorders should be conducted in standardized conditions to rule out major contribution of the experimental conditions. Such characterizations, combined with the evaluation of simultaneous social interactions and neurobiological studies, in various genetic models should improve our knowledge on the genetic contribution to the different aspects of mouse ultrasonic communication. Over a long term, it should give further light on some neurobiological bases of social communication in humans. We presently aim at providing simple protocols to reliably elicit ultrasonic vocalizations during development and in adulthood for both male and female mice in the laboratory. Such protocols should ease the standardization of recordings to more reliably compare ultrasonic vocalization emissions between strains and laboratories. It should also facilitate the setting up of such recordings in laboratories having no prior experience with mouse ultrasonic vocalizations recordings. We also highlight the current possibility to combine ultrasonic vocalizations data with detailed behavioral data collected simultaneously during social interactions in adult mice, to obtain crucial information on social impairments as well as on the context of emission of ultrasonic vocalizations. Such analyses will shed new light on the organization and functions of mouse ultrasonic vocalizations. Finally, we also advertise the possibility to share ultrasonic vocalization recordings with the whole scientific community on the mouseTube database (http://mousetube.pasteur.fr). Open access to audio recording data should boost knowledge on mouse ultrasonic communication by allowing scientists to compare their own data with ultrasonic vocalizations recorded in other laboratories (with similar or different strains/protocols), and/or to challenge their analysis methods with files recorded under different conditions.
Protocol
Ethics statement: Procedures involving animal subjects have been approved by the Comité d'Ethique en Expérimentation animale (CETEA) n°89 at the Institut Pasteur, Paris.
1. Animal Preparation
To record pup isolation calls, obtain pregnant females from the mouse strain of interest. Note: Breed heterozygous males and females to obtain at least 10 litters including wild-type, heterozygous and knock-out pups to obtain robust control animals.
- Obtain two categories of adult mice to record vocalizations during same-sex interactions.
- Obtain at least 12 males or 12 females of each genotype as test mice from the strain of interest (to take into account inter-individual variability). Note: This protocol is well-adapted for adults, but it can be adjusted to juveniles, with reduced isolation time before the experiment9. This test works for males or females. However, avoid testing males from mouse strains that display a clear aggressive phenotype in the male-male social interaction test.
- To maximize the amount of affiliative interactions, isolate the test animals before the experiments. House males individually for 3 weeks (to reduce aggressive interactions to a minimum14,17) and females for 3 days (E. Ey, unpublished data) to increase their social motivation.
- Obtain males or females from strains representative of the genetic background of the test strain to use them as new-comers (e.g., as interacting mice, see 3.1.3). For instance, if the mutant strain being studied has been generated on the C57BL/6J background, use C57BL/6J mice as new-comers. Calculate the number of animals needed so that each of these mice is not used more than 2 times a day as new-comer. House them in groups.
- Obtain two categories of adult mice to record male vocalizations in presence of an estrus female.
- Obtain at least 12 sexually mature males from each genotype of the strain of interest (to take into account inter-individual variability). Note: If the males have never had experience with females before, put them in individual cages and leave them spend one night with a female two days before the test to increase their motivation to emit ultrasonic vocalizations6.
- Obtain sexually mature females from the background strain of the recorded males. For instance, if the mutant males being studied have been generated on the C57BL/6J background, use C57BL/6J females. Calculate the number of females needed so that each of these mice is not used more than 3 times a day. House them in groups.
2. Pup Isolation Calls
- Pup Identification
- Three days before the predicted day of birth, isolate the pregnant females.
- Check the females for birth every morning and every evening. Note the day of birth as P0.
- Identify the pups at P1 using long-lasting paw tattoos (subcutaneous injection of green tattoo paste with a 0.3 mm x 13 mm [30 G ½"] needle). Create a code with one, two, three or four paws marked. Be as quick as possible, to minimally disturb the pups, and put them back in the nest as soon as possible.
- Set up the Cage to Record Pup Isolation Calls.
- Use either a self-made soundproof chamber (Figure 1A) or a simple Styrofoam box. Place a thermometer within the box to monitor the temperature for each recording. Make sure that the temperature remains between 18 °C and 22 °C.
- Place a microphone at the top (through a hole in the top of the box). Adjust the height of the microphone so that the membrane of the microphone is 12-15 cm above the bottom of the box where the pup will lie down. Connect the microphone to the sound card, and the sound card to the computer.
- To adjust the gain of the sound card, conduct a recording trial with a pup that will not be used in the experiment. Put the pup in the same conditions as in the experiment (see 2.3.1). Close the door. Adjust the gain on the sound card, so that it is on the maximum value (to have the highest amplitude possible for the vocalizations) but without overloading (check on the live spectrogram display on the recording software). Note: A few calls might be overloaded if the amplitude of the majority of the calls is kept at the highest level possible.
- Register the gain level for each recording session and do not change it between pups/litters/sessions. Variations in gain levels would lead to inaccurate call detection and acoustic variables measures with the same thresholds in the analyses using automatic detection and measurements (see 5.1 to 5.4).
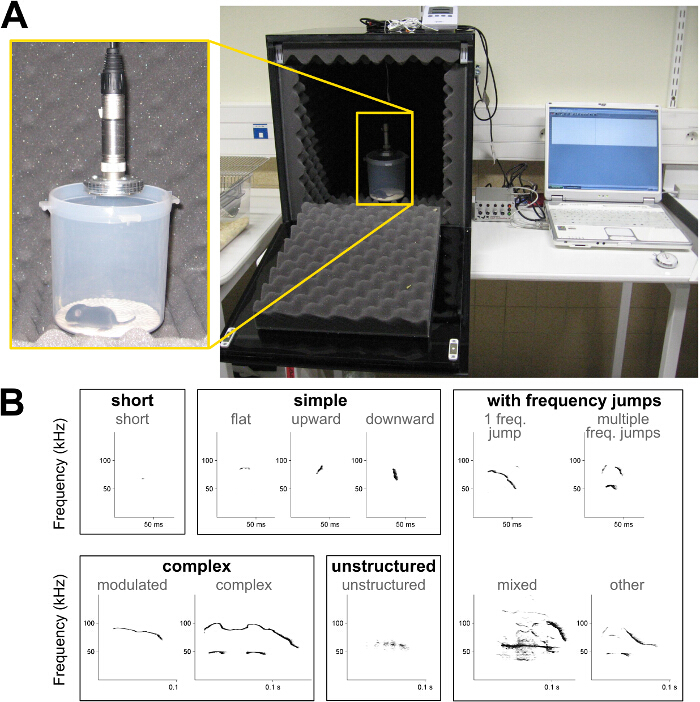 Figure 1: Set up for recording isolation calls from mouse pups and spectrograms of ultrasonic vocalizations. (A) Example of a self-made sound-proof chamber to record pup isolation calls. (B) Spectrograms of the different call types used in the present call type classification; see description in Table 1. Please click here to view a larger version of this figure.
Figure 1: Set up for recording isolation calls from mouse pups and spectrograms of ultrasonic vocalizations. (A) Example of a self-made sound-proof chamber to record pup isolation calls. (B) Spectrograms of the different call types used in the present call type classification; see description in Table 1. Please click here to view a larger version of this figure.
- Conduct the recording of pup isolation calls every two days. Conduct recordings in the morning for pups born in the night, and in the afternoon for pups born during the day to avoid categorizing in the same age class pups with half a day of age difference. This is most noticeable for the very young stages P2 and P4.
- Take one pup in the litter. Place it as quickly and gently as possible in a plastic recipient washed with ethanol 10% and dried (diameter: 9 cm; height: 10 cm to prevent older pups from escaping from the area covered by the microphone). Put the recipient just under the microphone.
- Close the box as quickly and silently as possible. Start the recording of pup ultrasonic vocalizations in the recording software (16-bit format, 300 kHz sampling frequency to capture sound amplitude up to 150 kHz with a high quality).
- After the required time of recording has elapsed (up to 5 min), stop the recording. Take the pup out of the box. Write down the paw tattoos of the pup.
- Take the axillary temperature of the pup with a probe-thermometer. Mark the pup on its back with a tiny point with a smell-less pen (water ink), to recognize more easily the already recorded pups in the nest when the next one is chosen and to avoid manipulating all pups each time a new one is chosen. Put the pup back in the nest.
- Wash the plastic recipient and the plastic covering its bottom with 10% ethanol and dry it well before putting the next pup inside.
- Choose the next pup in the litter and repeat 2.3.
Check body weight, motor coordination, negative geotaxis, and developmental marks (for details of the reduced test battery please see the method sections in Schmeisser et al.18 and in Ey et al.19) after a period of rest of 1 hr to allow the pups to recover after the exhausting emission of ultrasonic vocalizations. Use another cohort of animals if the complete developmental test battery such as in Chadman et al.20 and Scattoni et al.21 is conducted.
Repeat these recordings every two days between P2 and P12 to characterize pup vocal behavior and development throughout their first two weeks of life.
3. Ultrasonic Vocalizations during Same-sex Social Interactions
- Vocalization Recordings
- Prepare a test cage (50 x 25 cm x 30 cm3; Plexiglas, 100 lux [low intensity white light]) cleaned with soap water, dried and filled with 2 cm fresh bedding in the soundproof chamber.
- Place the microphone so that vocalizations emitted from all corners of the cage can be recorded. Fix the microphone in a corner of the test cage (either on the cage or on a tripod) and adjust the angle microphone-cage bottom to cover the cage's entire surface. Note: Ultrasounds are very directional.
- Place a video camera on the top of the soundproof chamber to capture the whole surface of the test cage. Note: Check that the microphone is not hiding a corner of the test cage on the video.
- Before testing, adjust the gain of the soundcard with a spare male and a spare female that will not be used in the experiment later. Put these animals in the test cage in the recording chamber. Adjust the gain level on the sound card to maximize the amplitude of the recorded vocalizations but to minimize overloading as seen in the live spectrogram display on the recording software. Note: The gain depends on the distance between the microphone and the vocalizing animals.
- Introduce the animal to be tested (male or female; it will be called the "occupant") in the test cage on fresh bedding. Leave it habituate to the test cage in the soundproof chamber for 20 min to maximize its interest for the unknown conspecific introduced in 3.1.3.
- After this habituation time, introduce the 2nd animal for the interaction (male or female, same sex as the occupant, but different ear marks / paw tattoo to identify them later; it will be called the "new-comer").
- Start recording the ultrasonic vocalizations (16-bit format, 300 kHz sampling frequency to capture sound amplitude up to 150 kHz with a high quality) and the video to capture the introduction of the new-comer in the test cage. Start recording the ultrasonic vocalizations during habituation (occupant alone) if a comparison between the baseline level of vocalization emission during cage exploration and the social interaction is needed.
- Synchronize manually/visually the audio and video recordings by pressing on the time watch ("bip" sound near the microphone) exactly when the hind paws of the new-comer mouse touch the ground.
- Leave the two animals interact for the desired time (for instance 4 min, a duration sufficient to collect enough ultrasonic vocalizations).
- Put the occupant and the new-comer back in their respective home cages. Empty the used bedding from the test cage, wash it with soap water and dry it with paper towels. Put fresh bedding and place it back in the soundproof chamber for the next test.
4. Male Vocalizations During Interaction with an Estrus Female
- Early in the morning the day of testing the males, take vaginal smears from each female to determine their sexual status within the estrus cycle.
- Hold the female by the tail and maintain her on the cage grid. Use a pipette to rinse the vagina several times with 20 µl PBS (i.e., inject and recollect the same 20 µl PBS several times). Recollect the 20 µl PBS with the same pipette tip. Use sterile PBS, to avoid any infection if females are to be tested for several consecutive days.
- Spread the PBS containing the suspension of vaginal cells on a slide. Put four samples on one slide (identify the individuals at the side of the slide with a pencil). Let the slides dry before conducting the staining.
- Work under the laboratory fume hood.
- Prepare one bath of pure May-Grünwald, one bath of phosphate buffer solution (0.1 M) and one bath of Giemsa R (1/20 in phosphate buffer solution).
- Put the slides in the bath of pure May-Grünwald for 3 min, then rinse them in the bath of phosphate buffer solution for 1 min and finally transfer them for 10 min in the bath of Giemsa R (1/20 in phosphate buffer solution).
- After that, rinse the slides again in the bath of phosphate buffer solution for 10 sec and let them dry.
- Examine the stained slides under the microscope. Females that can be used during the day are those whose samples present only large cornified epithelial cells (without nucleus, stained in blue; full estrus).
Put the males in the test room at least 30 min before testing them.
- Vocalization Recordings
- Repeat 3.1 if necessary.
- Introduce the male to be tested (on fresh bedding). Leave him habituate to the test cage in the soundproof chamber for 10 min.
- After this habituation time, introduce a female in estrus (among the ones selected from the staining).
- Start recording the ultrasonic vocalizations and the video to capture the introduction of the female in the test cage. Start recording the ultrasonic vocalizations during habituation (male alone) if a comparison between the baseline level of vocalization emission during cage exploration and the social interaction is needed.
- Synchronize manually/visually the audio and video recordings by pressing on the time watch ("bip" sound near the microphone) exactly when the hind paws of the female mouse touch the ground.
- Leave the two animals interact for the desired time (for instance 4 min, a duration sufficient to collect enough ultrasonic vocalizations).
- Put the male and the female back in their respective home cages. Empty the used bedding from the test cage, wash it with soap water and dry it with paper towels. Put fresh bedding and place it back in the soundproof chamber for the next test. Note: It is optimal to use each estrus female only one time each day (but if necessary it can be used up to 3 times on the same day but not in a row).
5. Variables to Be Extracted
- Prepare audio files for the analyses. Note: The procedure below is specific to Avisoft SASLab Pro and may change according to the software used.
- Cut the files so that they start exactly at the "bip" of the time watch, and end after the desired duration (5 min for pup recordings, 4 min for adult recordings).
- Filter out the amplitude under 30 kHz by using a high-pass filter (Edit>Filter>FIR Time Domain Filter; High Pass with 30 kHz frequency cut off). Use batch processing to filter all files of interest (Actions>Batch Processing>FIR filter).
- Identify each ultrasonic vocalization by labelling them with the software.
- Use automatic detection for pup recordings (Tools>Labels>Create section labels from waveform events). Adjust threshold, hold time and margin for the most accurate detection. Manually check the detection and adjust labels if necessary (recommended).
- Use visual detection (manual insertion of labels) for adult recordings with background noise (select the vocalizations, click right, and insert section label from marker).
- Create the spectrogram. Activate automatic parameter measurements (Tools>Automatic Parameter Measurements>Automatic Parameter Measurements Set up).
- Check the "Enable automatic measurements", the "Compute parameters from entire spectrogram", and the "Automatic update" boxes. Select "Element separation": interactively (section labels).
- Check boxes to calculate the desired temporal parameters (Duration of element, Interval, Start/End time) and spectrum-based parameters (Peak frequency), and the location of measurements (Start of element, End of element, Mean, Max, Min).
- Copy the measurements and paste them in a spreadsheet. Note: For recordings conducted with adults, measurements of spectrum-based parameters might be impossible because of background noise. Use manual measurements of peak frequency by clicking on the different frequency values directly on the spectrogram and pasting the values manually in a table.
Determine call rate, i.e., number of calls per minute by first determining the number of vocalizations emitted (total number of labels). Then, calculate the call rate by dividing the total number of vocalizations recorded by the duration (in minutes) of the file.
- Determine temporal organization, i.e., distribution of time intervals between calls to determine sequence organization.
- Calculate the time intervals between the end of the vocalization n and the start of the vocalization n+1 using the start/end time of each label.
- Establish the distribution density of the time intervals between ultrasonic vocalizations.
- Determine call repertoire, i.e., define the call types present in the recording. Use the example of classification presented in Table 1 and Figure 1B.
- When labeling each vocalization in 5.1.3, write the name of the call type in the label.
- Calculate the exact number and the proportion of each call type to build the vocal repertoire.
- Determine acoustic characteristics for each vocalization, i.e., duration, peak frequency (i.e., frequency with the highest amplitude) at the beginning and the end of the call, maximum and minimum peak frequency, and mean frequency if automatic measurement is possible (Figure 1B).
- Use the automatic parameter measurements function in the software to measure automatically duration, peak frequency characteristics (e.g., start, end, mean, maximum, minimum) in pup recordings.
- Use the duration of the label as the duration of the vocalization for adult recordings. Measure manually on the spectrogram window the peak frequency characteristics (e.g., start, end, maximum, minimum).
- Couple ultrasonic vocalization data and social interaction data (MiceProfiler plugin from the ICY platform22).
- Make sure to synchronize as precisely as possible the audio and the video recordings as described in the protocol.
- Encode the video of the social interaction using the Mice Profiler Tracker plugin of the ICY platform as described in de Chaumont et al.22. Start the tracking exactly when the hind paws of the introduced animal touch the ground.
- Upload the encoded video file and its corresponding xml file (generated by Mice Profiler Tracker) in the Mice Profiler Video Label Maker plugin of the ICY platform as described in de Chaumont et al.22. Note: The Mice Profiler Video Label Maker plugin will automatically link the video file and the text file generated from the analysis of the audio file if they have the same name (see: http://icy.bioimageanalysis.org/plugin/Mice_Profiler_Video_Label_Maker).
- After checking that the mouse scale is correct, click on "Create USV stats" for each file to obtain the number and the proportion of vocalizations emitted during each social event in a separated file.
6. Uploading Files on the mouseTube Database
Make sure the files are on a storage server which can be accessed from outside the institution. Note: Servers hosted in some institutions with high security levels will need a specific configuration to be accessible by people connecting from outside the institution.
Go to the mouseTube website (http://mousetube.pasteur.fr). Log in (login and password are attributed to each user by the administrators).
Check whether the mouse strain recorded already exists in the mouseTube database by clicking on the "Strains" button. If not, ask the administrators to create it.
Create subjects using the "Subjects>Create" button. Enter the identification codes of the animals recorded. Gather them in groups to ease later retrieval of data.
Enter the description of the protocol used to record ultrasonic vocalizations using the "Protocols>Create" button.
Create an experiment for each recording session using the "Experiments>Create" button. Specify the protocol, the group of individuals which has been recorded, the hardware and software used and their specificities. Note: The experiment gathers all the metadata corresponding to the vocalization files.
Create the link to the vocalization files using the "Vocalisations>Create" button. Select the experiment within the list. Copy and paste the url of the vocalization file (this link begins with http://…) into the corresponding field of the “Files to link” column. Validate the entries by clicking on the button “Create a link between mouseTube and the files”. Note: It is not required to fill each box for each file at the same time.
If necessary, modify the links entered at any moment, and add details in the "Notes" section. Do not hesitate to write down notes to give more details. For instance, if a link toward a video file that was recorded simultaneously with the audio file has been entered, this can be specified in the "Notes".
Representative Results
With the present protocols, we characterized the vocal behavior of mice lacking ProSAP1/Shank2, a gene associated with autism spectrum disorders (ASD)23-25. ASD are characterized by deficits in social communication and stereotyped behaviors1. Our Shank2-/- mice displayed hyperactivity, increased anxiety and atypical vocal communication18,26. Indeed, we noted that Shank2-/- mice displayed an atypical developmental profile in their emission rate of pup isolation calls in comparison with the typical inverted U-shaped curve in their wild-type littermates. Shank2-/- mice displayed an increased call rate at P4 and decreased call rate at P6 in comparison with their wild-type littermates (Figure 2). We also observed a decreased call rate in female interactions involving a Shank2-/- female in comparison with interactions involving a wild-type littermate (Figure 2). We examined the repertoire of the 5 different call categories. It appeared to be different between pups (for instance here P2, P6 and P10) and adults (Figure 3). Genotype-related differences were significant mostly in adulthood. During social interactions involving adult Shank2-/- males or females with a C57BL/6N female, more short calls and unstructured calls were recorded in comparison with interactions involving their wild-type littermates (Figure 3D and E). Less complex calls and frequency jumps calls were also recorded during interactions with a C57BL/6N female involving adult Shank2-/- females in comparison with interactions involving Shank2+/+ females (Figure 3E). Finally, we also measured manually acoustic variables. There was no significant genotype-related difference during development. In contrast, the duration of calls recorded during interactions involving adult Shank2-/- females were shorter than those recorded during interactions involving their wild-type littermates (Figure 4A). We also highlighted that the peak frequency of ultrasonic vocalizations increased during pup development without significant genotype-related difference26. During interactions involving Shank2-/- males or females with a C57BL/6N female, ultrasonic vocalizations had a lower peak frequency in comparison with calls recorded during interactions involving their wild-type littermates (Figure 4B).
In addition, the present protocol also allowed to study the context of emission of ultrasonic vocalizations by combining the data from audio recordings to the behavioral data extracted from MiceProfiler (ICY software, Institut Pasteur, Paris). For instance, in female-female interactions, most ultrasonic vocalizations were emitted when animals were in contact and more specifically the occupant sniffing the new-comer's ano-genital region, or at least the occupant being behind the new-comer. Mice also emitted many ultrasonic vocalizations when the occupant approached the new-comer (Figure 5, upper panel). Less vocalizations were recorded when the occupant Shank2-/- mice were in physical contact with the new-comer (e.g., sniffing the ano-genital region of the new-comer) than when the occupant was a wild-type mouse. Less vocalizations were triggered when the occupant behind the new-comer was a Shank2-/- mouse than when it was a wild-type mouse. More vocalizations were also recorded when the new-comer was in the visual field of the occupant mouse, and more so in the wild-types than in the mutants (Figure 5, lower panel).
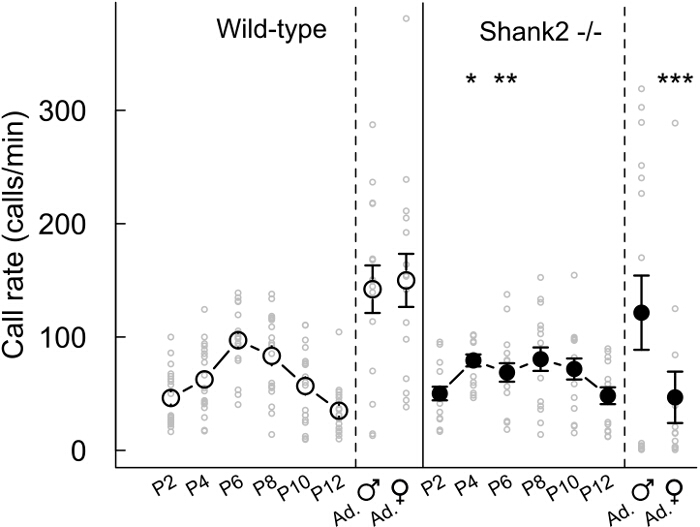 Figure 2: Emission rate of ultrasonic vocalizations during development and in adult male and female Shank2-/- mice and wild-type littermates. Call rate of pups (every two days from P2 to P12, n = 18-19 Shank2+/+, n = 15-16 Shank2-/-) and adults during male-estrus female interactions (n = 15 Shank2+/+, n = 16 Shank2-/-) and female-female interactions (n = 15 Shank2+/+, n = 13 Shank2-/-) in wild-type mice (left panel) and Shank2-/- mice (right panel). Data are presented as mean+/-SEM and individual points (non-paired Wilcoxon tests: *p <0.05, **p <0.01, ***p <0.001). Please click here to view a larger version of this figure.
Figure 2: Emission rate of ultrasonic vocalizations during development and in adult male and female Shank2-/- mice and wild-type littermates. Call rate of pups (every two days from P2 to P12, n = 18-19 Shank2+/+, n = 15-16 Shank2-/-) and adults during male-estrus female interactions (n = 15 Shank2+/+, n = 16 Shank2-/-) and female-female interactions (n = 15 Shank2+/+, n = 13 Shank2-/-) in wild-type mice (left panel) and Shank2-/- mice (right panel). Data are presented as mean+/-SEM and individual points (non-paired Wilcoxon tests: *p <0.05, **p <0.01, ***p <0.001). Please click here to view a larger version of this figure.
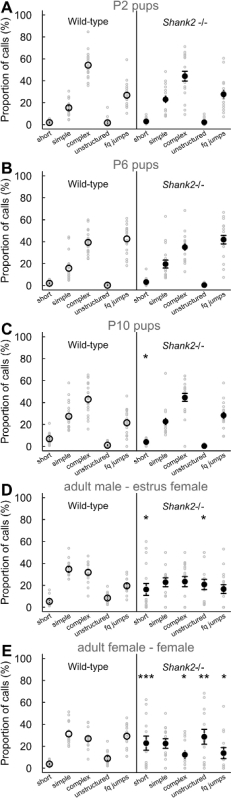 Figure 3: Vocal repertoire of Shank2-/- mice and wild-type littermates. Proportions of the five different call types emitted by P2 pups (A; n = 20 Shank2+/+, n = 18 Shank2-/-), P6 pups (B; n = 19 Shank2+/+, n = 18 Shank2-/-), P10 pups (C; n = 20 Shank2+/+, n = 18 Shank2-/-), adult males with an estrus female (D; n = 16 Shank2+/+, n=16 Shank2-/-) and adult females with another female (E; n = 15 Shank2+/+, n=13 Shank2-/-) in wild-type mice (left panels) and Shank2-/- mice (right panels). Data are presented as mean+/-SEM and individual points (chi-squared tests: *p <0.05, **p <0.01, ***p <0.001). Please click here to view a larger version of this figure.
Figure 3: Vocal repertoire of Shank2-/- mice and wild-type littermates. Proportions of the five different call types emitted by P2 pups (A; n = 20 Shank2+/+, n = 18 Shank2-/-), P6 pups (B; n = 19 Shank2+/+, n = 18 Shank2-/-), P10 pups (C; n = 20 Shank2+/+, n = 18 Shank2-/-), adult males with an estrus female (D; n = 16 Shank2+/+, n=16 Shank2-/-) and adult females with another female (E; n = 15 Shank2+/+, n=13 Shank2-/-) in wild-type mice (left panels) and Shank2-/- mice (right panels). Data are presented as mean+/-SEM and individual points (chi-squared tests: *p <0.05, **p <0.01, ***p <0.001). Please click here to view a larger version of this figure.
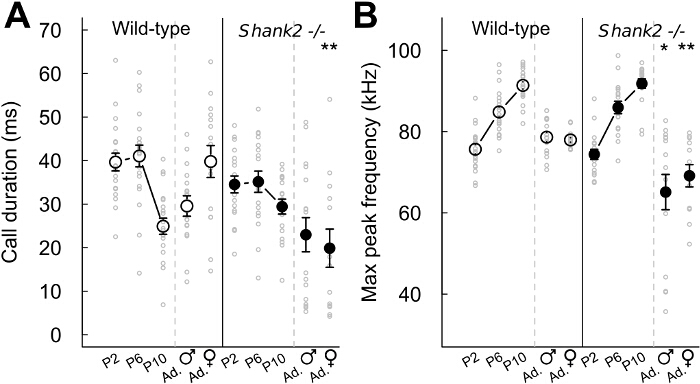 Figure 4: Acoustic variables extracted from ultrasonic vocalizations in Shank2-/- mice and wild-type littermates. (A) Duration of all call types confounded emitted by P2 pups (n = 20 Shank2+/+, n = 18 Shank2-/-), P6 pups (n = 19 Shank2+/+, n = 18 Shank2-/-), P10 pups (n = 20 Shank2+/+, n = 18 Shank2-/-), adult males with an estrus female (n = 16 Shank2+/+, n = 16 Shank2-/-) and adult females with another female (n = 15 Shank2+/+, n = 13 Shank2-/-) in wild-type mice (left panel) and Shank2-/- mice (right panel). (B) Maximum peak frequency measured on all call types confounded in P2 pups, P6 pups, P10 pups, adult males with an estrus female and adult female with another female (same Ns as above). Data are presented as mean+/-SEM and individual points (non-paired Wilcoxon tests: *p <0.05, **p <0.01, ***p <0.001). Please click here to view a larger version of this figure.
Figure 4: Acoustic variables extracted from ultrasonic vocalizations in Shank2-/- mice and wild-type littermates. (A) Duration of all call types confounded emitted by P2 pups (n = 20 Shank2+/+, n = 18 Shank2-/-), P6 pups (n = 19 Shank2+/+, n = 18 Shank2-/-), P10 pups (n = 20 Shank2+/+, n = 18 Shank2-/-), adult males with an estrus female (n = 16 Shank2+/+, n = 16 Shank2-/-) and adult females with another female (n = 15 Shank2+/+, n = 13 Shank2-/-) in wild-type mice (left panel) and Shank2-/- mice (right panel). (B) Maximum peak frequency measured on all call types confounded in P2 pups, P6 pups, P10 pups, adult males with an estrus female and adult female with another female (same Ns as above). Data are presented as mean+/-SEM and individual points (non-paired Wilcoxon tests: *p <0.05, **p <0.01, ***p <0.001). Please click here to view a larger version of this figure.
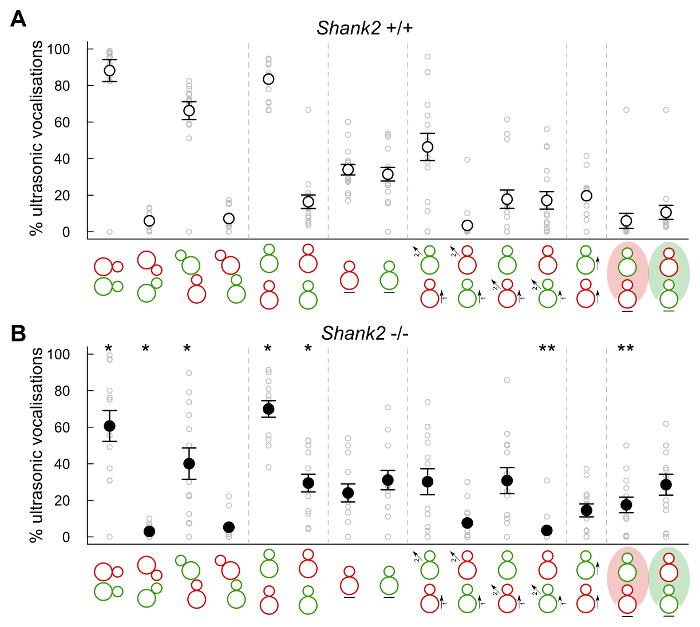 Figure 5: Contexts of emission of mouse ultrasonic vocalizations in female-female adult social interactions. Proportion of ultrasonic vocalizations emitted by pairs involving a Shank2+/+ with a C57BL/6N mouse (n = 16, A) and pairs involving a Shank2-/- with a C57BL/6N mouse (n = 13, B) during the following types of behavioral events (red: occupant, green: new-comer): social contacts, oro-oral contact, ano-genital sniffing from the occupant mouse, ano-genital sniffing from the new-comer mouse, occupant behind new-comer, new-comer behind occupant, immobility of occupant, immobility of new-comer, approach from the occupant & escape from the new-comer, approach from the new-comer & escape from the occupant, approach & escape from the occupant, approach & escape from the new-comer, occupant following the new-comer, new-comer in the vision field of occupant, occupant in the vision field of new-comer. Data are presented as mean+/-SEM and individual points (non-paired Wilcoxon tests: *p <0.05, **p <0.01). Unpublished data. Please click here to view a larger version of this figure.
Figure 5: Contexts of emission of mouse ultrasonic vocalizations in female-female adult social interactions. Proportion of ultrasonic vocalizations emitted by pairs involving a Shank2+/+ with a C57BL/6N mouse (n = 16, A) and pairs involving a Shank2-/- with a C57BL/6N mouse (n = 13, B) during the following types of behavioral events (red: occupant, green: new-comer): social contacts, oro-oral contact, ano-genital sniffing from the occupant mouse, ano-genital sniffing from the new-comer mouse, occupant behind new-comer, new-comer behind occupant, immobility of occupant, immobility of new-comer, approach from the occupant & escape from the new-comer, approach from the new-comer & escape from the occupant, approach & escape from the occupant, approach & escape from the new-comer, occupant following the new-comer, new-comer in the vision field of occupant, occupant in the vision field of new-comer. Data are presented as mean+/-SEM and individual points (non-paired Wilcoxon tests: *p <0.05, **p <0.01). Unpublished data. Please click here to view a larger version of this figure.
| Call types | Description |
| short | duration ≤5 msec and frequency range ≤6.25 kHz |
| simple | duration >5 msec and frequency range ≤6.25 kHz (flat), or frequency modulation in only one direction (upward or downward) with frequency range >6.25 kHz |
| complex | frequency modulations in more than one direction and frequency range >6.25 kHz (modulated), or inclusion of one or more additional frequency component (harmonic or non-linear phenomena, but no saturation) but no constraint on frequency range (complex) |
| frequency jumps | inclusion of one jump (one frequency jump) or more jumps (frequency jumps, others) in frequency without time gap between the consecutive frequency components, with (mixed) or without any noisy part within the pure tone call |
| unstructured | no pure tone component identifiable; “noisy” calls |
Table 1: Characteristics of five types of mouse ultrasonic vocalizations. Examples of criteria of duration, frequency range, frequency modulations and frequency jumps used to determine 5 different call types within mouse ultrasonic vocalizations.
Discussion
The protocol presented here provides standardized and reliable ways to collect mouse ultrasonic vocalizations in the laboratory. These very constrained situations present the advantage of standardization. They are used with success to compare strains or genotypes within strains18,19,26,27. As presented in the representative results, these methods allow the identification of atypical social communication in mice mutated for Shank2, a gene associated with autism spectrum disorders. Comparisons between mouse strains, between different contexts or even between laboratories will be triggered by the availability of larger datasets on the mouseTube database. This tool should boost studies on mouse ultrasonic vocalizations by allowing multivariate analyzes.
The protocols described here are optimized to test mice of different genotypes within a strain, as it is done in the majority of studies modeling the genetic contribution to neuropsychiatric disorders. It is recommended to experimentally design each study to have the best controls possible. Indeed, litter effects might mask or artificially inflate genetic effects28,29. It is therefore advisable to include littermate controls for each genotype. Breeding heterozygous parents should therefore be favored, since it will allow the correct matching of mutant and control mice within a litter. This justifies the paw tattoo marking of all pups (blinded to genotype) to track individuals throughout the recordings every two days. Genotyping is done at weaning, by taking tail samples. When recording pup isolation calls from P2 on, we would not recommend taking tail samples already in pups, since this operation includes supplementary manipulation and stress very close in time to a recording session.
The protocols suggested here to elicit ultrasonic vocalizations in adults does not allow clear identification of the emitter of the vocalizations. This explains why we manipulate the motivation of the test animal. Indeed, the test mice are isolated and not the new-comer and the test animals habituate for a long time to the test cage during same-sex interactions. In male-female interactions, the introduced female is not isolated and the test male habituates for shorter time since motivation might be higher in this sexual context. These manipulations of motivation should maximize the probability of the test mouse emitting the vocalizations and not the introduced one. To record male ultrasonic vocalizations in a sexual context, a simple cotton swab with fresh (i.e., not frozen) urine of an estrus female can also be introduced in the cage30. This method allows the assignment of ultrasonic vocalizations to the test male with 100% certainty but it prevents collecting any specific information about the actual social context of emission of these vocalizations. Therefore, we favor the protocol described here (with a freely-moving estrus female). We also recommend to always use introduced mice from the same strain when testing mice from a mutant strain and to analyze the data as a pair of mice vocalizing. One recent study promotes the use of triangulation to localize the emitter31. In this study, females were found to also emit ultrasonic vocalizations during encounters with a male. This might be explained by the fact that they were isolated for at least two weeks before the recording session. The generalization of the use of the triangulation proposed in this study should nevertheless allow identification of the emitter of the vocalizations in most cases if video recordings are properly synced.
The isolation calls from pups recorded during development are not disturbed by background noise from the bedding. Usually an automatic analysis works very well to extract the main variables. In contrast, vocalizations recorded from adults are disturbed by background noise from the animals moving in the bedding. Automatic analysis might fail, and therefore manual analysis should be used. Nevertheless, adding bedding in the test cage should provide conditions that are less stressful for the animals than bare soil (that mice do not like). Further efforts in the community are concentrated on improving the automatic detection of ultrasonic vocalizations under various conditions, even those implying background noises. For instance, the VoICE software allows to analyze vocalizations that had been manually selected for the absence of background noise32. In this software, the extraction of the acoustic variables is automatic but needs the initial manual selection.
It should be noted that the inter-individual variability is very important in the vocal behavior of mice. For instance, the call rate of adult males in presence of an estrus female is very distributed (Figure 1). We suggest these standardized protocols to elicit ultrasonic vocalizations already to limit the variability related to the experimental context. Nevertheless, we would like to point out the importance of presenting not only the mean and SEM for the data, but most importantly the individual points in samples of small size33. It is also very relevant — if not necessary — to record at least 12 individuals of each group/genotype to gather representative data. In many cases, the inter-individual variability should not be hidden (usually it cannot be), and it might be of high importance to identify individuals carrying the genetic mutation studied but not displaying any atypical phenotype. Such individuals could provide clues about compensations, which might open new pathways for therapies of genetic disorders.
In most behavioral characterizations of mouse models for neuropsychiatric disorders, vocal behavior and social contacts are considered apart (e.g., 19,27,34,35). Recent analysis methods now provide a semi-automatic detailed characterization of the social events and sequences of events during an interaction (using MiceProfiler for instance)36, as well as the possibility to combine this analysis with data from audio recordings. The main advantage of this method is to provide a comprehensive view of the social communication in mouse models of ASD, to more precisely identify which aspects of social communication are affected. In the present protocol the synchronization is still manual but this can be improved by triggering the video recording through the audio recording software. This type of analyses should become the standard to provide a more comprehensive view of social communication deficits in mouse models of neuropsychiatric disorders. In addition, up to now, vocal signals are mostly analyzed from the emitter side (i.e., tests are built to favor the emission of vocal signals by the tested mouse, as in the present protocols). The focus should now also be set on the receiver of these signals, to better identify the functions of these acoustic signals. This should be done by evaluating also the behavior of the new-comer mice in the present protocols in adults (using MiceProfiler for instance)36, by using playback experiments16, or by setting up new protocols. Indeed, the present protocols provide very constrained situations that might not reflect the exact ethological conditions of vocalization emission in mice. The spontaneous emission of ultrasonic vocalizations will have to be better-characterized using continuous audio and video recordings to shed more light on the spontaneous vocal behavior of mice.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the Fondation de France; by the ANR FLEXNEURIM [ANR09BLAN034003]; by the ANR [ANR- 08-MNPS-037-01-SynGen]; by Neuron-ERANET (EUHF-AUTISM); by the Fondation Orange; by the Fondation FondaMentale; by the Fondation de France; by the Fondation Bettencourt-Schueller. The research leading to this article has also received support from the Innovative Medicine Initiative Joint Undertaking under grant agreement no. 115300, resources of which are composed of financial contribution from the European Union's Seventh Framework Program (FP7/2007-2013) and EFPIA companies' in kind contribution. We thank Julie Lévi-Strauss for helpful comments on the manuscript and six anonymous reviewers whose comments noticeably improved the manuscript.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) 2013.
- Ey E, Leblond CS, Bourgeron T. Behavioral Profiles of Mouse Models for Autism Spectrum Disorders. Autism Res. 2011;4(1):5–16. doi: 10.1002/aur.175. [DOI] [PubMed] [Google Scholar]
- Bourgeron T, Jamain S, Granon S. Animal Models of Autism - Proposed Behavioral Paradigms and Biological Studies. Contemporary Clinical Neuroscience: Transgenic and Knockout Models of Neuropsychiatric Disorders. 2006. pp. 151–174.
- Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: A tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2009;33(4):508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV, Perkel DJ. The role of ultrasonic vocalizations in mouse communication. Cur. Opin. Neurobiol. 2014;28:115–120. doi: 10.1016/j.conb.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriaga G, Zhou EP, Jarvis ED. Of mice, birds, and men: the mouse ultrasonic song system has some features similar to humans and song-learning birds. PLOS ONE. 2012;7:e46610. doi: 10.1371/journal.pone.0046610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt K, Schreiweis C, Minge C, Pääbo S, Fischer J, Enard W. A humanized version of Foxp2 does not affect ultrasonic vocalization in adult mice: Ultrasonic vocalization of "humanized" FoxP2 mice. Genes Brain Behav. 2015. [DOI] [PubMed]
- Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J. Am. Assoc. Lab. Anim. Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- Panksepp JB, et al. Affiliative Behavior, Ultrasonic Communication and Social Reward Are Influenced by Genetic Variation in Adolescent Mice. PLOS ONE. 2007;2 doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippelius H-M, Schleidt WM. Ultraschall-Laute bei jungen Mäusen. Naturwissenschaften. 1956;43:502. [Google Scholar]
- Whitney G, Coble JR, Stockton MD, Tilson EF. Ultrasonic emissions: do they facilitate courtship of mice. J. Comp. Physiol. Psychol. 1973;84:445–452. doi: 10.1037/h0034899. [DOI] [PubMed] [Google Scholar]
- Holy TE, Guo ZS. Ultrasonic songs of male mice. PLOS Biol. 2005;3:2177–2186. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio JC, Whitney G. Ultrasonic vocalizing by adult female mice (Mus musculus) J. Comp. Psychol. 1985;99:420–436. [PubMed] [Google Scholar]
- Chabout J, et al. Adult Male Mice Emit Context-Specific Ultrasonic Vocalizations That Are Modulated by Prior Isolation or Group Rearing Environment. PLOS ONE. 2012;7(1):e29401. doi: 10.1371/journal.pone.0029401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. Female mice respond to male ultrasonic "songs" with approach behaviour. Biol. Lett. 2009;5:589–592. doi: 10.1098/rsbl.2009.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Moles A, Schwarting RKW, D'Amato FR. Lack of social exploratory activation in male -opioid receptor KO mice in response to playback of female ultrasonic vocalizations. Soc. Neurosci. 2011;6:76–87. doi: 10.1080/17470911003765560. [DOI] [PubMed] [Google Scholar]
- Granon S, Faure P, Changeux J-P. Executive and social behaviors under nicotinic receptor regulation. Proc. Nat. Acad. Sci. 2003;100(16):9596–9601. doi: 10.1073/pnas.1533498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser MJ, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486(7402):256–260. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- Ey E, et al. Absence of Deficits in Social Behaviors and Ultrasonic Vocalizations in Later Generations of Mice Lacking Neuroligin4. Genes Brain Behav. 2012;11:928–941. doi: 10.1111/j.1601-183X.2012.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK, et al. Minimal Aberrant Behavioral Phenotypes of Neuroligin-3 R451C Knockin Mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual Repertoire of Vocalizations in the BTBR T plus tf/J Mouse Model of Autism. PLOS ONE. 2008;3 doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Chaumont F, Coura RD-S, et al. Computerized video analysis of social interactions in mice. Nat. Methods. 2012;9:410–417. doi: 10.1038/nmeth.1924. [DOI] [PubMed] [Google Scholar]
- Leblond CS, et al. Genetic and Functional Analyses of SHANK2 Mutations Suggest a Multiple Hit Model of Autism Spectrum Disorders. PLOS Genet. 2012;8(2):e1002521. doi: 10.1371/journal.pgen.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkel S, et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat. Genet. 2010;42:489–491. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- Pinto D, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey E, et al. The autism ProSAP1/Shank2 mouse model displays quantitative and structural abnormalities in ultrasonic vocalisations. Behav. Brain Res. 2013;256:677–689. doi: 10.1016/j.bbr.2013.08.031. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T plus tf/J mice during three types of social encounters. Genes Brain Behav. 2010;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev. Psychobiol. 1997;30(2):141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lazic SE, Essioux L. Improving basic and translational science by accounting for litter-to-litter variation in animal models. BMC Neurosci. 2013;14(1):37. doi: 10.1186/1471-2202-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F, Musolf K, Penn DJ. Freezing urine reduces its efficacy for eliciting ultrasonic vocalizations from male mice. Physiol. Behav. 2009;96:602–605. doi: 10.1016/j.physbeh.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Neunuebel JP, Taylor AL, Arthur BJ, Egnor SR. Female mice ultrasonically interact with males during courtship displays. eLife. 2015;4 doi: 10.7554/eLife.06203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett ZD, Day NF, Peñagarikano O, Geschwind DH, White SA. VoICE: A semi-automated pipeline for standardizing vocal analysis across models. Sci. Rep. 2015;5:10237. doi: 10.1038/srep10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissgerber TL, Milic NM, Winham SJ, Garovic VD. Beyond Bar and Line Graphs: Time for a New Data Presentation Paradigm. PLOS Biol. 2015;13(4):e1002128. doi: 10.1371/journal.pbio.1002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Nat. Acad. Sci. U. S. A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won H, et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- Ferhat A-T, Le Sourd A-M, de Chaumont F, Olivo-Marin J-C, Bourgeron T, Ey E. Social Communication in Mice - Are There Optimal Cage Conditions? PLOS ONE. 2015;10(3):e0121802. doi: 10.1371/journal.pone.0121802. [DOI] [PMC free article] [PubMed] [Google Scholar]


