Abstract
TRUE gene silencing (termed after tRNase ZL-utilizing efficacious gene silencing) is one of the RNA-directed gene silencing technologies, which utilizes an artificial small guide RNA (sgRNA) to guide tRNA 3′ processing endoribonuclease, tRNase ZL, to recognize a target RNA. sgRNAs can be taken up by cells without any transfection reagents and can downregulate their target RNA levels and/or induce apoptosis in human cancer cells. We have screened an sgRNA library containing 156 heptamer-type sgRNAs for the effect on viability of human myeloma and leukemia cells, and found that 20 of them can efficiently induce apoptosis in at least one of the cancer cell lines. Here we present a protocol for screening of a heptamer-type sgRNA library for potential therapeutic drugs against blood cancers. The protocol includes how to construct the sgRNA library, how to assess the effect of each sgRNA on cell viability, and how to further evaluate the effective sgRNAs by flow cytometry. Around 2,000 hits would be expected to be obtained by screening the full-scale sgRNA library composed of 16,384 heptamers.
Keywords: Medicine, Issue 112, TRUE gene silencing, tRNase ZL, heptamer-type sgRNA, sgRNA library, screening, RNA therapy, cancer, therapeutic agent
Introduction
TRUE gene silencing (termed after tRNase ZL-utilizing efficacious gene silencing) is one of the RNA-directed gene silencing technologies1. This technology has been developed based on properties of tRNase ZL (or 3′ tRNase), tRNA 3′ processing endoribonuclease: it can cleave any target RNA at any expected site under the direction of an artificial small guide RNA (sgRNA) by recognizing a pre-tRNA-like or micro-pre-tRNA-like structure formed between the target RNA and the sgRNA2-9. sgRNA, which is usually 7-31 nt in length, is categorized into four groups, 5′-half-tRNA, heptamer RNA, 14-nt linear RNA, and hook RNA.
We have demonstrated the efficacy of TRUE gene silencing by introducing various artificially-designed sgRNAs into living cells10-15. We have also shown that sgRNAs can be taken up by cells without any transfection reagents and can downregulate their target RNA levels and/or induce apoptosis in human cancer cells16-18. TRUE gene silencing appears to work on the intracellular and intercellular gene regulatory network system via tRNase ZL and natural sgRNAs19-21.
We have constructed an sgRNA library containing 156 heptamer-type sgRNAs to search for potential therapeutic heptamer-type sgRNAs for blood cancers22. We have screened it for the effect on viability of human myeloma and leukemia cells, and found that 20 of them can efficiently induce apoptosis in at least one of the cancer cell lines. And 4 of them have been shown to reduce tumor growth rates in mouse xenograft experiments.
Here we present a protocol for screening of a heptamer-type sgRNA library for potential therapeutic drugs against blood cancers. The protocol includes how to construct the sgRNA library, how to assess the effect of each sgRNA on cell viability, and how to further evaluate the effective sgRNAs by flow cytometry. Analysis for sgRNA's cellular target RNAs and evaluation of effective sgRNAs in mouse xenograft models can be performed according to conventional methods17,22, although these are not included in this protocol.
Protocol
1. Construction of a Heptamer-type sgRNA Library
Select a subset of the 16,384 (47) 7-nt sequences unless the full-scale library is to be constructed. NOTE: The sequences can be random and/or designed to target specific cellular RNAs. For the latter, find hairpin structures resembling the T-arm of tRNA in a target RNA with the aid of an appropriate computer program and/or visually, and select sequences complementary to 7-nt sequences immediately downstream of the hairpin structures4,10,17,18.
Synthesize each of the selected heptamers as a fully 2′-O-methylated, 5′- and 3′-phosphorylated RNA with a DNA/RNA synthesizer. Use the phosphoramidite method on the controlled-pore glass (CPG) support, and leave the 5′-terminal 4,4′-dimethoxytrityl (DMT) protecting group on in the last cycle (the addition of the 5′ phosphate) of the synthesis23. NOTE: Custom synthetic RNA can be also obtained from oligonucleotide suppliers.
After completion of the last synthesis cycle, transfer the CPG with the synthesized oligonucleotide from the column to a glass vial with a Teflon-liner screw cap, add 1 ml of 29% ammonium hydroxide, and incubate it at 55 °C for 8 hr to cleave the oligonucleotide from the CPG and remove the protecting groups from the bases and phosphates.
Evaporate ammonium hydroxide with a centrifugal evaporator at 40 °C and re-dissolve the oligonucleotide in 0.2 ml of 0.1 M n-hexylammonium acetate.
Purify the synthetic oligonucleotide through reverse-phase high-performance liquid chromatography using a polystyrene-divinylbenzene column with a buffer containing 10-50% acetonitrile/0.1 M n-hexylammonium acetate. Run the column at 2.0 ml/min for 25 min at 80 °C.
Add 0.25-0.5 ml of concentrated acetic acid to the fractionated sample (1-2 ml) to make a 20% acetic acid solution, and incubate this solution for 1 hr at room temperature to remove the DMT group.
Dry the sample with a centrifugal evaporator at 40 °C, re-dissolve it in 1 ml of 29% ammonium hydroxide, and incubate the solution for 15 min at room temperature to remove the side chain of the 5′ phosphate.
Reduce the volume (1 ml) of the sample to about 0.3 ml with a centrifugal evaporator at 40 °C.
Dialyze the entire concentrated sample against distilled water (500 ml) using a dialysis tube of molecular weight cut-off 1,000 at 4 °C overnight.
Transfer the dialyzed sample from the dialysis tube to a 1.5-ml tube with a pipette, dry it with a centrifugal evaporator at 40 °C, and subsequently re-dissolve it in water-for-injection-grade water, the volume of which should be small enough to make a greater than 100 µM solution.
Measure an optical density of the RNA sample at 260 nm with a spectrophotometer and adjust its concentration to 100 µM by adding water-for-injection-grade water. Store the RNA solution at below -20 °C before use.
2. Screening of the sgRNA Library for Cell Viability
Prepare 103 cells/99 μl/well (e.g., HL60 and RPMI-8226) on a 96-well dish in RPMI-1640 media supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin solution. Prepare wells containing the media only for background subtraction. To eliminate edge effects, add sterile water to empty wells surrounding the sample wells.
Add 1 μl of heptamer-type sgRNA (100 μM) dissolved in water-for-injection-grade water to each well. Assay each sgRNA in triplicate. Incubate the plate at 37 °C in a 5% CO2 humidified incubator for 3 days.
Add an appropriate volume of a commercially available WST-8 solution to each well. Incubate the plate at 37 °C in the same incubator for 1 to 4 hr.
Measure absorbance at 450 nm with a microplate reader. NOTE: In most cases, linearity between absorbance and viable cell number appears to be maintained unless the absorbance value exceeds 1.0.
3. Evaluation of Effective sgRNAs by Flow Cytometry
Prepare 103 cells/99 µl/well (e.g., HL60) on a 96-well dish in RPMI-1640 media supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin solution. Prepare at least 10 wells for one sgRNA to be tested. To eliminate edge effects, add sterile water to empty wells surrounding the sample wells.
Add 1 µl of heptamer-type sgRNA (100 µM) dissolved in water-for-injection-grade water to each well. Incubate the plate at 37 °C in a 5% CO2 humidified incubator for 3 days.
Collect the cells treated with the same sgRNA from at least 10 wells into a 5-ml tube with a pipette. Spin the tube for 5 min in a centrifuge at 300 x g, and discard the media.
Add 2 ml of 1x phosphate buffered saline (PBS) to the tube, and re-suspend the cells. Spin the tube for 5 min in a centrifuge at 300 x g, and discard PBS. Add 150 µl of 1x annexin V binding buffer to the tube, and re-suspend the cells.
Add 2 µl of phycoerythrin (PE)-conjugated annexin V solution and 1 µl of 7-aminoactinomycin D (7AAD) solution to the tube, mix them well, and incubate the tube for 15 min at room temperature in the dark. Analyze the sample using a flow cytometer24.
Representative Results
The six heptamer-type sgRNAs 5′-AUCUUCA-3′ (H1885), 5′-ACACACA-3′ (H3277), 5′-GGGGGCG-3′ (H10927), 5′-GGGGCCC-3′ (H10944), 5′-GCCCCCG-3′ (H12287), and 5′-CACCAGC-3′ (H13260) were chemically synthesized as 2′-O-methyl RNA containing 5′- and 3′-phosphates.
These sgRNAs were examined for the effects on viability of a human leukemia cell line, HL60, and a human myeloma cell line, RPMI-8226. Representative results of the cell viability assays are shown in Figure 1. The four sgRNAs H1885, H3277, H10927, and H13260 were very effective and reduced the viability of HL60 and RPMI-8226 cells by >80% and >70%, respectively. The heptamers H3277 and H10927 were also tested for the effects on non-cancerous HEK293 cell viability, and have been shown to hardly affect the cell viability22.
The flow cytometry with annexin V and 7AAD double staining was performed for HL60 cells treated with the heptamer H1885, H3277, H10927, or H13260. In each sgRNA, a total cell number (56-95%) in early and late apoptotic stages was much higher than that (4-6%) in mock (Figure 2). These observations suggest that the reduction of HL60 cell viability is due to apoptosis.
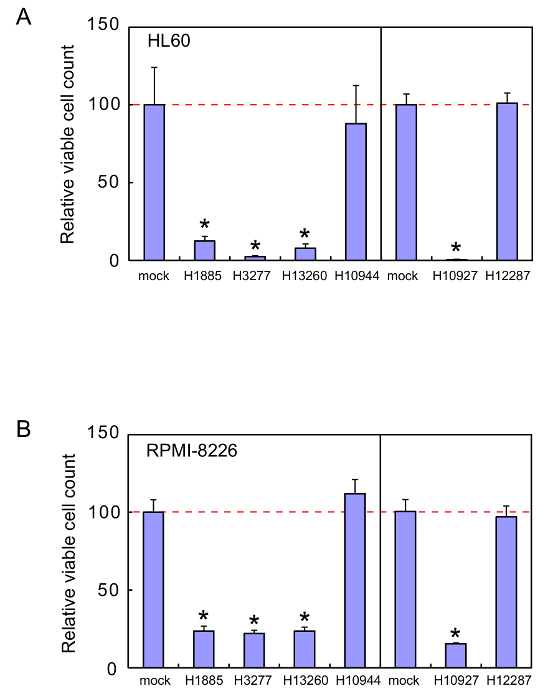 Figure 1. Cell viability assays. Cell viability was measured 72 hr after HL60 cells (A) or RPMI-8226 cells (B) were cultured in the absence or presence of 1 µM of the naked heptamer-type sgRNA H1885, H3277, H10927, H10944, H12287, or H13260. The relative living cell numbers in the absence of sgRNAs are adjusted to 100. Error bars indicate SD (n = 3). *, P < 0.002. Reprinted from Leukemia Research, Vol. 38, Takahashi M. et al., Screening of a heptamer-type sgRNA library for potential therapeutic agents against hematological malignancies, pp 808-815, Fig. 1, Copyright (2014), with permission from Elsevier. Please click here to view a larger version of this figure.
Figure 1. Cell viability assays. Cell viability was measured 72 hr after HL60 cells (A) or RPMI-8226 cells (B) were cultured in the absence or presence of 1 µM of the naked heptamer-type sgRNA H1885, H3277, H10927, H10944, H12287, or H13260. The relative living cell numbers in the absence of sgRNAs are adjusted to 100. Error bars indicate SD (n = 3). *, P < 0.002. Reprinted from Leukemia Research, Vol. 38, Takahashi M. et al., Screening of a heptamer-type sgRNA library for potential therapeutic agents against hematological malignancies, pp 808-815, Fig. 1, Copyright (2014), with permission from Elsevier. Please click here to view a larger version of this figure.
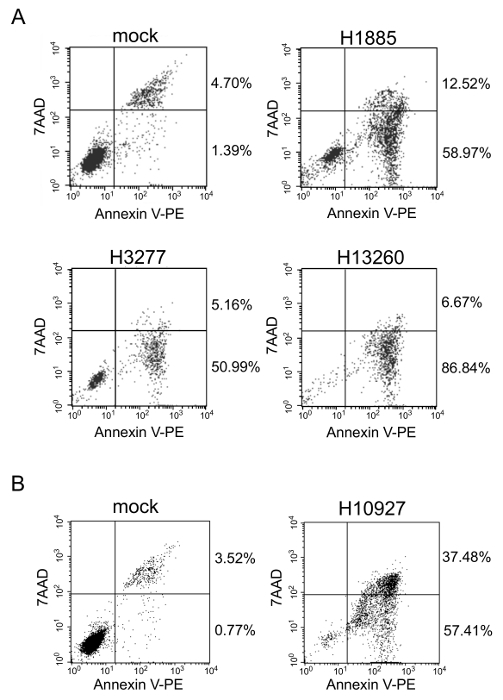 Figure 2. Flow cytometry. With respect to HL60 cells, flow cytometry was performed with annexin V and 7AAD double staining. The cells were analyzed 72 hr after cultured in the absence or presence of 1 µM of the naked heptamer-type sgRNA H1885 (A), H3277 (A), H13260 (A), or H10927 (B). Reprinted from Leukemia Research, Vol. 38, Takahashi M. et al., Screening of a heptamer-type sgRNA library for potential therapeutic agents against hematological malignancies, pp 808-815, Fig. 2, Copyright (2014), with permission from Elsevier. Please click here to view a larger version of this figure.
Figure 2. Flow cytometry. With respect to HL60 cells, flow cytometry was performed with annexin V and 7AAD double staining. The cells were analyzed 72 hr after cultured in the absence or presence of 1 µM of the naked heptamer-type sgRNA H1885 (A), H3277 (A), H13260 (A), or H10927 (B). Reprinted from Leukemia Research, Vol. 38, Takahashi M. et al., Screening of a heptamer-type sgRNA library for potential therapeutic agents against hematological malignancies, pp 808-815, Fig. 2, Copyright (2014), with permission from Elsevier. Please click here to view a larger version of this figure.
Discussion
In most cases, fully 2′-O-methylated, 5′- and 3′-phosphorylated heptamer-type sgRNAs dissolved in water-for-injection-grade water (in the concentration of 100 µM) appear to be stable at below -20 °C for at least one year, judging from their activity to induce apoptosis in cancer cells. However, their stability would change depending on their sequence, quality, and purity. In some cases, 5′- or 3′-phosphate of a part of sgRNA molecules appears to be removed spontaneously during storage, judging from mass spectrometry analysis, although this may not affect their activity seriously.
When a hit sgRNA is obtained by the library screening, its cellular target RNAs can be found by transcriptome analysis through a DNA microarray and/or next-generation sequencing. The data obtained so far, however, suggest that downregulation levels of target RNAs via heptamer-type sgRNAs are usually moderate regardless of the presence or absence of transfection reagents10,12,14,17,18.
The advantage of heptamer-type sgRNAs targeting BCL2 and WT1 mRNAs is that they can efficiently induce apoptosis in human leukemia cells in spite of weak downregulation of the target mRNAs17,18, suggesting the presence of additional RNA targets and/or additional mechanisms other than TRUE gene silencing.
Since even the screening of the library containing only 156 heptamer-type sgRNAs can make 20 hits, around 2,000 hits would be expected to be obtained by screening the full-scale library composed of 16,384 heptamers22,25.
For future applications, sgRNAs other than the heptamer type can be more suitable for TRUE gene silencing11-13,15,16, although heptamer-type sgRNAs are easier, more accurate and cheaper to synthesize than longer sgRNAs. Once the therapeutic sgRNA candidate is obtained, it is easy to proceed to animal model experiments.
Disclosures
Niigata University of Pharmacy and Applied Life Sciences has a pending patent application on a subset of the heptamer-type sgRNAs.
Acknowledgments
This work was supported by Adaptable and Seamless Technology Transfer Program through Target-driven R&D, Japan Science and Technology Agency, the Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private Schools of Japan, and the JSPS KAKENHI Grant Numbers 24300342 and 15H04313.
References
- Scherer L, Rossi JJ. Therapeutic potential of RNA-mediated control of gene expression: Options and designs. In: Morris KV, editor. RNA and the Regulation of Gene Expression: A hidden layer of complexity. Caister Academic Press; 2008. pp. 201–226. [Google Scholar]
- Nashimoto M. Conversion of mammalian tRNA 3′ processing endoribonuclease to four-base-recognizing RNA cutters. Nucleic Acids Res. 1995;23:3642–3647. doi: 10.1093/nar/23.18.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashimoto M. Specific cleavage of target RNAs from HIV-1 with 5′ half tRNA by mammalian tRNA 3′ processing endoribonuclease. RNA. 1996;2:523–534. [PMC free article] [PubMed] [Google Scholar]
- Nashimoto M, Geary S, Tamura M, Kasper R. RNA heptamers that directs RNA cleavage by mammalian tRNA 3′ processing endoribonuclease. Nucleic Acids Res. 1998;26:2565–2571. doi: 10.1093/nar/26.11.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashimoto M. Anomalous RNA substrates for mammalian tRNA 3′ processing endoribonuclease. FEBS Lett. 2000;472:179–186. doi: 10.1016/s0014-5793(00)01462-9. [DOI] [PubMed] [Google Scholar]
- Takaku H, Minagawa A, Takagi M, Nashimoto M. A candidate prostate cancer susceptibility gene encodes tRNA 3′ processing endoribonuclease. Nucleic Acids Res. 2003;31:2272–2278. doi: 10.1093/nar/gkg337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaku H, Minagawa A, Takagi M, Nashimoto M. The N-terminal half-domain of the long form of tRNase Z is required for the RNase 65 activity. Nucleic Acids Res. 2004;32:4429–4438. doi: 10.1093/nar/gkh774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaku H, Minagawa A, Takagi M, Nashimoto M. A novel four-base-recognizing RNA cutter that can remove the single 3′ terminal nucleotides from RNA molecules. Nucleic Acids Res. 2004;32:e91. doi: 10.1093/nar/gnh092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata HS, Takaku H, Takagi M, Nashimoto M. The T loop structure is dispensable for substrate recognition by tRNase ZL. J. Biol. Chem. 2005;280:22326–22334. doi: 10.1074/jbc.M502048200. [DOI] [PubMed] [Google Scholar]
- Tamura M, Nashimoto C, Miyake N, Daikuhara Y, Ochi K, Nashimoto M. Intracellular mRNA cleavage by 3′ tRNase under the direction of 2′-O-methyl RNA heptamers. Nucleic Acids Res. 2003;31:4354–4360. doi: 10.1093/nar/gkg641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habu Y, et al. Inhibition of HIV-1 gene expression by retroviral vector-mediated small-guide RNAs that direct specific RNA cleavage by tRNase ZL. Nucleic Acids Res. 2005;33:235–243. doi: 10.1093/nar/gki164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A, et al. Gene-silencing by the tRNA maturase tRNase ZL under the direction of small guide RNA. Gene Ther. 2007;14:78–85. doi: 10.1038/sj.gt.3302841. [DOI] [PubMed] [Google Scholar]
- Elbarbary RA, Takaku H, Tamura M, Nashimoto M. Inhibition of vascular endothelial growth factor expression by TRUE gene silencing. Biochem. Biophys. Res. Commun. 2009;379:924–927. doi: 10.1016/j.bbrc.2008.12.173. [DOI] [PubMed] [Google Scholar]
- Sano T, Takahashi M, Nozaki T, Takahashi Y, Tamura M, Nashimoto M. Expanding the utility of heptamer-type sgRNA for TRUE gene silencing. Biochem. Biophys. Res. Commun. 2011;416:427–432. doi: 10.1016/j.bbrc.2011.11.091. [DOI] [PubMed] [Google Scholar]
- Iizuka S, Oridate N, Nashimoto M, Fukuda S, Tamura M. Growth inhibition of head and neck squamous cell carcinoma cells by sgRNA targeting the cyclin D1 mRNA based on TRUE gene silencing. PLoS One. 2014;9(114121) doi: 10.1371/journal.pone.0114121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, et al. Elimination of specific miRNAs by naked 14-nt sgRNAs. PLoS One. 2012;7(38496) doi: 10.1371/journal.pone.0038496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, et al. A naked RNA heptamer targeting the human Bcl-2 mRNA induces apoptosis of HL60 leukemia cells. Cancer Lett. 2013;328:362–368. doi: 10.1016/j.canlet.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Watanabe N, et al. Induction of apoptosis of leukemic cells by TRUE gene silencing using small guide RNAs targeting the WT1 mRNA. Leukemia Res. 2013;37:580–585. doi: 10.1016/j.leukres.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Elbarbary RA, et al. Modulation of gene expression by human cytosolic tRNase ZL through 5′-half-tRNA. PLoS One. 2009;4:5908. doi: 10.1371/journal.pone.0005908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbarbary RA, et al. Human cytosolic tRNase ZL can downregulate gene expression through miRNA. FEBS Lett. 2009;583:3241–3246. doi: 10.1016/j.febslet.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Ninomiya S, et al. Potential small guide RNAs for tRNase ZL from human plasma, peripheral blood mononuclear cells, and cultured cell lines. PLoS One. 2015;10:0118631. doi: 10.1371/journal.pone.0118631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, et al. Screening of a heptamer-type sgRNA library for potential therapeutic agents against hematological malignancies. Leukemia Res. 2014;38:808–815. doi: 10.1016/j.leukres.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Solid-phase oligonucleotide synthesis. ATD Bio. 2015. Available from: http://www.atdbio.com/content/17/Solid-phase-oligonucleotide-synthesis.
- FACSCalibur Instructions For Use. BD Bio. 2007. Available from: http://www.bdbiosciences.com/documents/BD_FACSCalibur_instructions.pdf.
- Shivarov V. TRUE gene silencing for hematologic malignancies. Leukemia Res. 2014;38:729. doi: 10.1016/j.leukres.2014.04.014. [DOI] [PubMed] [Google Scholar]


