Abstract
Mucins, the heavily-glycosylated proteins lining mucosal surfaces, have evolved as a key component of innate defense by protecting the epithelium against invading pathogens. The main role of these macromolecules is to facilitate particle trapping and clearance while promoting lubrication of the mucosa. During protein synthesis, mucins undergo intense O-glycosylation and multimerization, which dramatically increase the mass and size of these molecules. These post-translational modifications are critical for the viscoelastic properties of mucus. As a result of the complex biochemical and biophysical nature of these molecules, working with mucins provides many challenges that cannot be overcome by conventional protein analysis methods. For instance, their high-molecular-weight prevents electrophoretic migration via regular polyacrylamide gels and their sticky nature causes adhesion to experimental tubing. However, investigating the role of mucins in health (e.g., maintaining mucosal integrity) and disease (e.g., hyperconcentration, mucostasis, cancer) has recently gained interest and mucins are being investigated as a therapeutic target. A better understanding of the production and function of mucin macromolecules may lead to novel pharmaceutical approaches, e.g., inhibitors of mucin granule exocytosis and/or mucolytic agents. Therefore, consistent and reliable protocols to investigate mucin biology are critical for scientific advancement. Here, we describe conventional methods to separate mucin macromolecules by electrophoresis using an agarose gel, transfer protein into nitrocellulose membrane, and detect signal with mucin-specific antibodies as well as infrared fluorescent gel reader. These techniques are widely applicable to determine mucin quantitation, multimerization and to test the effects of pharmacological compounds on mucins.
Keywords: Molecular Biology, Issue 112, Mucins, high-molecular-weight glycoproteins, SDS-agarose gel, electrophoresis, vacuum blotter, nitrocellulose membrane, double staining, fluorescence
Introduction
Mucins are normally produced by mucosal surfaces that line cavities exposed to the external environment (e.g., respiratory, digestive, reproductive tracts, ocular surface) as well as internal organs (e.g., pancreas, gallbladder, mammary glands). The presence of these glycoproteins maintains surface hydration and forms a physical barrier against pathogens. Although mucin production is essential to mucosal health, mucin hyperconcentration and/or aberrant mucus properties can lead to duct obstruction, bacterial colonization and chronic inflammation, which can cause irreversible tissue damage. A similar cascade of events are observed in several diseases, e.g., cystic fibrosis1, chronic otitis media2 and cervicovaginal infection3. Therefore, it is important to understand the role of mucins in health and disease and to establish routine protocols for protein identification.
To date, 19 mucins genes have been identified and encode for large polypeptide chains ranging from 1,200 (e.g., MUC1) to 22,000 (e.g., MUC16) amino acids. The mucin gene family can be divided into two subtypes: the membrane-associated mucins, involved in cell signaling and surface shielding, and the gel-forming mucins, responsible for the viscoelastic properties of mucus gels. Membrane-associated mucins are mostly monomeric and attach to the cell surfaces via a hydrophobic membrane-spanning domain. In contrast, gel-forming mucins possess several von Willebrand factor (vWF)-like and cysteine-rich domains that are essential for the formation of dynamic polymeric networks. Large glycans are attached to serine and threonine residues distributed throughout the apomucin. These dense O-linked oligosaccharides can contribute up to 80% of the molecular weight4. Intra- and inter-molecular disulfide bonds connecting mucin monomers ensure the integrity of the mucin gel network. As a result of heavy glycosylation and multimerization, mucins are among the largest molecules in the animal world and cannot be analyzed by standard gel electrophoresis using conventional SDS-PAGE polyacrylamide gel and standard protein ladders. These methods resolve/separate proteins with molecular weights lower than 250 kDa while mucin monomers can reach up to 2 MDa in the case of MUC16. However, high-molecular-weight protein ladders can be used to study small mucin monomers (i.e., MUC1).
A variety of techniques can be applied to study mucin size, conformation and interaction. Traditionally, biochemical characterization of mucins is accomplished by mucin isolation via isopycnic density-gradient centrifugation in denaturing buffer, followed by size-exclusion chromatography and immunodetection (e.g., slot blotting)5. Dynamic and/or multi-angle light scattering provide information on the oligomeric state of mucin-rich samples1. In addition, rate-zonal centrifugation coupled with immunodetection and transmission electron microscopy are commonly used to determine the macromolecular conformation of mucins6. Mass spectrometry is also used to quantify mucins, detect proteolytic cleavage and analyze oligosaccharide composition1,7,8. Such techniques are costly, time consuming and often require large volumes and/or high concentrations of sample. The methodology described herein, i.e., mucin separation by electrophoresis, is reproducible, low cost and can be used in high-throughput studies to provide relative mucin quantitation and investigate polymer assembly. However, this assay requires high-affinity, high-specificity mucin antibodies that may not be available for rare mucins (e.g., MUC19) or certain species (e.g., pig, ferret).
Agarose Western blotting is suitable to resolve a wide variety of mucin-rich samples with concentrations ranging from 50 µg/ml (e.g., cell washes) to 5 mg/ml (e.g., sputum). This assay was introduced in the 1990s and was only performed in few specialized laboratories9,10. Initially, this technique helped identify subpopulations of mucin monomers in human respiratory secretions11,12 and confirmed the oligomerization process in goblet cells, which consists of dimer formation in the endoplasmic reticulum followed by dimer multimerization in the Golgi apparatus13. More recently, the generation of polyclonal antibodies against murine mucins facilitated studies on small animal models (e.g., mucin deficient, βENaC, OVA-challenged mouse models) and opened a new field of research for preclinical studies testing pharmacological compounds aimed at removing mucus from the lungs14-17. As a result of an increasing interest in mucin biology and the generation of novel, more specific mucin antibodies, we describe herein the methodology to separate mucins by agarose gel electrophoresis, vacuum transfer to nitrocellulose and two-color infrared fluorescent detection.
Protocol
1. Prepare Buffers for Mucin Gel Western Blotting
- Prepare 1 liter of 50x TAE (Tris-acetate-EDTA) buffer.
- In 700 ml of distilled water (dH2O) add 242 g of Tris base (0.4 M), 57.1 g of glacial acetic acid (weigh liquid) (0.2 M) and 14.61 g of ethylenediaminetetraacetic acid (EDTA) (50 mM).
- Adjust pH to 8.0 and make the volume up to 1 liter with dH2O.
- Prepare 10 ml of 10x loading buffer.
- Prepare 5 ml of 1x TAE buffer. To do this, add 5 ml of glycerol (50%), 25 mg of bromophenol blue (0.25%), and 100 mg of sodium dodecyl sulfate (SDS) (1%).
- Prepare 2 liters of 20x sodium saline citrate (SSC) buffer.
- In 1.5 liters of dH2O, add 350.6 g of NaCl (3 M) and 176.4 g of trisodium citrate (Na3C6H5O7) (0.3 M). Adjust pH to 7.0 and make up the volume to 2 liters with dH2O.
- Prepare 1 liter of 1x TAE-0.1% SDS buffer.
- Add 20 ml of 50x TAE to 980 ml of dH2O. Add 1 g of SDS to make a 0.1% SDS-TAE solution.
2. TAE-SDS Agarose Gel Preparation
Pour sufficient volume (i.e., 150 ml) of 1x TAE-0.1% SDS buffer in a microwavable Erlenmeyer flask to make a 5 - 7 mm-thick gel. Add 0.8% (1.2 g) agarose powder.
Microwave flask in 30 sec intervals with intermittent swirling until agarose is completely dissolved (usually 1 - 3 min). Use hot hand pads during this process. Be careful of eruptive boiling while swirling.
Allow the agarose solution to cool down for 5 - 10 min. Note: The agarose solution will be ready to be poured when bottom of the flask can be left on the palm for 5 sec.
Prepare electrophoresis casting tray (10 x 15 cm) by sealing the edges with tape and placing well comb in position.
Pour agarose solution slowly in the casting tray to avoid the formation of bubbles. Remove bubbles using a pipette tip. Wait for the gel to cool and completely solidify. Once solidified, remove the comb slowly to avoid collapsing the wells by suction and remove the tape from the edges of the tray.
Place agarose gel into the gel box and fill the electrophoresis unit with 1x TAE-0.1% SDS buffer until the gel is fully covered.
3. Sample Loading and Electrophoresis Separation
Note: In our laboratory, we commonly use samples from both mouse and humans, including bronchoalveolar lavage fluid (BALF; both species), cell washings from human bronchial epithelial cells (HBE), and human saliva and sputum samples. Samples can be denatured in 6 M urea upon collection or stored for short periods of time (6 hr) by adding proteinase cocktail inhibitor.
Prepare samples for loading by adding 10% of loading dye to each sample (i.e., 30 µl of sample and 3 µl of dye). Homogenize samples by pipetting up and down. Briefly spin down tubes at 5,000 rpm to collect droplets.
- Carefully load equal volume of samples into the wells.
- Pre-wet tips and/or use positive displacement pipettes to facilitate loading of highly viscous samples. Slowly aspirate 80 - 90% of sample-dye solution (i.e., 27 - 30 µl) to avoid pipetting bubbles.
- Slowly load at the bottom of the wells and progressively move the pipet tip upward. For samples that remain attached to the pipette tip, dispense at a 45° angle and elongate straight above the well or gently use the side of the well to break the stringy mucus. Do not overload the wells.
Connect the electrodes from the gel box to the electrophoresis power generator. Ensure the electrodes are connected properly (i.e., red lead plugged to the positive output of the generator) to allow negatively charged mucins to run towards the positive electrode.
Run the gel at 80 Volts for 90 min for a single comb gel, or 60 - 75 min for a double comb gel to prevent overlap between the two rows.
Turn generator power OFF and disconnect the electrodes from the power source.
Carefully remove the gel from the gel box with one hand on either side of the casting tray to ensure the gel remains in place.
4. Reduce Agarose Gel for Efficient Mucin Transfer
Carefully place the gel flat in a glass container. Rinse the gel with dH2O to remove traces of TAE-SDS buffer.
Prepare 1 liter of 4x SSC buffer by adding 200 ml of 20x SSC into 800 ml of dH2O. Make 200 ml of 10 mM dithiothreitol (DTT) 4x SSC solution by adding 309 mg of fresh DTT into 200 ml of 4x SSC buffer.
Soak the gel with DTT-4x SSC buffer and cover. Gently rock for 20 min (10 rotations per minute). Rinse gel with DTT-free 4x SSC buffer.
5. Vacuum Blotter Assembly and Sample Transfer to a Nitrocellulose Membrane
- Prepare vacuum blotter for transfer.
- Carefully cut nitrocellulose membrane at dimensions slightly wider than the gel (i.e., 10 x 15 cm).
- Wet porous mat with dH2O and place it smooth-side-up in the blotter.
- Wet nitrocellulose membrane with DTT-free 4x SSC buffer and place it at the center of the porous mat.
- Cover the porous mat with the rubber gasket, leaving the opening of the gasket precisely aligned with the nitrocellulose membrane. If porous mat is still visible, carefully adjust the membrane to ensure no gap remains between gasket and membrane.
- Carefully place the agarose gel on top of the membrane. Ensure gel forms a tight seal with the gasket and the wells of the gel are positioned on the membrane for transfer.
- Avoid the formation of bubbles between the membrane and the gel. To minimize the formation of bubbles, squirt few drops of 4x SSC buffer on the membrane and slide the gel from top to bottom to flush any formed bubbles. Avoid moving the gel on the membrane as proteins will begin to blot immediately.
Carefully cover the gel with 4x SSC buffer.
- Begin mucin transfer by suction.
- Turn vacuum blotter ON and set pressure at 40 - 50 mBar. Add a few drops of 4x SSC buffer on the gel every 5 - 10 min to prevent the gel from drying out. Run transfer for 90 min. Optional: Upon completion, mark the wells with a pencil for orientation.
Dispose of the gel and retrieve nitrocellulose membrane using plastic tweezers on the edge of the membrane.
6. Membrane Blocking and Detection
Rinse membrane immediately with 1x phosphate-buffered saline (PBS). Do not let the membrane dry out.
Immerse the membrane into 50 ml of 3% milk-PBS blocking buffer and place on a rocker at room temperature for 1 hr. After incubation, discard the blocking buffer.
Immerse membrane in 1% milk-PBS primary antibody solution. Incubate the membrane in the primary antibody solution overnight on a rocker at 4 °C. After incubation, discard the primary antibody solution. Dilute the primary antibodies to a 1:2,000 ratio of antibody solution to blocking buffer. Note: For samples originating from mice, we use UNC 222 rabbit anti-Muc5b and goat anti-Green Fluorescent Protein (GFP). For samples originating from humans, we use H300 anti-MUC5B and 45M1 anti-MUC5AC primary antibodies.
Wash membrane 3 times with PBS for 10 min on a rocker.
Immerse membrane in 1% milk-PBS secondary antibody solution. Dilute the secondary antibodies to a 1:7,500 ratio of antibody solution to blocking buffer (e.g., 700 anti-rabbit and 680 anti-goat) and incubate at room temperature for 1 hr on a rocker. Protect from light by incubating in a non-transparent container. Discard the secondary antibody solution after 1 hr.
Wash membrane 3 times with PBS for 5 min. Rinse membrane with dH2O to remove salt.
Read the membrane on an infrared fluorescence system according to manufacturer's instructions.
Representative Results
We show representative results of mucin expression following agarose gel electrophoresis in BALF from the lungs of mice (Figure 1). In this example, we used the agarose gel to show upregulation of mucin production following IL-13 treatment of the Tg-Muc5ac mouse model. The Western blot shows a visual representation of mucin expression, which can be used for a quantitative analysis of multimer or monomer band signal intensity (Figure 2). This method can also be used to show expression of mucins in human bronchial epithelial (HBE) cell washings and human sputum samples. This method can be used to show the reduction of mucins following treatment with reducing agents. We show representative results from HBE washings treated with increasing concentrations of DTT (Figure 3). We quantified the loss of multimer signal intensity following mucin reduction with treatment with a reducing agent (Figure 4).
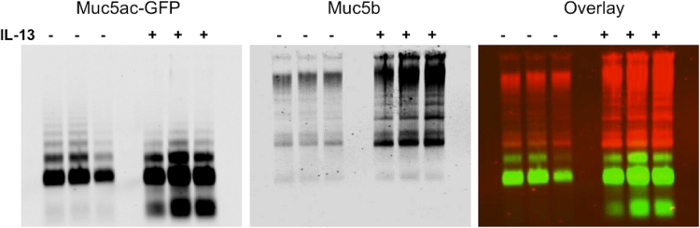 Figure 1. Upregulation of Mucin Production Following IL-13 Treatment of the Tg-Muc5ac/Green Fluorescent Protein (GFP) mouse model. Mice overexpressing Muc5ac tagged with GFP were instilled with PBS (-) or IL-13 (+) to induce goblet cell metaplasia and increase mucin production in the lungs (n = 3 per group). BALF were harvested and separated via mucin agarose Western blotting. Membrane was probed with a rabbit polyclonal anti-Muc5b (red) and a goat polyclonal anti-GFP (green). The infrared system can be used to show the results as an overlay or as a single channel with the option to change images to black and white. Please click here to view a larger version of this figure.
Figure 1. Upregulation of Mucin Production Following IL-13 Treatment of the Tg-Muc5ac/Green Fluorescent Protein (GFP) mouse model. Mice overexpressing Muc5ac tagged with GFP were instilled with PBS (-) or IL-13 (+) to induce goblet cell metaplasia and increase mucin production in the lungs (n = 3 per group). BALF were harvested and separated via mucin agarose Western blotting. Membrane was probed with a rabbit polyclonal anti-Muc5b (red) and a goat polyclonal anti-GFP (green). The infrared system can be used to show the results as an overlay or as a single channel with the option to change images to black and white. Please click here to view a larger version of this figure.
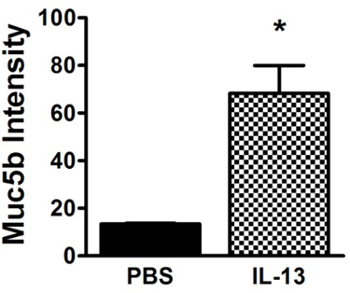 Figure 2. Quantitative Analysis of Mucin Agarose Gel Showing Upregulation of Mucin Secretion in IL-13-Treated Mice. Muc5b signal intensity was measured for each lane of the mucin gel shown in Figure 1. Results show that mucin secretion was increased 5.1-fold over PBS-treated mice in IL-13-treated animals (n = 3, p <0.005). Data shown as mean ±SEM. Please click here to view a larger version of this figure.
Figure 2. Quantitative Analysis of Mucin Agarose Gel Showing Upregulation of Mucin Secretion in IL-13-Treated Mice. Muc5b signal intensity was measured for each lane of the mucin gel shown in Figure 1. Results show that mucin secretion was increased 5.1-fold over PBS-treated mice in IL-13-treated animals (n = 3, p <0.005). Data shown as mean ±SEM. Please click here to view a larger version of this figure.
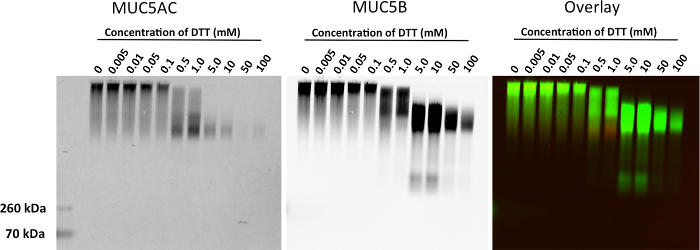 Figure 3. Reduction of MUC5AC and MUC5B Mucins in HBE Washes Treated with DTT. HBE washes were treated with 0, 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 50 and 100 mM DTT at 37 °C for 30 minutes. Membrane was probed with a rabbit polyclonal anti-MUC5B (green) and a mouse polyclonal anti-MUC5AC (red). Please click here to view a larger version of this figure.
Figure 3. Reduction of MUC5AC and MUC5B Mucins in HBE Washes Treated with DTT. HBE washes were treated with 0, 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 50 and 100 mM DTT at 37 °C for 30 minutes. Membrane was probed with a rabbit polyclonal anti-MUC5B (green) and a mouse polyclonal anti-MUC5AC (red). Please click here to view a larger version of this figure.
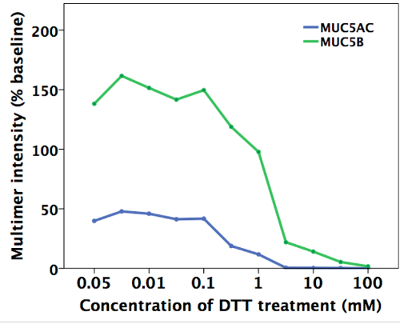 Figure 4. Quantitative Analysis of Mucin Agarose Gel Showing Disappearance of the Multimeric Band in HBE Washes Treated with DTT. MUC5AC and MUC5B signal intensity was measured for each lane of the mucin gel. Please click here to view a larger version of this figure.
Figure 4. Quantitative Analysis of Mucin Agarose Gel Showing Disappearance of the Multimeric Band in HBE Washes Treated with DTT. MUC5AC and MUC5B signal intensity was measured for each lane of the mucin gel. Please click here to view a larger version of this figure.
Discussion
The protocol of mucin Western blotting described in this video combines conventional techniques used in molecular biology to separate and transfer large macromolecules, such as DNA, with regular techniques for protein detection, i.e., immunoblotting. The same technique could be applied to study the biology of complex glycosaminoglycans, such as the breakdown of high-molecular-weight hyaluronic acid18. Although this technique could be used in a broad range of assays, successful agarose Western blotting relies on a multitude of steps that require meticulous and time-dependent actions. In order to maximize success, some critical steps should be reviewed.
Sample preparation and gel loading are critical steps for accurate results. Mucin-rich samples, i.e., sputa, are, by nature, adherent to experimental tubing, i.e., pipet tips, centrifuge tubes, filters. The use of positive displacement pipets and pre-wetting pipet tips can facilitate the loading of viscoelastic material into the wells. Such samples require particular attention due to heightened buoyancy and stickiness of these materials. Concentrated samples (above 5 mg/ml) will poorly migrate through the pore size of a 0.8% agarose gel and may require further dilution and/or denaturation in up to 6 M urea. However, avoid running samples denatured in guanidinium chloride (GuHCl) as guanidinium will precipitate with SDS. Hence, samples stored in GuHCl require an additional dialysis step. For non-polymeric status studies, partial reduction (0.5 mM DTT for 5 min) will greatly improve the migration of concentrated samples (see Figure 3).
Protein transfer to the nitrocellulose membrane is a key step for this protocol and, if not executed with precision, can lead to stained membrane, weak signal and false quantitative results. Nitrocellulose membranes are delicate and easily contaminated and should always be handled with gloves and care to avoid unspecific binding and damage. Placement of the gel onto the membrane requires precise alignment to generate a tight seal and prevent transfer buffer leakage, which would result in stains and reduce transfer efficiency. Formation of bubbles between gel and membrane will prevent protein transfer and should be carefully avoided.
Optimum immunodetection relies on high-affinity, high-specificity antibodies against the protein backbone of the target mucin. Recently, novel immunization strategy, antigen design and screening technologies yield to increased success with the generation of mucin antibodies and provided new tools to study mucin biology. In order to detect two different mucins on one blot, i.e., MUC5AC and MUC5B, the two primary antibodies must be derived from different host species, i.e., rabbit and goat. Also, the secondary antibodies need to be labeled with different fluorophores to be detected in both 700 nm- and 800 nm-channel.
Relative mucin abundance and mucin size can be quantitated directly with imaging software. Fluorescent signal is directly proportional to the amount of target mucin, allowing quantification of a broad range of samples, from low (e.g., cell washes) to high (e.g., sputum) mucin concentration. However, absolute mucin concentration requires the purification of mucin standards, which was not described in this protocol as it is a lengthy and difficult process that was described in Abdullah et al.19. In addition, agarose Western blotting provides the possibility to perform mucin shift assays to study the process of polymerization or depolymerization. Electrophoretic mobility of mucins depends on their polymeric state, i.e., migration of large polymers is delayed, and can be quantified using the imaging software. For instance, reducing agents will induce a mobility shift of the signal that correlates with changes in the biophysical properties of the mucus. Such assay can be used to study mucin multimerization process13 but also to test pharmacological agents aimed at affecting the mucin network11. To conclude, mucin agarose Western blotting coupled with fluorescence labeling has significantly changed our approach to study mucins by producing high qualitative and quantitative data.
Common problems with agarose mucin Western blotting can result in the following outcomes: low signal intensity, high background and/or the appearance of punctates on the membrane. Several troubleshooting strategies can be used to improve mucin gel images. Signal intensity can be increased by loading larger sample volumes and/or increasing primary and secondary antibody concentrations. Typically, high background signal is attributable to insufficient blocking and/or washings, which can be resolved by performing longer block/wash incubations, as well as using optimized blocking buffers that are commercially available. The appearance of punctates can be the result of extended membrane incubations at room temperature, which facilitates bacterial growth. Punctates may also be caused by nitrocellulose membrane damage from the DTT soaking solution (step 4.1) and can be avoided by rinsing the agarose gel thoroughly before transfer and/or decreasing DTT concentration to 5 mM.
The main limitation of agarose mucin Western blotting is the lack of protein ladder for high-molecular-weight proteins and mucin standards, which hinders absolute mucin quantification. Most of the results published from mucin agarose gels are comparative studies for size and concentration. Size exclusion chromatography with light scattering coupled with refractive index is another technique commonly used to determine precise molecular weight and concentration of macromolecules. However, this technique is costly and lacks specificity for mucins, hence, measures other large molecules included in the samples, e.g., DNA. In addition, size exclusion chromatography is unable to differentiate between mucin types, i.e., MUC5AC versus MUC5B, and measures and average concentration and size from the mixture of mucins included in a given specimen.
Other techniques can be used to study mucins including dynamic and multi-angle light scattering1, isopycnic and rate-zonal centrifugations6, electron microscopy, mass spectrometry1,7,8. However, with the investigation of novel pharmacological agents targeting mucin molecules, it is imperative to develop reliable laboratory techniques to study mucin biology. Agarose mucin Western blotting is a relatively easy and inexpensive technique that can be used to answer important questions regarding mucin processes, i.e., change in mucin burden, size and ratio. This assay will be used in future applications to test drug efficacy and toxicity for novel molecules aimed at removing adherent mucus in in vitro and in vivo models.
Disclosures
The authors have no conflict to disclose.
Acknowledgments
The authors would like to acknowledge Dr. John Sheehan and Dr. Lubna Abdullah for their guidance and mentoring that were central in the completion of this work. This work was supported by funds from the National Institutes of Health (P01HL108808, UH2HL123645) and the Cystic Fibrosis Foundation Therapeutics, Inc. (EHRE07XX0). Kathryn Ramsey is supported by an NHMRC Early Career Research fellowship.
References
- Henderson AG, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124(7):3047–3060. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preciado D, et al. MUC5B Is the predominant mucin glycoprotein in chronic otitis media fluid. Pediatr Res. 2010;68(3):231–236. doi: 10.1203/PDR.0b013e3181eb2ecc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, et al. IgG in cervicovaginal mucus traps HSV and prevents vaginal herpes infections. Mucosal Immunol. 2014;7(5):1036–1044. doi: 10.1038/mi.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1(3):183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton DJ, et al. Mucus glycoproteins from 'normal' human tracheobronchial secretion. Biochem J. 1990;265(1):179–186. doi: 10.1042/bj2650179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesimer M, Makhov AM, Griffith JD, Verdugo P, Sheehan JK. Unpacking a gel-forming mucin: a view of MUC5B organization after granular release. Am J Physiol Lung Cell Mol Physiol. 2010;298(1):L15–L22. doi: 10.1152/ajplung.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesimer M, Sheehan JK. Mass spectrometric analysis of mucin core proteins. Methods Mol Biol. 2012;842:67–79. doi: 10.1007/978-1-61779-513-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Post S, Thomsson KA, Hansson GC. Multiple enzyme approach for the characterization of glycan modifications on the C-terminus of the intestinal MUC2mucin. J Proteome Res. 2014;13(12):6013–6023. doi: 10.1021/pr500874f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton DJ, Carlstedt I, Sheehan JK. Identification of glycoproteins on nitrocellulose membranes and gels. Methods Mol Biol. 1994;32:119–128. doi: 10.1385/0-89603-268-X:119. [DOI] [PubMed] [Google Scholar]
- Thornton DJ, Carlstedt I, Sheehan JK. Identification of glycoproteins on nitrocellulose membranes and gels. Mol Biotechnol. 1996;5(2):171–176. doi: 10.1007/BF02789065. [DOI] [PubMed] [Google Scholar]
- Sheehan JK, et al. Physical characterization of the MUC5AC mucin: a highly oligomeric glycoprotein whether isolated from cell culture or in vivo from respiratory mucous secretions. Biochem J. 2000;347 Pt 1:37–44. [PMC free article] [PubMed] [Google Scholar]
- Thornton DJ, Howard M, Khan N, Sheehan JK. Identification of two glycoforms of the MUC5B mucin in human respiratory mucus. Evidence for a cysteine-rich sequence repeated within the molecule. J Biol Chem. 1997;272(14):9561–9566. doi: 10.1074/jbc.272.14.9561. [DOI] [PubMed] [Google Scholar]
- Sheehan JK, et al. Identification of molecular intermediates in the assembly pathway of the MUC5AC mucin. J Biol Chem. 2004;279(15):15698–15705. doi: 10.1074/jbc.M313241200. [DOI] [PubMed] [Google Scholar]
- Ehre C, et al. Overexpressing mouse model demonstrates the protective role of Muc5ac in the lungs. Proc Natl Acad Sci U S A. 2012;109(41):16528–16533. doi: 10.1073/pnas.1206552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livraghi A, et al. Airway and lung pathology due to mucosal surface dehydration in {beta}-epithelial Na+ channel-overexpressing mice: role of TNF-{alpha} and IL-4R{alpha} signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. J Immunol. 2009;182(7):4357–4367. doi: 10.4049/jimmunol.0802557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino MB, et al. The ER stress transducer IRE1beta is required for airway epithelial mucin production. Mucosal Immunol. 2013;6(3):639–654. doi: 10.1038/mi.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LP, et al. Chronic exposure to beta-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol. 2008;38(3):256–262. doi: 10.1165/rcmb.2007-0279RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakonstantinou E, et al. COPD exacerbations are associated with pro-inflammatory degradation of hyaluronic acid. Chest. 2015. [DOI] [PubMed]
- Abdullah LH, Wolber C, Kesimer M, Sheehan JK, Davis CW. Studying mucin secretion from human bronchial epithelial cell primary cultures. Methods Mol Biol. 2012;842:259–277. doi: 10.1007/978-1-61779-513-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]


