Abstract
Exosome research in the last three years has greatly extended the scope towards identification and characterization of biomarkers and their therapeutic uses.
Exosomes have recently been shown to contain microRNAs (miRs). MiRs themselves have arisen as valuable biomarkers for diagnostic purposes. As specimen collection in clinics and hospitals is quite variable, miRNA isolation from whole bile varies substantially. To achieve robust, accurate and reproducible miRNA profiles from collected bile samples in a simple manner required the development of a high-quality protocol to isolate and characterize exosomes from bile. The method requires several centrifugations and a filtration step with a final ultracentrifugation step to pellet the isolated exosomes. Electron microscopy, Western blots, flow cytometry and multi-parameter nanoparticle optical analysis, where available, are crucial characterization steps to validate the quality of the exosomes. For the isolation of miRNA from these exosomes, spiking the lysate with a non-specific, synthetic miRNA from a species like Caenorhabditis elegans, i.e., Cel-miR-39, is important for normalization of RNA extraction efficiency. The isolation of exosome from bile fluid following this method allows the successful miRNA profiling from bile samples stored for several years at -80 °C.
Keywords: Medicine, Issue 112, Exosomes, extracellular vesicles, Molecular Biology, Bile, microRNA, electron microscopy, Western Blot, nanoparticle optical analysis
Introduction
Like other biological fluids, i.e., breast milk, plasma or urine, bile contains exosomes, lipid rich vesicles1-4. Exosomes can induce or alter biological functions in recipient cells5, 6, a form of cell-cell communication that might be part of their normal function4, 7-9. Exosomes can contain miRNA species which can provide a valuable source of biomarkers for diagnosis10. miRNAs profiles have gained substantial attention in recent years as diagnostic and prognostic markers for a variety of diseases11-14.
miRNA itself can be found in biological fluids, possibly released by dead cells and the amount of shed cells can be greatly influenced by an underlying disease. The miRNA profile of exosomes does not necessarily reflect the miRNA profile of the originating cell6, 10, 15-17, yet exosomes may carry miRNA signatures that might be characteristic for the parental cell like a tumor cell. Tumor-derived exosomes have been identified in the plasma of patients with lung adenocarcinoma, prostate cancer and other tumors8, 16, 18.
The goal of this method is the reliable and robust isolation of exosomes from human bile specimens, largely independent of their prior handling procedures. It was developed to utilize bile as a reliable source of miRNA for the potential diagnosis of diseases of the bile duct such as cholangiocarcinoma19. It consist of several steps of centrifugation with increasing speeds to isolate exosomes and might be applicable to other biological fluids.
Protocol
Obtaining the bile from patients by endoscopic retrograde cholangiopancreatography (ERCP) requires the approval of a human subjects study protocol by the Institutional Review Board. All work presented here was approved by the Johns Hopkins University Institutional Review Board.
1. Exosome Isolation from Bile
- Perform endoscopic retrograde cholangiopancreatography (ERCP). Place the patient in the supine position and intubate.
- Pass a duodenoscope through the mouth of the patient and insert it into the second part of the duodenum and identify the major papilla.
- Selectively cannulate the common bile duct by inserting a triple lumen sphincterotome pre-loaded with a 0.035 inch (0.9 mm) hydrophilic guidewire.
- Advance the guidewire under fluoroscopic guidance to the left hepatic duct since on occasion it can be difficult to differentiate the cystic duct from the right hepatic duct, then advance the sphincterotome 5 cm into the bile duct to acquire a stable position.
- Remove the guidewire, attach a 10 ml syringe to the guidewire port through the Luer lock and aspirate the bile using 10 ml of negative pressure.
- Remove the syringe containing the bile to proceed with the ERCP by reinserting the guidewire and injecting contrast agent through the injection port to opacify the biliary tree.
- Transfer the collected bile into a 15 ml tube on ice and keep on ice until ready to proceed with the centrifugation step. Caution: Wear gloves to protect yourself and the samples! Treat the bile as a potential source of infection as it can contain Hepatitis A viral particles!
- Keep the sample(s) on ice. Either proceed with step 1.2 or centrifuge at 500 x g at 4 °C for 10 min and then freeze at -80 °C until further use. Note: Samples stored this way for several years have routinely been used with good results19.
Transfer 400 µl of bile into a microcentrifuge tube and collect cells and cell debris by centrifugation at 300 x g for 10 min at 4 °C.
Remove the supernatant by pipetting, transfer to a fresh microcentrifuge tube and centrifuge the sample(s) at 16,500 x g for another 20 min at 4 °C to clear the supernatant of further debris. Discard the tubes in the biohazard waste. Alternatively, spin the samples in an ultracentrifuge instead.
Collect the supernatant and filter it in a biosafety cabinet with a syringe through a 0.2 µm polyethersulfone (PES) membrane filter which has good flow rates and low protein binding to remove any particles larger than 200 nm left.
Pipet the filtered supernatant to ultracentrifuge tubes and add PBS so that the tubes are more than 2/3 full. If the tubes contain less liquid than that, the tubes will collapse during centrifugation! Tubes can be washed, autoclaved and reused several times.
Pellet the exosomes through ultracentrifugation at 120,000 x g for 70 min at 4 °C. After the centrifugation look at the bottom of the ultracentrifuge tube to sometimes see small yellow pellets; these are the exosomes.
Discard the supernatant by carefully decanting it and disinfect the waste by adding bleach to a final concentration of 10% v/v and let it stand for 20 min.
For downstream experiments, either process the pellet(s) in a small volume of the appropriate solution (i.e., radioimmunoprecipitation assay (RIPA) buffer for protein isolation, RNA lysis buffer for miR isolation) or resuspend it in 50-150 µl of phosphate buffered saline (PBS) which can be either stored at -80 °C for future use (quantitative real-time polymerase chain reaction (qRT-PCR) or protein extraction etc.) or used for characterization of the exosome preparation by TEM or nanoparticle optical analysis.
2. Exosome Identification by Transmission Electron Microscopy (TEM) or CryoEM
Resuspend extracellular vesicles in 100 µl of PBS by pipetting. Ideally, use exosomes that have not been stored at -20 °C or -80 °C, especially when performing the isolation for the first time.
Adsorb a 20 µl aliquot (about 2 µg) to 400 mesh carbon-coated Parlodion copper grids for 2 min and allow to dry at RT.
Fix the exosome in 1% (v/v) glutaraldehyde (EM-grade) for 5 min at RT by placing drops carefully on the dried preparation.
Wash the grids twice with water for 5 min and then contrast stain EVs with 1% phosphotungstic acid (PTA) for 30 sec.
Acquire images with an electron microscope with an acceleration voltage of 80 kilovolts starting at magnifications of 20,000X and increasing to 100,000X when determining the size of the particles. Note: Other preps like layering onto Formvar carbon-coated 200 mesh copper or nickel grids and contrast staining like 1% or 2% uranyl acetate for 10-15 min are also possible.
For CryoEM, mix a 20 µl aliquot (about 2 µg) with 60 µl Protein G gold 10 nm (1:3 ratio). This will help to determine the exosome diameter.
Transfer the samples onto glow-discharged C-flat holey carbon grids and remove excess liquid by blotting. Freeze the grids by dipping them immediately into liquid ethane.
Mount the grids in a cryoholder in liquid nitrogen.
Acquire the images at 200 kV with magnifications of 20,000X and 100,000X.
3. Exosome Identification by Multi-parameter Nanoparticle Optical Analysis
Resuspend the isolated exosomes in 100 µl of PBS by pipetting.
Dilute exosomes in 600 µl PBS at several different ratios (like 1:50, 1:100, 1:300, 1:600 and 1:1,200).
Visualize the exosomes by laser light scattering with the appropriate instrument and software in real-time according to manufacturer's protocol20.
Increase the level of the camera in capture mode until particles become visible, then adjust the focus to make the particles look smooth.
While in standard mode, decrease the camera to the lowest level that the particles can still be seen, if necessary adjust camera shutter and gain to achieve this.
Visually check to ensure 20-100 particles are visible on the screen, if not flush the unit and adjust the dilution in PBS.
Then adjust the capture duration to between 30-60 sec. After the video is captured, adjust the processing parameters until all particles are identified on the screen and process the video file.
4. Exosome Characterization by Western Blot
Lyse exosome pellets from 2-5 ml of bile in 100 µl of ice-cold radioimmunoprecipitation assay (RIPA) buffer with a protease inhibitor by pipetting up and down thoroughly and mix lysates vigorously for 30 sec on a vortex to solubilize the proteins effectively.
While most of the time not necessary, use pulse-sonication of the samples on ice with 2 pulses for 10 sec each to ensure full lysis.
Pellet insoluble material by centrifugation at 15,000 x g at 4 °C for 5 min and transfer supernatant to a clean tube.
Determine the protein concentration with a method of choice (i.e., bicinchoninic acid (BCA), Coomassie, modified Lowry, 660 nm Protein Assay, etc.) according to manufacturer's protocol. The method is not as important as long as the same method is used consistently to achieve results that can be compared across experiments.
Load 50 µg of protein per sample in Laemmli buffer after heating the sample at 95 °C for 5 min into a well of a polyacrylamide gel for electrophoresis (PAGE).
Electrophorese the samples on the PAGE gels for 1.5 hr and transfer the separated proteins on to a nylon membrane.
Block the membrane(s) with 5% nonfat milk in Tris-buffered Saline with 0.1% Tween 20 (TBS-T) for 1 hr, then incubate with exosome-specific antibodies like anti-CD63 and anti-TSG-101 at a dilution of 1:500 preferably O/N at 4 °C.
Wash the membranes with TBS-T 3 x 10 min, then incubate with an appropriate, matching HRP-conjugated secondary antibody in 5% nonfat milk in TBS-T for 1-2 hr at RT.
Wash the membrane(s) 3 x 5 min with TBS-T and detect the specific proteins with chemiluminescence reagents according to manufacturer's protocol.
Image the membranes with film or a digital imaging system according to manufacturer's protocol.
5. MicroRNA Isolation from the Isolated Exosomes
Note: Isolation of miRNA from bile exosomes is carried out using a modified miRNA isolation based on a commercially available kit, but any other RNA isolation method can be used or adapted.
Add 300 µl lysis/building buffer to the exosome pellet (isolated from 400 µl bile), vortex, pipet or pass through a needle/syringe to completely lyse the exosomes to obtain a homogenous lysate.
Add 1/10 (30 µl) volume of miRNA Homogenate Additive, and mix well by vortexing or inverting the tube several time.
Incubate the mix for 10 min on ice.
Add a volume of acid-phenol:chloroform equal to the initial homogenate (300 µl).
Add 5 µl of 5 fmol/µl cel-miR 39 and vortex for 30-60 sec.
Centrifuge for 5 min at 10,000 x g at RT to separate the aqueous (upper) and organic (lower) phases.
Carefully aspirate the upper phase by pipetting and transfer to a fresh tube while noting the volume transferred. Discard the organic phase in the appropriate organic waste container.
To enrich for small RNAs add 1/3 volume of 100% ethanol to the recovered aqueous phase and mix by thoroughly vortexing or inverting the tube several times.
Pipet the lysate/ethanol mixture onto the filter cartridge, up to 700 µl at a time.
Centrifuge the cartridges for 15 sec at a maximum of 10,000 x g or apply vacuum to pass the mixture through the filter.
Collect the filtrate and if the lysate/ethanol mixture exceeds 700 µl, transfer the flow-through to a fresh tube and repeat the procedure to collect all the filtrates. These filter contains RNA fractions devoid of small RNAs and can be used to recover these larger RNAs.
Add 2/3 volume of RT 100% ethanol to the total filtrate and mix thoroughly by vortexing.
Pipet the filtrate/ethanol mixture onto a second filter cartridge. Like before the maximum volume that can be applied at once is 700 µl.
Centrifuge for 15 sec at 10,000 x g or apply vacuum as described in step 5.10.
Discard the flow-through, and repeat until all of filtrate/ethanol mixture has been filtered.
Apply 700 µl miRNA Wash Solution 1 to the filter cartridge, centrifuge for 5-10 sec or apply vacuum and discard the flow-through.
Wash the filter with 500 µl wash solution 2/3 as done in step 5.16.
Repeat the wash a second time with 500 µl of wash solution 2/3 as in step 5.16, discard the flow-through and place the filter back in the collection tube.
Spin the filter cartridge for 1 min at 10,000 x g to remove all residual fluid from the filter.
Transfer the filter cartridge into a fresh collection tube and apply 100 µl of hot (95 °C) nuclease free water to the center of the filter.
Spin the assemblies for 20-30 sec at maximum speed to recover the RNA.
Collect the eluate and use immediately or store at -80 °C.
Representative Results
Since exosomes are too small to be detected by regular microscopy or flow cytometry, electron microscopy or nanoparticle optical analysis has to be performed. The nanoparticle optical analysis has the advantage over electron microscopy that it is also quantitative and provides size distribution and concentration. The instrument introduces a finely focused laser beam through a glass prism into the sample. The Brownian motion of the isolated vesicles is captured via an EMCCD high sensitivity camera and tracked on a frame-by-frame basis. The nanoparticle tracking analysis (NTA) software measures this Brownian movement frame to frame to calculate the size, mode and distribution of the bile exosome preparations. Figure 1 shows the result of a typical NTA analysis of exosome isolated from a human bile sample.
Alternatively, electron microscopy can be utilized to confirm the size of the isolated particles, albeit without quantification. Figure 2A shows the typical result of transmission electron microscopy for exosome isolated from human bile.
For further verification of the exosomal nature of the isolated particles, Western blots probing for the presence of proteins like Tsg101 or tetraspanin CD63, shown in Figure 2B, have to be used. Exosome-specific proteins do not exist per se, but exosomes are enriched in tetraspanins, especially CD9, CD63, CD81 and CD82 with CD63 and CD81 referred to as classical exosome markers, and proteins involved in exosome formation like TSG101 and Alix21. Other proteins like heat shock protein cognate 70 (Hsc70) and 73 (Hsc73), as well as major histocompatibility complex (MHC) class II molecules can also be found enriched in exosomes with some like Hsc73 and the peripheral membrane-associated protein milk fat globule - epidermal growth factor -factor 8 (Mfge8) being quite specific for exosomes secreted from dendritic cells21.
Figure 3 shows the typical real-time amplification plots of a variety of miRNA species extracted from exosomes of human bile. Since exosomes, unlike cells, lack reliable standards like 18S or 28S rRNA or housekeeping genes like GAPDH or β-actin for normalization, the spiking with a synthetic miRNA is important. The synthetic miRNA should be derived from a different species like cel-miR 39 from Caenorhabditis elegans to enable one to normalize the expression levels effectively.
For reproducibility it is critical that the bile is processed as soon as possible and not frozen prior to processing as these conditions lead to degradation of miRNAs present in bile. On the other hand, once isolated, exosomes are very stable and quite resistant as storage at RT for 48 hr or up to three freeze-thaw cycles cause negligible effects on the levels of at least two species of miRNA, although the stability for the miRNA of interest has to be determined empirically.
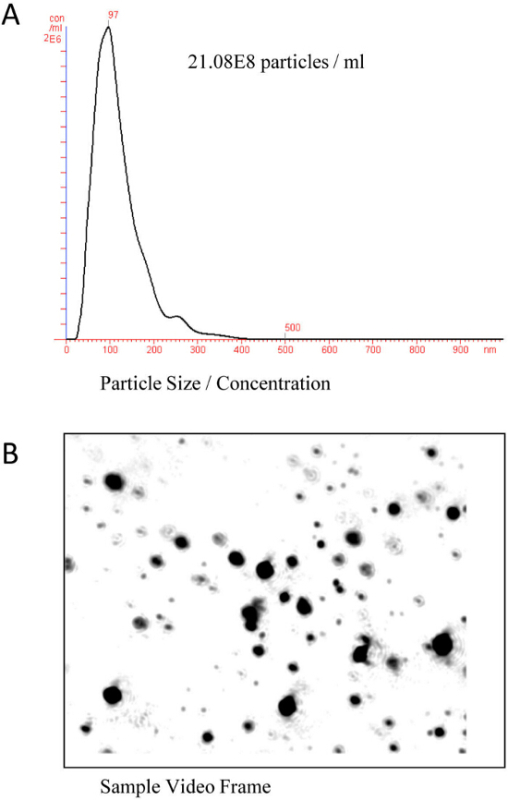 Figure 1.(Nanoparticle) NTA analysis to characterize human biliary exosomes. Bile exosomes were diluted in PBS at the ratio of 1:600. (A) Size distribution and concentration of EVs isolated from human bile. The average size was determined to be approximately 97 nm for this sample (x-axis: exosomes size, y-axis: exosomes concentration for each size). (B) Sample snap shot of the same sample analyzed in A. The size range shows vesicles ranging from 30-110 nm. Please click here to view a larger version of this figure.
Figure 1.(Nanoparticle) NTA analysis to characterize human biliary exosomes. Bile exosomes were diluted in PBS at the ratio of 1:600. (A) Size distribution and concentration of EVs isolated from human bile. The average size was determined to be approximately 97 nm for this sample (x-axis: exosomes size, y-axis: exosomes concentration for each size). (B) Sample snap shot of the same sample analyzed in A. The size range shows vesicles ranging from 30-110 nm. Please click here to view a larger version of this figure.
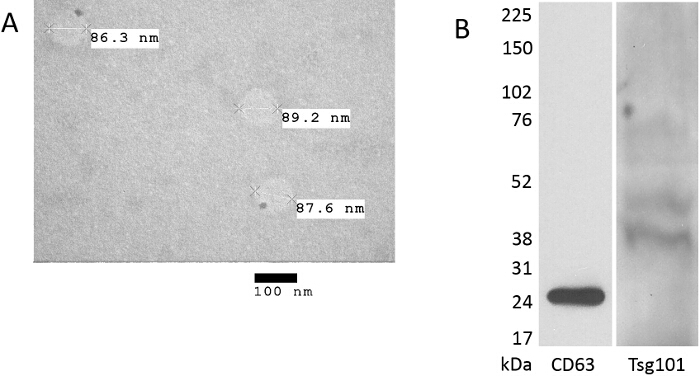 Figure 2. Verification of the exosomal nature of the preparation by Transmission electron microscopy and Western blot. (A) Transmission electron microscopy (TEM) of spherical structures present in human bile 70-110 nm in size. Scale bar is 100 nm. (B) Western blot analysis of biliary exosomes shows presence of the typical exosome marker proteins TSG101 and CD63. Please click here to view a larger version of this figure.
Figure 2. Verification of the exosomal nature of the preparation by Transmission electron microscopy and Western blot. (A) Transmission electron microscopy (TEM) of spherical structures present in human bile 70-110 nm in size. Scale bar is 100 nm. (B) Western blot analysis of biliary exosomes shows presence of the typical exosome marker proteins TSG101 and CD63. Please click here to view a larger version of this figure.
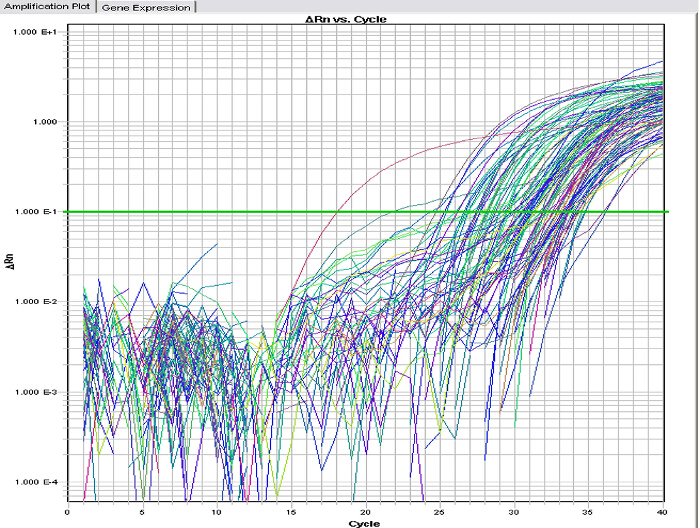 Figure 3. Real-time PCR of miRNA species isolated from bile exosomes. Real-time PCR of a miR array demonstrates the amplification of multiple miR species extracted from human biliary exosomes (x-axis, PCR cycle number; y-axis, relative intensity). Please click here to view a larger version of this figure.
Figure 3. Real-time PCR of miRNA species isolated from bile exosomes. Real-time PCR of a miR array demonstrates the amplification of multiple miR species extracted from human biliary exosomes (x-axis, PCR cycle number; y-axis, relative intensity). Please click here to view a larger version of this figure.
Discussion
To reliably utilize exosomes isolated from bile, it is important to employ consistent high quality isolation methods to obtain high quality samples in return. The methodology defined in this paper is a well-established way to isolate exosomes and miRNA from human bile. It highlights several crucial steps in the characterization of the isolated exosomes which at a minimum should comprise electron microscopy or nanoparticle optical analysis and Western blots.
The most crucial step in the isolation of exosomes, at least when it comes to miRNA stability, is to process fresh bile as soon as possible. Prolonged storage of whole bile at RT or even a single freeze-thaw cycle can significantly reduce the miRNA content while in contrast the isolated exosomes are very stable even when stored at RT or undergoing several freeze-thaw cycles. As long as the samples in question have been stored at -80 °C, successful isolation of exosomes and miRNA from bile can be performed with great reproducibility to identify miRNA signatures in the samples. It is recommended to initially start with fresh bile to ensure proper miRNA integrity. This is an important consideration when attempting to build up a collection of bile samples over time as would be the case for patient samples. It enables one to process samples that have been collected over months or even years to be processed and evaluated at the same time.
This greatly enhances the use of bile for diagnostic purposes, either to look for miRNA signatures that confirm the presence of a disease state like cancer or the response of a disease to therapeutic interventions. In fact, results from clinical samples have been used to establish miRNA signatures as biomarkers for cholangiocarcinoma19.
The first centrifugation step removes intact cells that are present in bile. In the second, higher speed centrifugation cell debris, apoptotic bodies and other larger organelles are pelleted. To ensure that only particles smaller than 200 nm are collected in the ultracentrifugation step, the filtration step with a low protein binding filter is important. Some protocols omit this step, but we think it is necessary. After the ultracentrifugation at 120,000 x g the pellet obtained contains fairly pure exosomes that can either be processed immediately or after resuspension in PBS be stored at -80 °C. A limitation of this method is that the pellet obtained is only highly enriched in exosomes but this is true for other enrichment methods such as size exclusion and polymeric precipitation. Further processing might entail another purification step by sucrose gradient or binding to antibody-coated magnetic beads. If the source of the exosomes (like for example plasma) contains precipitates as a result of clotting, this extra purification might be necessary as these particulates can often have the size of exosomes. In particular, the immuno-purification leads to a more exosome marker enriched preparation. The disadvantage is that this further reduces the amount of exosomes and/or miRNA present, and for most purposes does not justify the time and resource consuming process. If, however, Western blot analysis detects the presence of microsome contamination through an endoplasmic reticulum protein such as calnexin, further purification, in particular immuno-isolation based, is advised. In our experience this is rarely the case for bile, if one uses other sources of biological fluids an immune-based purification step would be recommended.
The use of differential ultracentrifugation to isolate and enrich exosomes from bile fluid is a relatively fast and cost-effective method. While it requires the use of an ultracentrifuge, preferably with a swing bucket rotor, these devices are commonly found in many institutions. Further purification via density centrifugation are possible with the same equipment and the tubes are reusable several times after cleaning and sterilization. While polymer-based precipitation requires only the use of microcentrifuges or standard centrifuges to pellet the precipitates at speed of 10,000 x g or less, it generally co-isolates non-vesicular contaminants including lipoproteins. While lipoproteins are not generally present in bile, the cost of the often proprietary and patented polymers make the isolation expensive in comparison. The polymers also are sometimes incompatible with downstream applications such as mass spectrometry, but when downstream analysis is compatible with the polymer (RNA or protein isolation), the method is easy, fast and does not require specific equipment.
Size exclusion chromatography has the downside of long running times and the requirement of special equipment, but allows the precise separation of large and small particles. Immunoaffinity purification yields the highest purity of exosomal vesicles and even allows the isolation of specific exosomal fractions such as epithelial derived, but this comes at the cost of total yield. Furthermore, it is limited to small samples volumes requiring a first enrichment step if a large volume is to be processed and the isolated exosomes may lose functional activity or exhibit altered biological function due to the bound antibodies.
It is important to consider the downstream application(s) and availability of specialized equipment when it comes to the isolation of exosomes. The characterization of the isolated/enriched particles is important regardless of the isolation method used. Ultracentrifugation has so far been a "gold standard" used by many laboratories due to its robust and fast (and cost-effective if an ultracentrifuge is available) enrichment of exosomal particles.
Disclosures
None of the authors have competing financial interests.
Acknowledgments
This study was supported by a K08 Award (DK090154-01) from the National Institutes of Health (NIH; to F.M.S.).
References
- Admyre C, et al. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007;179(3):1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- Aushev VN, et al. Comparisons of microRNA patterns in plasma before and after tumor removal reveal new biomarkers of lung squamous cell carcinoma. PLoS One. 2013;8(10):e78649. doi: 10.1371/journal.pone.0078649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Dov IZ, et al. Urine microRNA as potential biomarkers of autosomal dominant polycystic kidney disease progression: description of miRNA profiles at baseline. PLoS One. 2014;9(1):e86856. doi: 10.1371/journal.pone.0086856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta. 2012;1820(7):940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010;285(23):17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel DM, van de Garde MD, Middeldorp JM. Viral miRNAs exploiting the endosomal-exosomal pathway for intercellular cross-talk and immune evasion. Biochim. Biophys. Acta. 2011;1809(11-12):715–721. doi: 10.1016/j.bbagrm.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Bessho K, et al. Integrative genomics identifies candidate microRNAs for pathogenesis of experimental biliary atresia. BMC Syst. Biol. 2013;7 doi: 10.1186/1752-0509-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin GA, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc. Natl. Acad. Sci. U.S.A. 2004;101(32):11755–11760. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AL, et al. A differential microRNA profile distinguishes cholangiocarcinoma from pancreatic adenocarcinoma. Ann. Surg. Oncol. 2014;21(1):133–138. doi: 10.1245/s10434-013-3240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008;110(1):13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Rabinowits G, Gercel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin. Lung Cancer. 2009;10(1):42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat.Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance RS, Drake RR, Troyer DA. Multiple recognition assay reveals prostasomes as promising plasma biomarkers for prostate cancer. Expert Rev. Anticancer Ther. 2011;11(9):1341–1343. doi: 10.1586/era.11.134. [DOI] [PubMed] [Google Scholar]
- Li L, et al. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology. 2014;60(3):896–907. doi: 10.1002/hep.27050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel DA, Giordano K. Microparticle sizing and counting using light scattering methods. Semin. Thromb. Hemost. 2010;36(8):824–832. doi: 10.1055/s-0030-1267036. [DOI] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]


