Abstract
Four to six week old, male Wistar rats were used to produce animal models of liver fibrosis. The process requires four weeks of administration of 10 mg/kg dimethylnitrosamine (DMN), given intraperitoneally for three consecutive days per week. Intraperitoneal injections were performed in the fume hood as DMN is a known hepatoxin and carcinogen. The model has several advantages. Firstly, liver changes can be studied sequentially or at particular stages of interest. Secondly, the stage of liver disease can be monitored by measurement of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) enzymes. Thirdly, the severity of liver damage at different stages can be confirmed by sacrifice of animals at designated time points, followed by histological examination of Masson's Trichome stained liver tissues. After four weeks of DMN dosing, the typical fibrosis score is 5 to 6 on the Ishak scale. The model can be reproduced consistently and has been widely used to assess the efficacy of potential anti-fibrotic agents.
Keywords: Medicine, Issue 112, dimethylnitrosamine, liver, fibrosis, animal model, rat, preclinical studies
Introduction
DMN is a potent liver specific toxin. Its metabolism, tissue distribution, and ability to cause injury to livers of rats was reported by Magee1, and the mechanism of hepatocyte damage and cell death by apoptosis was described by Pritchard and Butler2. Intermittent administration of this compound was reported to induce liver fibrosis in dogs and rats3,4.
The mechanisms and morphologic changes of liver fibrosis have been extensively investigated using this model. In early studies using the rat, 3-week treatment with DMN produced centrilobular hemorrhagic necrosis followed by micronodular cirrhosis without steatosis5. It was shown that in early fibrosis, the collagen formed was more cross linked with type III being more prominent than type I6. In common with other causes, DMN-induced fibrotic changes were associated with an increase in Kupffer cells; hepatic macrophages that reside in the sinusoids. These cells morph into myofibroblasts and produce excessive amounts of extracellular matrix which is the primary problem in fibrosis7.
In terms of cellular signaling, Nakamura et al. demonstrated that TGF-β plays a critical role in the progression of liver fibrosis8. They used an adenovirus expressing a truncated type II TGF-β receptor, to specifically inhibit TGF-β signaling. Liver fibrosis in these rats was spectacularly halted during DMN treatment when compared to control groups. Other studies have confirmed that suppression of TGF-β leads to alleviation of liver fibrosis development9,10. The model was also used in a global gene profiling study to identify other fibrosis markers and proteins which could be used as drug targets for anti-fibrotic therapy11.
Other chemical agents used to induce liver fibrosis include thioacetamide (TAA) and carbon tetrachloride (CCl4). TAA was used first in rats and later in mice12. The advantages of this model include: ease of chemical administration in drinking water, the reproducibility of the model with characteristic micronodular cirrhosis and biochemical changes. The disadvantages include: the long time period of 3 months for liver fibrosis to develop and the lack of understanding of the molecular mechanism for induction of liver fibrosis. As for CCl4, its use has declined for the following reasons: it does not mimic human liver disease, has harmful effects on the ozone layer, causes pain and distress to animals, is very toxic to humans and requires extra precautions in its handling and disposal13,14.
Prolonged bile duct obstruction (by surgical intervention) as an experimental model for liver cirrhosis was first reported by Kountouras et al.15. This method is nontoxic to humans and animals. However, the time required for liver fibrosis to develop varies. A review of 30 reports by Marques et al.16, found that it took from seven days to four weeks after surgery for liver fibrosis to develop. The pathological changes described mimic those of human chronic biliary fibrosis and the model would be more suited for researchers interested in this area.
In summary, the intermittent administration of a constant dose of DMN in the rat over 4 weeks produces liver fibrosis that mimics the human disease. Dosed rats show progressive development of liver damage and parenchymal fibrosis4,17. Disease progression and severity can be monitored via blood samples or sacrifice of animals at specific time points and the effect is highly reproducible18. Thus the model has been widely used to study the mechanisms of liver fibrosis and cirrhosis as well as to screen for potential anti-fibrotic agents10,19,20.
Protocol
All animal experiments were approved by the animal care and use committee of the School of Applied Science, Temasek Polytechnic.
1. Preparation of DMN
Pipette 200 µl of DMN (1 g/ml stock solution) and add 19.8 ml of PBS to prepare 10 mg/ml DMN solution for the injection. Note: DMN is carcinogenic. Use a fume hood to prepare the DMN and perform the animal injections.
2. Intraperitoneal Injection of DMN
Use male Wistar rats with an average weight of 150 - 200 g. Acclimate rats for 4 - 7 days before the start of the experiment.
Maintain rats at room temperature of 22 ± 1 °C, with 12 hr light and 12 hr dark cycles. Provide rat chow and water ad libitum.
Measure food and water intakes daily. Measure body weight weekly.
Calculate the amount of DMN for each rat based on a dose of 10 mg/kg body weight. Use a 1 ml syringe with a 25 gauge needle to draw the calculated volume of DMN solution into the syringe.
Restrain the rat for intraperitoneal injection21.
With the rat properly restrained, introduce the needle into the lower right quadrant of the abdomen, draw back on the plunger of the syringe (nothing should be aspirated) to check for the correct placement of needle within the abdominal space and slowly inject DMN solution.
When the dose of DMN has been administered, slowly withdraw the needle and dispose into the sharps bin.
Perform the intraperitoneal injections of DMN for 3 consecutive days a week for 4 weeks. Administer the injections at the same time each day. Re-calculate the dosage based on the body weight at the start of each week of injections.
Collect blood from the tail vein weekly to measure biochemical parameters: alanine aminotransferase (ALT) and aspartate aminotransferase (AST)22.
Measure serum ALT and AST using a commercial Vet Test Chemistry Analyzer.
3. Gross Examination and Harvest of Liver
Euthanize the rats by intraperitoneal injection of pentobarbital at a dose of 100 mg/kg body weight. Ensure death by checking for the lack of heartbeat.
Prepare for the post mortem and assess the condition of the animal as described by Parkinson et al.23 Place dead rats onto dissecting board in dorsal recumbency with the abdomen facing upwards.
Disinfect and moisten the skin with 70% ethanol.
Using scissors, incise the skin the full length of the ventrum from the anus to the chin, and reflect the skin. With the scissors, incise the abdominal wall from the anus to the xiphoid cartilage, to expose the abdominal viscera.
Examine the abdominal organs in situ to check for any abnormalities. Note any changes in color, size, location of organs and the presence of fluid within the peritoneal cavity. Examine the consistency of organs and note the presence of any odors.
- Using scissors and forceps, dissect the entire liver free of its ligaments and attachments.
- Begin at the hilus where it is attached to the diaphragm and work to free all the liver lobes of attachments. Carefully cut all ligaments and blood vessels.
Transfer the liver onto a petri dish and weigh the liver.
Using a scalpel, cut 2 - 5 mm thick sections of liver lobes. For consistency, always take samples from the same liver lobe. Take a wedge about 5 mm diameter in thickness, from the right lateral lobe24 about 1 cm from the edge of the lobe.Immediately place into 10% buffered formalin for fixation. Ensure the tissue to formalin volume is 1:10 or more.
Harvest and immediately freeze portions (using liquid nitrogen) of liver lobes at this point if required for other studies. As for (3.8), sample from the same liver lobe for consistency.
4. Processing, Embedding and Sectioning of the Liver
Fix the liver samples in 10% buffered formalin for a minimum of 24 hr.
After fixation, trim the liver, place into cassettes and load them into the basket of the automated tissue processor.
Place the basket with cassettes into the first station which contains 10% buffered formalin. Ensure the cassettes are entirely submerged. They can remain in this chamber for up to 12 hr until the cycle begins.
Program the tissue processor to rotate the cassettes for 1 hr each, through successive stations containing the following solutions: ethanol at concentrations of 50%, 70%, 90%, 100% (2 stations), xylene (2 stations) and liquid paraffin (2 stations).
Transfer the liver samples into the wax bath of the embedding station. Ensure that the embedding machine is switched on at least one hour earlier.
Using pre-warmed forceps (65 °C), remove each cassette from the wax bath and place onto the warm plate (65 °C) of the embedding machine. Dispense sufficient amount of liquid paraffin into the stainless steel base mold (pre-warmed to 65 °C) till half full. Transfer the section of liver from the cassette into the mold. Ensure the specimen is placed with the cut surface (to be examined microscopically) flat on the bottom of the mold.
Fill the mold completely with liquid paraffin, mount the empty cassette on top and place the mold onto the 4 °C plate of the embedding machine to cool for at least 30 min.
Check that the wax has cooled and solidified, and remove the paraffin block from the mold. The block should pop out easily and not stick or crack. If this happens, melt the block and repeat this step. The paraffin block can be sectioned once it solidifies. Blocks can be stored at room temperature for many years.
Place the paraffin block into the microtome and section at 10 µm thickness until the wax layer is removed sufficiently for the embedded liver tissue to be visible.
Change the setting on the microtome to cut at 5 µm thickness. Using forceps, pick up a well cut section by holding it at the edge and carefully float the section in the water bath at a temperature of 40 °C.
Gently place a clean glass slide under the floating section and lift it onto the surface of the glass slide. Place the slide onto a slide warmer at 37 °C overnight.
5. Masson Trichrome Staining
- Before applying the stains, treat the slides to remove the wax and rehydrate the tissues as follows:
- Immerse the sections in xylene for 5 min. Repeat 2x.
- Immerse the sections in 100% ethanol for 3 min. Repeat 1x.
- Immerse the sections for 1 min each in the following solutions of ethanol: 90%, 80% and 70%.
Rinse the slide under tap water to remove any existing ethanol on the slide.
Immerse slides in Iron Hematoxylin for 10 min to stain the nucleus.
Rinse the slides with water to remove the excess stain from the slide.
Immerse the slides in Biebrich Scarlet-Acid Fuchsin for 2 min to stain the cytoplasm.
Rinse the slides with water.
Place the slides in Phosphotungstic/Phosphomolybdic Acid Solution for 10 min to promote the uptake of Aniline blue. Change the Phosphotungstic/Phosphomolybdic Acid Solution after 2 rounds of use.
Place the slides in aniline blue solution for 10 min to stain the collagen fibers.
Rinse the excess stain with water and place the slides in 1% acetic acid solution for 1 min to allow differentiation to take place to render the color of the slide more delicate and transparent.
Rinse the slides with water and proceed to dehydrate the tissue sections. The dehydration steps are as follows:
Immerse the slides successively for 10 sec each time, in the following concentrations of ethanol: 70%, 80%, 95% and 100%.
Immerse the slides for 30 sec in 100% ethanol. Repeat 1x.
Rinse the slides through 3 stations of xylene for 5 min each to remove ethanol.
Mount a glass cover slip onto the specimen with mounting media (e.g., DPX mountant) and allow to dry. Note: DPX is a synthetic resin that dries quickly, preserves stain and protects the tissue section.
6. Fibrosis Scoring on Masson's Trichome Stained Sections of Liver
Have a veterinary pathologist assess the severity of fibrosis according to the fibrosis score25. It would be optimum if the fibrosis scoring is done by two or three pathologists independently in a blinded manner. In such a scenario, obtain a final score after discussion and group review to sort out any differences in scoring.
| Score | Description |
| 0 | No fibrosis |
| 1 | Fibrous expansion of some portal areas, with or without short fibrous septa |
| 2 | Fibrous expansion of most portal areas, with or without short fibrous septa |
| 3 | Fibrous expansion of most portal areas, occasional portal to portal (P-P) bridging |
| 4 | Fibrous expansion of portal areas with marked bridging (portal to portal (P-P) as well as portal to central (P-C) |
| 5 | Marked bridging (P-P and/or P-C) with occasional nodules (incomplete cirrhosis) |
| 6 | Cirrhosis, probable or definite |
Table 1: Fibrosis Score Used for Reading the Masson's Trichome Stained Liver Sections25.
Representative Results
DMN treated rats lose weight and become less vigorous with ruffled hair coat. There is significant loss in average body weights of DMN treated rats; first detectable after 2 weeks of DMN treatment, and this difference remains through weeks 3 and 4 after DMN treatment (Figure 1a). As the rats receive DMN over successive weeks, damage to the liver causes it to become smaller. The liver index; which is the percent of liver weight at final body weight was significantly lower for the DMN treated rats (Figure 1b).
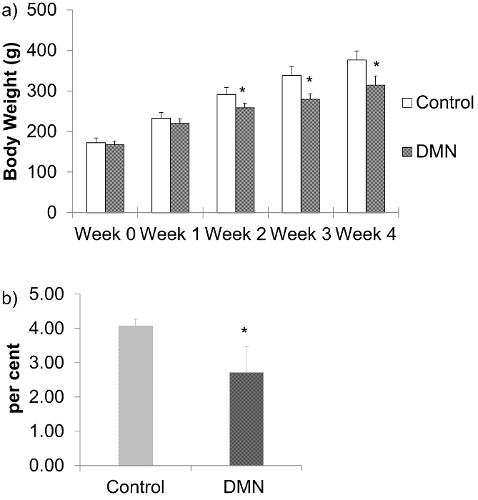 Figure 1: a) Body weights of DMN treated rats. The data are represented as the means ± SD (n = 6 - 8). *P < 0.05 compared with normal control group. b) Liver index; percent liver weight at final body weight of DMN treated rats after 4 weeks of DMN treatment. The data are represented as the means ± SD (n = 6 - 8). *P < 0.05 compared with normal control group. Please click here to view a larger version of this figure.
Figure 1: a) Body weights of DMN treated rats. The data are represented as the means ± SD (n = 6 - 8). *P < 0.05 compared with normal control group. b) Liver index; percent liver weight at final body weight of DMN treated rats after 4 weeks of DMN treatment. The data are represented as the means ± SD (n = 6 - 8). *P < 0.05 compared with normal control group. Please click here to view a larger version of this figure.
At sacrifice, after 4 weeks of DMN treatment, the liver is smaller and harder (Figure 2a) compared to those from aged matched control animals (Figure 2b). Fibrin may be present on the liver surface and adjacent liver lobes are adhered. About 20% of rats have ascites.
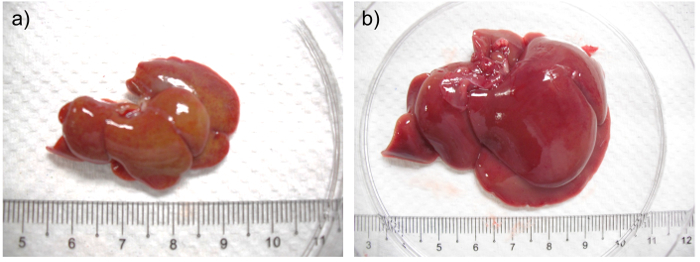 Figure 2:a) Liver from a rat after 4 weeks of DMN treatment. b) Liver from aged matched control rat. In (a) the liver is shrunken, firm and pale with a yellowish tinge when compared with the liver from its aged matched control. Please click here to view a larger version of this figure.
Figure 2:a) Liver from a rat after 4 weeks of DMN treatment. b) Liver from aged matched control rat. In (a) the liver is shrunken, firm and pale with a yellowish tinge when compared with the liver from its aged matched control. Please click here to view a larger version of this figure.
Injury to the liver causes increased permeability of the hepatocyte cell membrane. Increased serum ALT and AST are indicators of hepatocyte damage. Serum ALT (Figure 3a) and AST (Figure 3b) of the DMN treated group are significantly higher than the control group after weeks 2 and 4 of DMN injection. Serum ALT and AST levels typically increase after each week of DMN treatment.
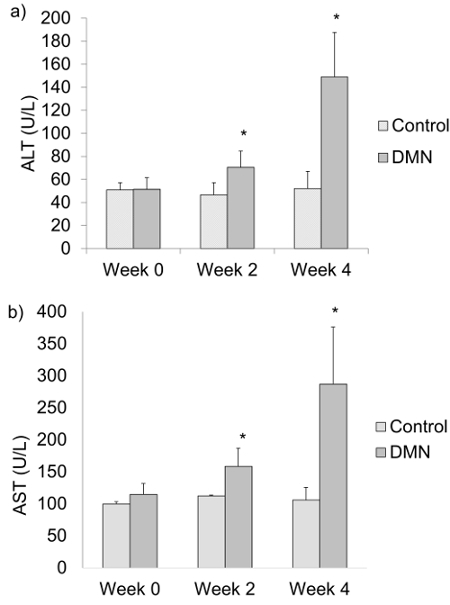 Figure 3:a) Serum alanine aminotransferase (ALT) and b) serum aspartate aminotransferase (AST) levels of DMN treated rats at weeks 0, 2 and 4 after the last DMN injection. The data are represented as the means ± SD (n = 6 - 8). *P < 0.05 compared with normal control group. Please click here to view a larger version of this figure.
Figure 3:a) Serum alanine aminotransferase (ALT) and b) serum aspartate aminotransferase (AST) levels of DMN treated rats at weeks 0, 2 and 4 after the last DMN injection. The data are represented as the means ± SD (n = 6 - 8). *P < 0.05 compared with normal control group. Please click here to view a larger version of this figure.
Histological examination of livers from DMN treated rats show that there is progressive increase and expansion of fibrous septa, with loss of hepatocytes, over time compared with control rats. Masson's Trichome stain is commonly used to highlight collagen deposits in liver tissue: these are stained blue.
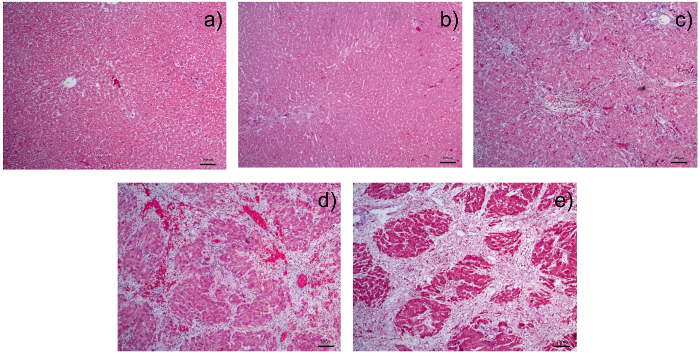
Figure 4: Photomicrographs of Liver Sections Stained with Masson's Trichome:a) liver section from a normal control rat; b) liver section from a rat after receiving 1 week of dimethylnitrosamine (DMN); c) liver section from a rat after receiving 2 weeks of DMN. There is fibrous expansion of most portal areas with occasional portal to portal bridging. d) Liver section from a rat after receiving 3 weeks of DMN. Note the fibrous expansion of portal areas with marked portal to portal as well as portal to central bridging. e) Liver section from a rat after receiving 4 weeks of DMN. There is cirrhosis with nodule formation. The control liver is from the group sacrificed together with rats after 4 weeks of DMN injection. There is a pattern of progressive increase of fibrosis score from 0 in (a); 2 in (b); 3 in (c) 4 in (d) to 5/6 in (e). All photomicrographs were taken at a magnification of 40X with 1 unit length of scale bar equivalent to 100 µm. Please click here to view a larger version of this figure.
Discussion
We have described a method to make an animal model of liver fibrosis and to assess the severity of liver fibrosis. It is important to deliver the correct dose of DMN and adhere to the schedule of weekly intraperitoneal injections. As the experiment progresses, it is crucial to weigh the rats and re-calculate the dose at the start of each week of DMN injections. Keep in mind that DMN is toxic and needs to be handled in the fume cabinet. We perform the intraperitoneal injections in the fume cabinet as well. With the need for repeated injections, the correct restraint and technique of injection is important to avoid introduction of contaminants and damage to internal organs. We rarely have mortality from the intraperitoneal injections.
Rats should be monitored 2x a day throughout the experimental period. During the last week of DMN injection, animals should be observed even more frequently and closely for moribund signs. This is the period when liver damage is most severe and affected rats will be inappetent, lose body weight and condition. 25 - 40% of rats can become severely sick during this period and die if there is no veterinary intervention. Hence close monitoring allows the researcher to assess the condition of the animal and plan appropriate termination of the experiment in order that organs can be freshly harvested from a euthanized rat instead of being lost to decay from unexpected death.
We have found weekly measurement of ALT and AST levels to be useful indicators of the progression of liver damage. We have also observed that AST is less specific than ALT and attribute this to its release from damaged erythrocytes and muscle tissue in addition to liver.
Ensure that sufficient volume of blood is collected to yield the required volume of serum for analysis. We typically collect 200 - 300 µl of blood per rat.
During the collection of liver tissue for histological examination, we recommend the sample be obtained from the same lobe. This is done after checking that the changes in the liver are uniform. DMN causes generalised changes in the liver. In the early stages of the study, we sampled from the left lateral and medial lobes as well as the right medial lobe. We did not observe any differences in the microscopic changes between these lobes.
Liver sections should be fixed in 10% buffered formalin for at least 24 hr and processed soon after. Prolonged immersion beyond a week can result in the tissue becoming brittle and difficult to section after paraffin embedding. Cutting suitable 5 µm thin sections requires practice and patience. Sections that are assessed to be suitable with the naked eye (Step 4.11) can be examined with the microscope to check that they do not have artefacts like folds or tears within the section. The staining procedure is best completed according to the time periods for slide immersion at each step without rest breaks. During the staining process, ensure that the sections are kept moist at all times and transferred from one staining solution to the next as soon as possible.
Evaluation of the Masson's Trichome stained sections for the degree of fibrosis requires the input of a veterinary pathologist. He/She is a crucial team member who should be part of the study from the beginning, as he/she would be able to advise and participate in the correct harvesting of organs at all stages of the experiment. Thus the current gold standard for liver fibrosis scoring is the assessment of appropriately stained tissue sections by a pathologist. Though it would be optimum to have the input of more than one pathologist, this may not be possible in smaller organizations. In such cases, the objectivity can be improved by the use of imaging technology26. We have found that results from imaging technology are comparable to pathological scoring (unpublished data), and recommend that it be used as a supplementary method of evaluation.
In summary, the DMN induced model of liver fibrosis has many advantages over other animal models. It is a comparatively easy model to make as it does not require surgical skills. DMN is environmentally safer and less toxic to humans and animals than CCl4 and takes a shorter time to induce disease than TAA. Compared to liver disease produced by CCl4, TAA, and bile duct ligation, DMN produces a closer representation of human liver fibrosis. For these reasons, we foresee that the model will continue to be used widely to study the mechanisms of liver fibrosis as well as to screen for potential anti-fibrotic agents.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
The authors acknowledge the funding support from the Ministry of Education, Singapore, grant number MOE2010-IF-1-025.
References
- Magee PN. Toxic liver injury; the metabolism of dimethylnitrosamine. Biochem J. 1956;64(4):676–682. doi: 10.1042/bj0640676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard DJ, Butler WH. Apoptosis-the mechanism of cell death in dimethylnitrosamine-induced hepatotoxicity. J Pathol. 1989;158(3):253–260. doi: 10.1002/path.1711580314. [DOI] [PubMed] [Google Scholar]
- Madden JW, Gertman PM, Peacock EE. Dimethylnitrosamine-induced hepatic cirrhosis: a new canine model of an ancient human disease. Surgery. 1970;68(1):260–267. [PubMed] [Google Scholar]
- Jenkins SA, Grandison A, Baxter JN, Day DW, Taylor I, Shields R. A dimethylnitrosamine-induced model of cirrhosis and portal hypertension in the rat. J Hepatol. 1985;1(5):489–499. doi: 10.1016/s0168-8278(85)80747-9. [DOI] [PubMed] [Google Scholar]
- Jézéquel AM, Mancini R, Rinaldesi ML, Macarri G, Venturini C, Orlandi F. A morphological study of the early stages of hepatic fibrosis induced by low doses of dimethylnitrosamine in the rat. J Hepatol. 1987;5(2):174–181. doi: 10.1016/s0168-8278(87)80570-6. [DOI] [PubMed] [Google Scholar]
- George J, Chandrakasan G. Molecular characteristics of dimethylnitrosamine induced fibrotic liver collagen. Biochim Biophys Acta, Protein Struct Mol Enzymol. 1996;1292(2):215–222. doi: 10.1016/0167-4838(95)00202-2. [DOI] [PubMed] [Google Scholar]
- Winwood PJ, Arthur MJ. Kupffer cells: their activation and role in animal models of liver injury and human liver disease. Semin Liver Dis. 1993;13(1):50–59. doi: 10.1055/s-2007-1007337. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sakata R, Ueno T, Sata M, Ueno H. Inhibition of transforming growth factorβ prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology. 2000;32(2):247–255. doi: 10.1053/jhep.2000.9109. [DOI] [PubMed] [Google Scholar]
- Shek FW, Benyon RC. How can transforming growth factor beta be targeted usefully to combat liver fibrosis? Eur J Gastroenterol Hepatol. 2004;16(2):123–126. doi: 10.1097/00042737-200402000-00001. [DOI] [PubMed] [Google Scholar]
- Wang JH, Shin JW, Son JY, Cho JH, Son CG. Antifibrotic effects of CGX, a traditional herbal formula, and its mechanisms in rats. J Ethnopharmacol. 2010;127(2):534–542. doi: 10.1016/j.jep.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Su LJ, Hsu SL, Yang JS, Tseng HH, Huang SF, Huang CYF. Global gene expression profiling of dimethylnitrosamine-induced liver fibrosis: from pathological and biochemical data to microarray analysis. Gene Expr. 2006;13(2):107–132. doi: 10.3727/000000006783991872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Machnik F, Zimmermann T, Schubert H. Thioacetamide-induced cirrhosis-like liver lesions in rats usefulness and reliability of this animal model. Exp Pathol. 1988;34(4):229–236. doi: 10.1016/s0232-1513(88)80155-5. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis. Lab Invest. 1985;53(2):166–186. http://www.ncbi.nlm.nih.gov/pubmed/3894794. [PubMed] [Google Scholar]
- Pritchard MT, Apte U. Models to Study Liver Regeneration. Liver Regeneration. 2015. pp. 15–40.
- Kountouras J, Billing BH, Scheuer PJ. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol. 1984;65(3):305–311. [PMC free article] [PubMed] [Google Scholar]
- Marques TG, Chaib E, et al. Review of experimental models for inducing hepatic cirrhosis by bile duct ligation and carbon tetrachloride injection. Acta Cir Bras. 2012;27(8):589–594. doi: 10.1590/s0102-86502012000800013. [DOI] [PubMed] [Google Scholar]
- Wasser S, Tan CE. Experimental models of hepatic fibrosis in the rat. Ann Acad Med Singapore. 1999;28(1):109–111. [PubMed] [Google Scholar]
- George J, Chandrakasan G. Biochemical abnormalities during the progression of hepatic fibrosis induced by dimethylnitrosamine. Clin Biochem. 2000;33(7):563–570. doi: 10.1016/s0009-9120(00)00170-3. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Ma YR, et al. Effects of Sho-saiko-to, a Japanese herbal medicine, on hepatic fibrosis in rats. Hepatology. 1999;29(1):149–160. doi: 10.1002/hep.510290108. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gao J, Zhang D, Zhang J, Ma J, Jiang H. New insights into the antifibrotic effects of sorafenib on hepatic stellate cells and liver fibrosis. J Hepatol. 2010;53(1):132–144. doi: 10.1016/j.jhep.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Machholz E, Mulder G, Ruiz C, Corning BF, Pritchett-Corning KR. Manual Restraint and Common Compound Administration Routes in Mice and Rats. J Vis Exp. 2012. [DOI] [PMC free article] [PubMed]
- Lee G, Goosens KA. Sampling Blood from the Lateral Tail Vein of the Rat. J Vis Exp. 2015. [DOI] [PMC free article] [PubMed]
- Parkinson CM, O'Brien A, Albers TM, Simon MA, Clifford CB, Pritchett-Corning KR. Diagnostic Necropsy and Selected Tissue and Sample Collection in Rats and Mice. J Vis Exp. 2011. [DOI] [PMC free article] [PubMed]
- Ruehl-Fehlert C, Kittel B, et al. Revised guides for organ sampling and trimming in rats and mice--part 1. Exp Toxicol Pathol. 2003;55(2-3):91–106. [PubMed] [Google Scholar]
- Ishak K, Baptista A, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- Sant'Anna LB, Sant'Anna N, Parolini O. Application of computer assisted image analysis for identifying and quantifying liver fibrosis in a experimental model. J Comput Interdiscip Sci. 2011;2(2):139–148. [Google Scholar]


