Abstract
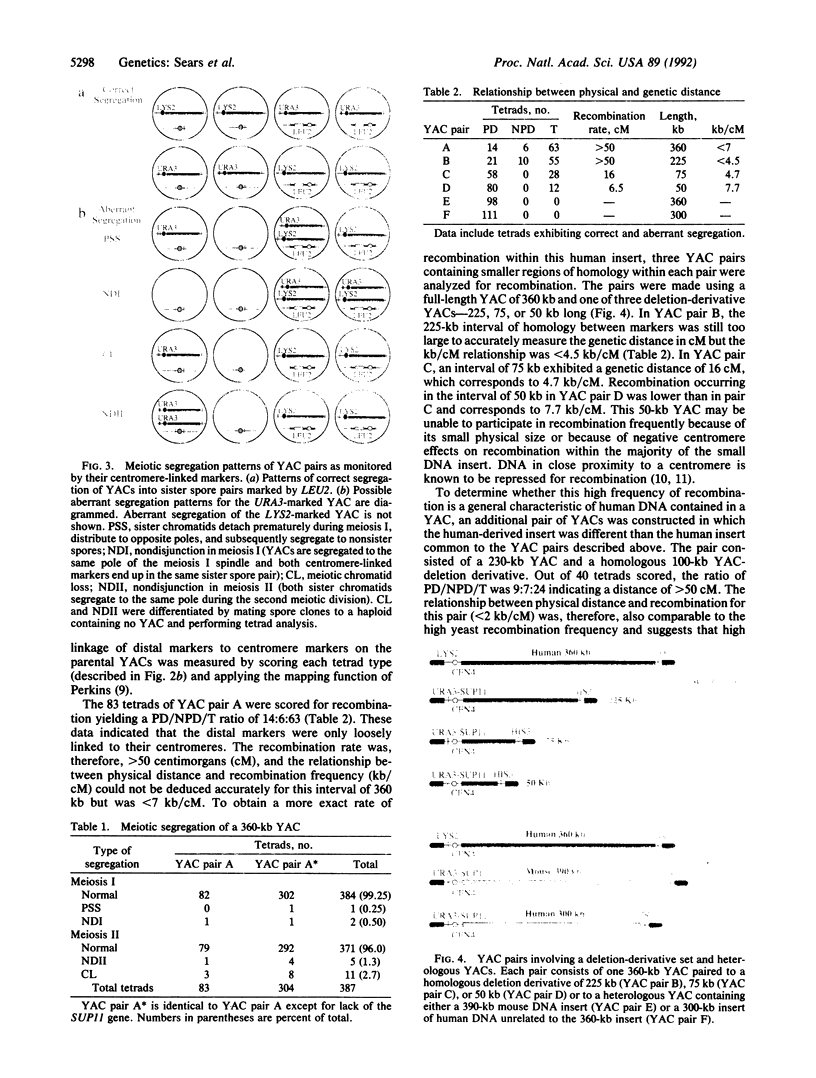
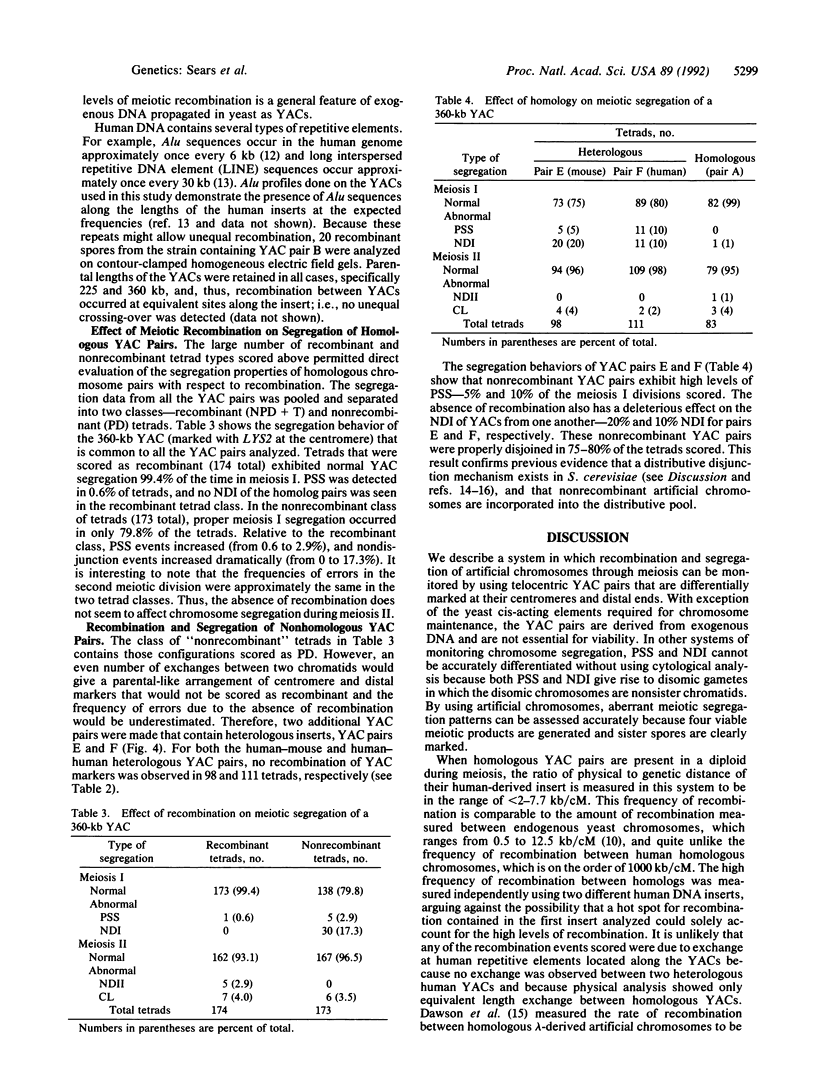
We have developed a system that utilizes human DNA-derived yeast artificial chromosomes (YACs) as marker chromosomes to study factors that contribute to the fidelity of meiotic chromosome transmission. Since aneuploidy for the YACs does not affect spore viability, different classes of meiotic missegregation can be scored accurately in four-viable-spore tetrads including precocious sister separation, meiosis I nondisjunction, meiotic chromatid loss, and meiosis II nondisjunction. Segregation of the homologous pair of 360-kilobase marker YACs was shown to occur with high fidelity in the first meiotic division and was associated with a high frequency of recombination within the human DNA segment. By using this experimental system, a series of YAC deletion derivatives ranging in size from 50 to 225 kilobases was analyzed to directly assess the relationship between meiotic recombination and meiosis I disjunction in a genotypically wild-type background. The relationship between physical distance and recombination frequency within the human DNA segment was measured to be comparable to that of endogenous yeast chromosomal DNA--ranging from less than 2.0 to 7.7 kilobases/centimorgan. Physical analysis of recombinant chromosomes detected no unequal crossing-over at dispersed repetitive elements distributed along the YACs. Recombination between YACs containing unrelated DNA segments was not observed. Furthermore, the segregational data indicated that meioses in which YAC pairs failed to recombine exhibited dramatically increased levels of meiosis I missegregation, including both precocious sister chromatid separation and nondisjunction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Carpenter A. T. Distributive segregation: motors in the polar wind? Cell. 1991 Mar 8;64(5):885–890. doi: 10.1016/0092-8674(91)90313-n. [DOI] [PubMed] [Google Scholar]
- Dawson D. S., Murray A. W., Szostak J. W. An alternative pathway for meiotic chromosome segregation in yeast. Science. 1986 Nov 7;234(4777):713–717. doi: 10.1126/science.3535068. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Green E. D., Olson M. V. Chromosomal region of the cystic fibrosis gene in yeast artificial chromosomes: a model for human genome mapping. Science. 1990 Oct 5;250(4977):94–98. doi: 10.1126/science.2218515. [DOI] [PubMed] [Google Scholar]
- Guacci V., Kaback D. B. Distributive disjunction of authentic chromosomes in Saccharomyces cerevisiae. Genetics. 1991 Mar;127(3):475–488. doi: 10.1093/genetics/127.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback D. B. Meiotic segregation of circular plasmid-minichromosomes from intact chromosomes in Saccharomyces cerevisiae. Curr Genet. 1989 Jun;15(6):385–392. doi: 10.1007/BF00376792. [DOI] [PubMed] [Google Scholar]
- Lambie E. J., Roeder G. S. A yeast centromere acts in cis to inhibit meiotic gene conversion of adjacent sequences. Cell. 1988 Mar 25;52(6):863–873. doi: 10.1016/0092-8674(88)90428-x. [DOI] [PubMed] [Google Scholar]
- McCormick M. K., Shero J. H., Cheung M. C., Kan Y. W., Hieter P. A., Antonarakis S. E. Construction of human chromosome 21-specific yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9991–9995. doi: 10.1073/pnas.86.24.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlon C. S., Lipchitz L. R., Collins I., Deshpande A., Devenish R. J., Green R. P., Klein H. L., Palzkill T. G., Ren R. B., Synn S. Analysis of a circular derivative of Saccharomyces cerevisiae chromosome III: a physical map and identification and location of ARS elements. Genetics. 1991 Oct;129(2):343–357. doi: 10.1093/genetics/129.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan W. J., Hieter P., Reeves R. H. Generation of deletion derivatives by targeted transformation of human-derived yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1300–1304. doi: 10.1073/pnas.87.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. D. Biochemical Mutants in the Smut Fungus Ustilago Maydis. Genetics. 1949 Sep;34(5):607–626. doi: 10.1093/genetics/34.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. F., Schmeckpeper B. J., Abdelrazik M., Comey C. T., O'Hara B., Rossiter J. P., Cooley T., Heath P., Smith K. D., Margolet L. Origin of the human L1 elements: proposed progenitor genes deduced from a consensus DNA sequence. Genomics. 1987 Oct;1(2):113–125. doi: 10.1016/0888-7543(87)90003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shero J. H., Koval M., Spencer F., Palmer R. E., Hieter P., Koshland D. Analysis of chromosome segregation in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:749–773. doi: 10.1016/0076-6879(91)94057-j. [DOI] [PubMed] [Google Scholar]
- Silverman G. A., Green E. D., Young R. L., Jockel J. I., Domer P. H., Korsmeyer S. J. Meiotic recombination between yeast artificial chromosomes yields a single clone containing the entire BCL2 protooncogene. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9913–9917. doi: 10.1073/pnas.87.24.9913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora S., Lucchini G., Magni G. E. Meiotic Diploid Progeny and Meiotic Nondisjunction in SACCHAROMYCES CEREVISIAE. Genetics. 1982 May;101(1):17–33. doi: 10.1093/genetics/101.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]






