Abstract
α-Synuclein is an abundant neuronal protein which localizes predominantly to presynaptic terminals, and is strongly linked genetically and pathologically to Parkinson’s disease and other neurodegenerative diseases. While the accumulation of α-synuclein in the form of misfolded oligomers and large aggregates defines multiple neurodegenerative diseases called “synucleinopathies”, its cellular function has remained largely unclear, and is the subject of intense investigation. In this review, I focus on the structural characteristics of α-synuclein, its cellular and subcellular localization, and discuss how this relates to its function in neurons, in particular at the neuronal synapse.
Keywords: α-synuclein, synapse, membranes, SNARE, neurotransmitter release, synaptic vesicles
HISTORY
α-Synuclein was named after its localization on synaptic vesicles and on nuclear envelopes isolated from the Torpedo electric organ [1]. In parallel, α-synuclein was identified as the non-amyloid-β component (NAC) found in amyloid plaques of Alzheimer’s disease patients [2]. The discovery of α-synuclein was soon followed by the identification of its close homologs β- and γ-synuclein [3–6]. Since then, α-synuclein has been linked to various devastating diseases, including Parkinson’s disease [7, 8], dementia with Lewy bodies [7, 8], multiple system atrophy [9–11], Alzheimer’s disease [12, 13], pantothenate kinase-associated neurodegeneration (PKAN; a.k.a. neurodegeneration with brain iron accumulation type I; formerly Hallervorden-Spatz syndrome) [14–16], Pick’s disease [17], diffuse Lewy body disease [18], Lewy body variant of Alzheimer’s disease [19], amyotrophic lateral sclerosis (ALS) [20, 21], ALS-Parkinsonism-dementia complex of Guam [22, 23], pure autonomic failure [24], frontotemporal dementia [25, 26], progressive supranuclear palsy [27, 28], corticobasal degeneration [29], and Krabbe disease [30], collectively termed “synucleinopathies”. In addition, genome-wide association studies have identified a higher risk of sporadic Parkinson’s disease for individuals with variations in the SNCA gene [31], highlighting α-synuclein’s genetic link to the disease. The physiological function of α-synuclein, however, has remained enigmatic.
α-SYNUCLEIN EXPRESSION & LOCALIZATION
α-Synuclein is a protein of 140 residues that is predominantly and ubiquitously expressed in the brain [4], in particular throughout the neocortex, hippocampus, olfactory bulb, striatum, thalamus, and cerebellum in the rat brain [32]. While initially described as a nuclear protein [33, 34], these reports have not been consistent. In contrast, the presynaptic localization of α-synuclein has become well established (see below). Yet, although α-synuclein is highly enriched in synaptic boutons which sprout from axons of different neurochemical phenotypes, α-synuclein is not present in all synaptic terminals, and, curiously, not all terminals accumulate the protein in neurodegenerative disorders [35], suggesting selective expression, targeting, and pathogenic vulnerability in certain neuronal populations. Furthermore, although highly enriched in the nervous system [2, 4], its expression is not limited to nervous tissues: significant amounts of α-synuclein have been detected in red blood cells [36], and low levels of expression have been found at mRNA and/or protein level also in other tissues [37–43], suggesting more general cellular functions in addition to its activity in the brain.
Out of the three synuclein family members, β-synuclein reveals the most brain-specific expression [44], and γ-synuclein the least [5]. Similar to α-synuclein, β- and γ-synucleins localize to synaptic terminals [4, 45, 46], and overlap with expression of α-synuclein in certain brain areas [5, 44, 47]. Although β- and γ-synuclein are absent from Lewy bodies, they co-localize with α-synuclein in spheroid-like neuronal inclusions in Parkinson’s disease, dementia with Lewy bodies and PKAN [7, 15]. The identification of polymorphisms in β- and γ-synuclein that predispose to dementia with Lewy bodies and diffuse Lewy body disease [18, 48], neurodegeneration in mutant β- and wild-type γ-synuclein transgenic mice [49–51], co-occurrence of β-synuclein in α-synuclein-containing Pick bodies in frontotemporal dementia [17], and the link of γ-synuclein to ALS, Gaucher’s disease, and Alzheimer’s disease [52–54], suggests that all synucleins may be involved in neurodegenerative diseases.
Within the nervous system, the expression of α-synuclein is developmentally regulated. α-Synuclein mRNA expression begins in late embryonic stages in rodents, reaches a peak in the first few postnatal weeks, and is then reduced [55, 56]. α-Synuclein protein levels increase during development and remain high during adulthood [56, 57], suggesting post-transcriptional regulation of its levels. α-Synuclein distributes from the soma to presynaptic terminals during early weeks of development in rodents [58, 59] and in humans [60, 61], where it associates with synaptic vesicles [1, 62]. Although it is still unclear how α-synuclein reaches the synapse, its preference for synaptic vesicle membranes [1, 62], and its affinity for the vesicular SNARE protein synaptobrevin-2 [63], synapsin III [64], or rab3A [65], may target it to presynaptic boutons. Strikingly, while highly concentrated in presynaptic terminals, α-synuclein is among the last proteins to reach the synapse [58, 66]. Together with its presence only in vertebrates [67], this suggests that α-synuclein has an activity required for a more complex cellular function that is not essential for basic neurotransmitter release or synapse development.
STRUCTURE OF α-SYNUCLEIN
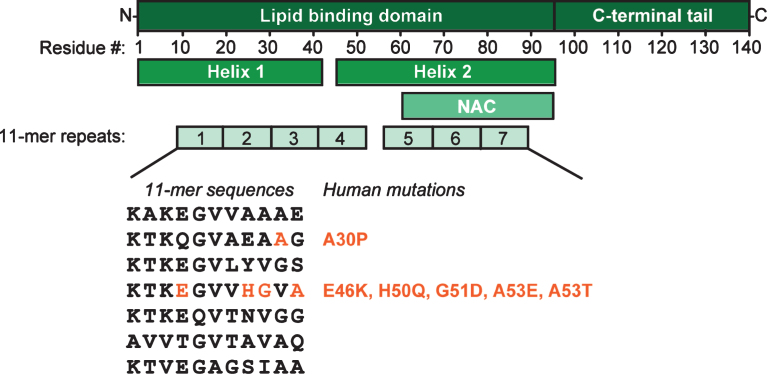
α-Synuclein has a remarkable and unique structure (Fig. 1). Its N-terminal sequence is divided into seven 11-mer repeats with a KTKGEV consensus sequence (residues 1–95), which, similar to apolipoproteins, form an amphipathic alpha-helix with 3 turns, and mediate association of α-synuclein with lipid membranes [68–72]. This region contains also the NAC domain (residues 60–95), an area believed to be responsible for α-synuclein aggregation [2] and sensing of lipid properties [73]. Curiously, all identified mutations associated with synucleinopathies are located in this region: A30P, E46K, H50Q, G51D, A53E, and A53T [74–80], five of which cluster within eight residues, suggesting that lipid binding or lack thereof may be linked to α-synuclein pathology. The C-terminus of α-synuclein (residues 96–140) is highly acidic and largely unstructured [68, 69, 81], target of various post-translational modifications [82], and believed to be responsible for (i) interactions with proteins (see below), (ii) ion, polycation and polyamine binding [83–86], (iii) modulation of membrane binding of α-synuclein [87, 88], and for (iv) protection of α-synuclein from aggregation [89–91].
Fig.1.
α-Synuclein domain structure. Upon binding to lipid membranes, the N-terminal domain of α-synuclein folds into two amphipathic helices; the C-terminal tail of α-synuclein does not contribute to membrane binding. The lipid binding domain can be divided into seven highly conserved 11-mer sequences. Helix 2 contains the aggregation-prone NAC-domain. All disease-linked mutations of α-synuclein are located in the second and fourth 11-mer stretch.
INTRACELLULAR POOLS OF α-SYNUCLEIN
α-Synuclein exists in a dynamic equilibrium between a soluble state and a membrane-bound state, with its secondary structure depending on its environment. The interaction between α-synuclein and lipid surfaces is believed to be key feature for mediating its cellular functions (Fig. 2). Soluble cytosolic α-synuclein is intrinsically unstructured and behaves like a natively unfolded protein [71, 92–95]. A debate has recently developed around α-synuclein’s soluble state, due to a proposed metastable tetrameric form of α-synuclein [96, 97]. While other studies have demonstrated that no such cytosolic tetramer exists in the central nervous system, in erythrocytes, mammalian cells, and in E.coli [94, 95, 98, 99], binding to cellular factors, such as lipids or membranes, can induce and stabilize such multimers [100], as endogenous multimers become unstable as the protein approaches purity [101].
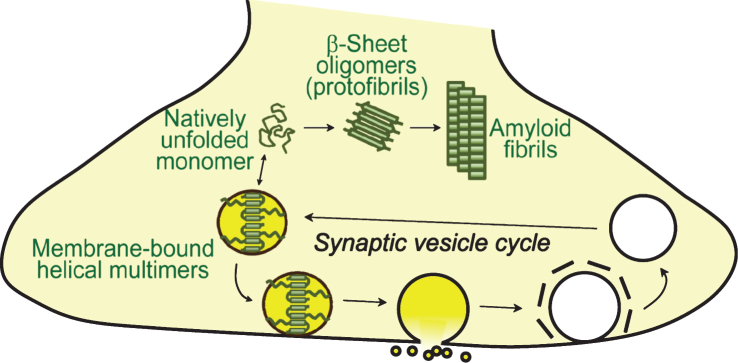
Fig.2.
Physiological and pathological conformations of α-synuclein at the synapse. Cytosolic α-synuclein is monomeric and natively unfolded. Upon binding to synaptic vesicles, the N-terminal residues of α-synuclein adopt a helical structure. Membrane binding of α-synuclein is associated with its multimerization, which is essential for its physiological function at the synapse. Pathologically, unfolded α-synuclein in the cytosol can convert into β-sheet containing oligomers (protofibrils) which eventually form amyloid fibrils.
In presence of lipid membranes, such as artificial liposomes, lipid droplets and lipid rafts, the N-terminal residues of α-synuclein adopt an alpha-helical structure which mediates binding of α-synuclein to membranes [68–71, 102–104]. Membrane binding is likely a cooperative effect of the 11-mer sequences, as truncation of the N-terminal domain reduces lipid binding drastically, and requires acidic head groups [102–106], such as phosphatidylethanolamine, phosphatidylserine or phosphatidylinositol. This suggests an interaction of the membrane headgroups with lysines found on opposite sides of the α-synuclein helix. Both, a single elongated alpha-helix, and a broken alpha-helix have been reported, depending on membrane curvature [68, 71, 72], and α-synuclein is able to transition between these two states [81, 107]: Upon binding to membranes with larger diameter (∼100 nm), α-synuclein adopts an elongated helix [68, 108–111]. In contrast, in presence of small and highly curved vesicles, α-synuclein adopts a broken helix conformation [71, 81, 112, 113], likely to adapt to the smaller liposome area. α-Synuclein preferentially binds to vesicles of smaller diameter [69, 114], and as such associates with synaptic vesicles in the brain [1, 62].
Recently, it was found that α-synuclein is N-terminally acetylated, mediated by attachment of an acetyl group to the alpha amino group of the first amino acid of α-synuclein [94, 95, 115, 116]. N-terminal acetylation of α-synuclein is seen both in healthy and Parkinson’s disease individuals, and increases its helical folding propensity, its affinity for membranes, and its resistance to aggregation [115–118], suggesting that N-terminal acetylation of α-synuclein could have important implications for both the native and pathological structures and functions of α-synuclein [119]. In addition, phosphorylation of α-synuclein regulates its structure, membrane binding, protein interactions, oligomerization, fibril formation, and neurotoxicity [120–125], although the exact kinases and phosphatases regulating (de)phosphorylation of α-synuclein remain unknown. Other post-translational modifications, such as ubiquitination [126, 127], sumoylation [128, 129], glycation [130–132], glycosylation [133, 134], nitration [135–137], and proteolysis [12, 89, 138, 139], can result in changes in protein charge and structure. This may lead to altered binding affinities with other proteins and lipids, but their functional significance remains unknown and controversial.
α-Synuclein folding stabilizes and protects its target membrane [140], and membrane-binding protects α-synuclein from aggregation [141–144], although membrane binding has also been reported to accelerate aggregation under oxidative stress [145]. Recently, alpha-helical multimers of α-synuclein have been reported upon binding of α-synuclein to membranes, which are required for its physiological function at the synapse, and protect α-synuclein from aggregation [100, 142, 146]. In contrast to these physiological conformations, in its pathologically relevant state, alpha -synuclein adopts a beta-sheet rich conformation which is accompanied by aggregation and fibril formation, and deposition into Lewy bodies [147–151]. These cytosolic aggregates are likely derived from the less stable, natively unfolded conformations of cytosolic α-synuclein [142].
α-SYNUCLEIN FUNCTION AT THE SYNAPSE
The normal function of α-synuclein remains enigmatic, despite more than 25 years of research. Assessing the normal function of α-synuclein has been challenging, because: (i) α-Synuclein is an intrinsically unstructured protein that cycles between a natively unfolded state in cytosol, and a helical multimeric state on membranes [71, 92–95, 100]; (ii) Overexpression of α-synuclein triggers toxic effects in humans [152, 153] and in animal models [154–156], that are much worse than the effects caused by loss of α-synuclein [157, 158]. This disconnection of the pathogenic activity of α-synuclein from its physiological function [159] complicates findings in overexpression models; (iii) Potential compensation of α-synuclein function by its isoforms β- and γ-synuclein complicate findings in knockout animals and necessitate simultaneous knockout of all isoforms or acute manipulation, such as done via viral injections. However, α-synuclein’s presynaptic localization and its interaction with highly curved membranes and synaptic proteins strongly suggests a regulatory function associated with the synapse, such as synaptic activity, synaptic plasticity, learning, neurotransmitter release, dopamine metabolism, synaptic vesicle pool maintenance, and/or vesicle trafficking (Fig. 3).
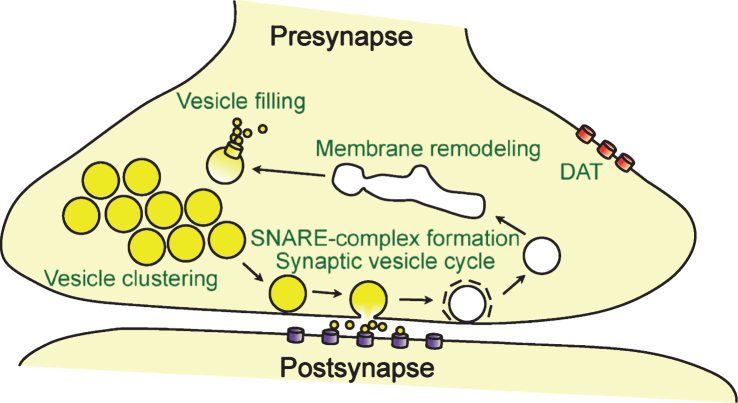
Fig.3.
Function of α-synuclein at the synapse. Shown are the synaptic processes that α-synuclein has been reported to affect, including membrane remodeling, modulation of the dopamine transporter DAT and vesicular monoamine transporter VMAT2, clustering of synaptic vesicles and maintaining synaptic vesicle pools, promoting SNARE-complex assembly, and modulating the release cycle of synaptic vesicles.
Protein interactions
α-Synuclein has been reported to interact with and affect a variety of proteins, mostly at the presynaptic terminal. This includes a controversial binding of phospholipase D [160–163], regulation of the membrane interaction of the G-protein rab3 [65], binding to the SNARE-protein synaptobrevin-2 and chaperoning SNARE-complex assembly [63, 159], binding and modulation of synapsin III [64], binding of VMAT2 [164], dopamine and serotonin transporters [165–167], and regulation of tyrosine hydroxylase [168–170]. While these interactions are compatible with a function at the presynaptic terminal, the reported localization of α-synuclein to mitochondria [171–173], endoplasmic reticulum [174, 175], Golgi [174, 175], and nuclei [1, 176] may arise from an altered subcellular distribution or spillover to other membranes, due to overexpression or during cell disruption. Overall, the functional significance of most of these findings remains unclear.
Lipid transport, lipid packing and membrane biogenesis
The similarity of α-synuclein with class A2 apolipoproteins and decreased brain palmitate, phosphatidylglycerol and cardiolipin metabolism in absence of α-synuclein [177–179] suggest a role in lipid metabolism, although lipidomic profiling of brains from synuclein transgenic and knockout mice revealed minimal effects of synuclein on lipid metabolism [180]. α-Synuclein has been reported to bind to fatty acids [181], and may thus serve as a fatty acid transporter between the cytosol and membrane compartments, while other studies suggest the contrary [182]. Furthermore, α-synuclein has been shown to induce membrane curvature and convert large vesicles into highly curved membrane tubules, cylindrical micelles and vesicles [183–187], driven by binding affinity, partition depth, and interleaflet order asymmetry [188]. In addition, α-synuclein has been reported to organize membrane components [189], to modulate phospholipid composition [190], and to be a specific inhibitor of phospholipase D1 and D2 in vitro andin vivo [160–162]. This suggests that α-synuclein may be involved in cleavage of membrane lipids and membrane biogenesis. Yet, the data on α-synuclein and phospholipase D inhibition are controversial [163]. Last, α-synuclein has been reported to sense lipid packing defects and to affect lipid packing [191, 192], and binding of α-synuclein to synaptic vesicles may stabilize them via stabilizing their intrinsically tight curvature [193].
Impact on dopamine metabolism and dopaminergic neurons
While many types of neurons are affected in Parkinson’s disease [194–196], a remarkable sign is the loss of dopaminergic neurons in the substantia nigra, and the resulting deficiency of dopamine signaling [197–199]. Despite tremendous strides in the understanding of α-synuclein function and dysfunction, the increased vulnerability of dopaminergic neurons to α-synuclein pathology remains unclear at the mechanistic level. α-Synuclein has been proposed to regulate homeostasis of monoamines in synapses, via interaction with the serotonin transporter [165]. It binds to and regulates the targeting and the activity of the dopamine transporter DAT [166, 167, 200], although its mode of action remains controversial [201–203]. α-Synuclein inhibits dopamine synthesis by inhibiting the expression and activity of tyrosine hydroxylase [154, 168–170, 204], likely via reducing the phosphorylation state of tyrosine hydroxylase and stabilizing dephosphorylated inactive tyrosine hydroxylase [168, 205–207]. In agreement, aging-related increases in α-synuclein expression in the substantia nigra negatively correlate to the expression of tyrosine hydroxylase [57]. In addition, α-synuclein affects the vesicular dopamine transporter VMAT2: Knockdown of alpha -synuclein increased the density of VMAT2 molecules per vesicle, while overexpression inhibits VMAT2 activity, interrupting dopamine homeostasis by causing increased cytosolic dopamine levels [164]. In agreement with a function in dopamine metabolism, absence of α-synuclein causes decreased reuptake of dopamine in the dorsal striatum [208], a 36% reduction in striatal dopamine, accompanied by a reduction in tyrosine hydroxylase-positive fibers in the striatum, decreased striatal levels of tyrosine hydroxylase and dopamine transporter [209], and a decrease in the number of dopaminergic neurons in the substantia nigra [210, 211]. In addition, α/β-synuclein double knockout mice display 20% reduced dopamine levels, with no change in dopamine uptake and release [212], a two-fold increase in extracellular dopamine levels upon striatal stimulation, and hyperactivity in a novel environment, which is reminiscent of mice expressing reduced levels of the dopamine transporter [213]. Overall, this suggests that dopaminergic neurons may have both, a higher need for α-synuclein function, and a higher susceptibility to α-synuclein dysfunction. Yet, the presence of α-synuclein in cells other than dopaminergic neurons suggests a more general activity in neuronal function.
Molecular chaperone activity
The biochemical structure of α-synuclein predicts a function as a molecular chaperone capable of binding to other intracellular proteins. This hypothesis was strengthened by three observations: First, alpha -synuclein shares structural and functional homology with the 14-3-3 family of molecular chaperone proteins [214]. Second, via its C-terminal domain, α-synuclein suppresses the aggregation of thermally denatured proteins [215–219], and overexpression of α-synuclein protects dopaminergic neurons from oxidative stress and apoptosis [220, 221]. Third, α-synuclein rescues the lethal neurodegeneration caused by knockout of the co-chaperone CSPα in mice by chaperoning assembly of synaptic SNARE-complexes [63, 222]. This function of α-synuclein is essential for long term functioning of neurons, since α-, β-, γ-synuclein triple-knockout mice have reduced SNARE-complex assembly, show neuropathological signs and reveal shortened survival [63, 223, 224]. This chaperone function is consistent with the lack of an acute effect of α-synuclein on cell survival and neurotransmitter release, and may become particularly important under stressful conditions and during the long life of a neuron.
Neurotransmitter release and synaptic plasticity
The presynaptic localization of α-synuclein, its interaction with synaptic vesicles [1, 62] and synaptobrevin-2 [63], its SNARE-complex chaperoning activity [63], and its changes during periods of song-acquisition-related synaptic rearrangements in birds [225] strongly argues for a role in neurotransmitter release and synaptic plasticity, although its precise function remains unclear. Yet, absence of α-synuclein in worms, flies and yeast suggests that α-synuclein is not required for synaptic transmission or membrane trafficking in general. In agreement, knockout of α-, α/β-, α/γ-, or α/β/γ-synucleins does not induce morphological changes in the brain [63, 157, 212, 224], although changes in synaptic protein levels [63, 212], changes in synapse structure and size [223], and impairments in survival [63, 223] have been reported in synuclein triple knockout mice. Together with neuromuscular pathology in mice lacking α-synuclein [226], and reduced working and spatial memory learning in α-synuclein knockout mice [227, 228], this suggests that α-synuclein contributes to the long-term operation of a neuron.
The effect of α-synuclein on neurotransmission and synaptic plasticity has been investigated both in knockout and under overexpressing conditions, where α-synuclein has been reported to both promote and inhibit neurotransmitter release, or have no effect at all. While some studies reported a lack of effect of α-synuclein on neurotransmitter release [63, 212, 229], others revealed an enhancement of synaptic transmission [223, 224, 230–234], or a decrease in release [157, 158, 213, 223, 235–237]. Two recent studies have reported an inhibitory effect of α-synuclein on synaptic vesicle endocytosis during intense stimulation, but not under basal levels [238, 239], while another study reported an enhancement of clathrin-mediated endocytosis by α-synuclein in neuronal and non-neuronal cells [240]. Whether the inconsistent results obtained for the effects of α-synuclein on neurotransmission and synaptic plasticity could be ascribed to the experimental models used and the investigated brain regions, needs to be determined. It seems to be clear, though, that α-synuclein is not required for basal neurotransmission, but plays an important role in maintaining neurons during intense neuronal activity and over their long lifetime.
How does α-synuclein exert its effect on the neurotransmission machinery? Within the presynaptic terminal, α-synuclein is highly mobile, as shown by photo-bleaching experiments, and α-synuclein disperses from synaptic vesicles upon stimulation [241, 242], similar to synapsin I [243]. Facilitated by its dynamic membrane-binding, this suggests that α-synuclein can be recruited to the site of high membrane-fusion activity, and that neural activity controls the normal function of α-synuclein at the nerve terminal. Indeed, α-synuclein attenuates the mobility of synaptic vesicle pools between presynaptic boutons and maintains the overall size of the recycling pools at individual synapses [244].
In vitro, α-synuclein inhibits docking of synaptic vesicle mimics with plasma membrane mimics [245, 246]. This inhibition is not caused by interfering with the fusion process itself, but is due to clustering of synaptic vesicle mimics, a process strongly dependent on α-synuclein’s ability to associate with lipids and synaptobrevin-2 [246]. α-Synuclein driven vesicle clustering has been initially reported in yeast [247, 248]. Recently, α-synuclein has been reported to cluster synaptic vesicles in neurons [146], which is likely mediated by α-synuclein’s ability to form multimers on the vesicle surface [100, 146]. This clustering activity of α-synuclein restricts synaptic vesicle motility [146], and thereby likely affects the kinetics of neurotransmitter release. Supportively, α-synuclein associates with specific subpopulations of synaptic vesicles [100, 249], and cooperatively regulates synaptic function with synapsin III in dopaminergic neurons [64]. In addition, α-synuclein knockout synapses reveal a selective deficiency of undocked vesicles without affecting docked vesicles [158], and knockdown of α-synuclein leads to a significant reduction in the distal pool of synaptic vesicles [66].
How does clustering of synaptic vesicles by α-synuclein multimers relate to increased SNARE-complex levels? α-Synuclein induced vesicle clustering may increase the local concentration of synaptic vesicles and thereby of the SNARE protein synaptobrevin-2. This clustering of synaptic vesicles at the active zone would promote the formation of neuronal SNARE-complexes by constraining additional synaptic vesicles close to the active zone. Supportively, the SNARE-complex assembly deficit in α/β/γ-synuclein triple knockout mice aggravates with increased synaptic activity [63].
Overall, the effect of α-synuclein on neurotransmitter release is likely not mediated by directly acting on the release machinery, but by affecting the spatial organization of distinct synaptic vesicle pools within the presynaptic terminal, possibly via α-synuclein multimerization, which is triggered by membrane binding and potentiates SNARE-complex assembly [100]. This activity of α-synuclein contributes to the long-term operation of the nervous system, suggesting that alterations in the physiological function of α-synuclein could promote the development of neuropathology in Parkinson’s disease and related disorders.
CONCLUSION
α-Synuclein is important for the normal function and integrity of synapses, and in the aging nervous system, dysfunction of α-synuclein becomes a predisposing factor for synaptic dysfunction and the development of neuropathology. Overexpression of α-synuclein triggers redistribution of the SNARE proteins SNAP-25, syntaxin-1 and synaptobrevin-2 in an age-dependent manner in the striatum [250], impairs proper vesicle trafficking and recycling [175, 248, 251, 252], and large α-synuclein oligomers inhibit SNARE-mediated vesicle fusion in vitro [253]. Furthermore, misfolded α-synuclein, in the form of oligomers and aggregates, is believed to be toxic [254, 255], and recent studies have revealed propagation of misfolded α-synuclein between neurons [256–259]. However, many questions remain unclear, including the causes of the selective vulnerability of dopaminergic neurons in Parkinson’s disease, the triggers for α-synuclein aggregation and pathology, and the role of aging in the pathogenesis of Parkinson’s disease. Understanding how α-synuclein localizes to and functions at the synapse, will provide a biological context to how it misfolds, which species of α-synuclein are toxic, how these species are released and taken up by neurons, and how they nucleate new aggregates in a healthy cell. It is clear that either too little or too much α-synuclein is deleterious for the brain. Thus, aiming at maintaining a healthy balance of α-synuclein in the brain is a worthwhile target for preventing synucleinopathies, while maintaining its normal brain function.
CONFLICT OF INTEREST
The author has none to declare.
ACKNOWLEDGMENTS
I thank Dr. Manu Sharma for critical review of this manuscript. This work was supported by an award from the Leon Levy Foundation, and by the American Parkinson Disease Association.
REFERENCES
- 1.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: A neuron-secific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakajo S, Omata K, Aiuchi T, Shibayama T, Okahashi I, Ochiai H, Nakai Y, Nakaya K, Nakamura Y. Purification and characterization of a novel brain-specific 14-kDa protein. J Neurochem. 1990;55:2031–2038. doi: 10.1111/j.1471-4159.1990.tb05792.x. [DOI] [PubMed] [Google Scholar]
- 4.Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- 5.Lavedan C, Leroy E, Dehejia A, Buchholtz S, Dutra A, Nussbaum RL, Polymeropoulos MH. Identification, localization and characterization of the human gamma-synuclein gene. Hum Genet. 1998;103:106–112. doi: 10.1007/s004390050792. [DOI] [PubMed] [Google Scholar]
- 6.Buchman VL, Hunter HJ, Pinon LG, Thompson J, Privalova EM, Ninkina NN, Davies AM. Persyn, a member of the synuclein family, has a distinct pattern of expression in the developing nervous system. J Neurosci. 1998;18:9335–9341. doi: 10.1523/JNEUROSCI.18-22-09335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galvin JE, Uryu K, Lee VM, Trojanowski JQ. Axon pathology in Parkinson’s disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc Natl Acad Sci U S A. 1999;96:13450–13455. doi: 10.1073/pnas.96.23.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 10.Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- 11.Gai WP, Power JH, Blumbergs PC, Blessing WW. Multiple-system atrophy: A new alpha-synuclein disease? Lancet. 1998;352:547–548. doi: 10.1016/s0140-6736(05)79256-4. [DOI] [PubMed] [Google Scholar]
- 12.Lewis KA, Su Y, Jou O, Ritchie C, Foong C, Hynan LS, White CL, 3rd, Thomas PJ, Hatanpaa KJ. Abnormal neurites containing C-terminally truncated alpha-synuclein are present in Alzheimer’s disease without conventional Lewy body pathology. Am J Pathol. 2010;177:3037–3050. doi: 10.2353/ajpath.2010.100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton RL. Lewy bodies in Alzheimer’s disease: A neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito Y, Kawai M, Inoue K, Sasaki R, Arai H, Nanba E, Kuzuhara S, Ihara Y, Kanazawa I, Murayama S. Widespread expression of alpha-synuclein and tau immunoreactivity in Hallervorden-Spatz syndrome with protracted clinical course. J Neurol Sci. 2000;177:48–59. doi: 10.1016/s0022-510x(00)00337-3. [DOI] [PubMed] [Google Scholar]
- 15.Galvin JE, Giasson B, Hurtig HI, Lee VM, Trojanowski JQ. Neurodegeneration with brain iron accumulation, type 1 is characterized by alpha-, beta-, and gamma-synuclein neuropathology. Am J Pathol. 2000;157:361–368. doi: 10.1016/s0002-9440(10)64548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arawaka S, Saito Y, Murayama S, Mori H. Lewy body in neurodegeneration with brain iron accumulation type 1 is immunoreactive for alpha-synuclein. Neurology. 1998;51:887–889. doi: 10.1212/wnl.51.3.887. [DOI] [PubMed] [Google Scholar]
- 17.Mori F, Hayashi S, Yamagishi S, Yoshimoto M, Yagihashi S, Takahashi H, Wakabayashi K. Pick’s disease: Alpha- and beta-synuclein-immunoreactive Pick bodies in the dentate gyrus. Acta Neuropathol. 2002;104:455–461. doi: 10.1007/s00401-002-0578-9. [DOI] [PubMed] [Google Scholar]
- 18.Nishioka K, Wider C, Vilarino-Guell C, Soto-Ortolaza AI, Lincoln SJ, Kachergus JM, Jasinska-Myga B, Ross OA, Rajput A, Robinson CA, Ferman TJ, Wszolek ZK, Dickson DW, Farrer MJ. Association of alpha-, beta-, and gamma-Synuclein with diffuse lewy body disease. Arch Neurol. 2010;67:970–975. doi: 10.1001/archneurol.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wirths O, Weickert S, Majtenyi K, Havas L, Kahle PJ, Okochi M, Haass C, Multhaup G, Beyreuther K, Bayer TA. Lewy body variant of Alzheimer’s disease: Alpha-synuclein in dystrophic neurites of A beta plaques. Neuroreport. 2000;11:3737–3741. doi: 10.1097/00001756-200011270-00029. [DOI] [PubMed] [Google Scholar]
- 20.Doherty MJ, Bird TD, Leverenz JB. Alpha-synuclein in motor neuron disease: An immunohistologic study. Acta Neuropathol. 2004;107:169–175. doi: 10.1007/s00401-003-0790-2. [DOI] [PubMed] [Google Scholar]
- 21.Takei Y, Oguchi K, Koshihara H, Hineno A, Nakamura A, Ohara S. alpha-Synuclein coaggregation in familial amyotrophic lateral sclerosis with SOD1 gene mutation. Hum Pathol. 2013;44:1171–1176. doi: 10.1016/j.humpath.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki M, Arai Y, Baba M, Iwatsubo T, Mori O, Katayama Y, Oyanagi K. Alpha-synuclein inclusions in amygdala in the brains of patients with the parkinsonism-dementia complex of Guam. J Neuropathol Exp Neurol. 2000;59:585–591. doi: 10.1093/jnen/59.7.585. [DOI] [PubMed] [Google Scholar]
- 23.Forman MS, Schmidt ML, Kasturi S, Perl DP, Lee VM, Trojanowski JQ. Tau and alpha-synuclein pathology in amygdala of Parkinsonism-dementia complex patients of Guam. Am J Pathol. 2002;160:1725–1731. doi: 10.1016/s0002-9440(10)61119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arai K, Kato N, Kashiwado K, Hattori T. Pure autonomic failure in association with human alpha-synucleinopathy. Neurosci Lett. 2000;296:171–173. doi: 10.1016/s0304-3940(00)01623-2. [DOI] [PubMed] [Google Scholar]
- 25.Wilhelmsen KC, Forman MS, Rosen HJ, Alving LI, Goldman J, Feiger J, Lee JV, Segall SK, Kramer JH, Lomen-Hoerth C, Rankin KP, Johnson J, Feiler HS, Weiner MW, Lee VM, Trojanowski JQ, Miller BL. 17q-linked frontotemporal dementia-amyotrophic lateral sclerosis without tau mutations with tau and alpha-synuclein inclusions. Arch Neurol. 2004;61:398–406. doi: 10.1001/archneur.61.3.398. [DOI] [PubMed] [Google Scholar]
- 26.Yancopoulou D, Xuereb JH, Crowther RA, Hodges JR, Spillantini MG. Tau and alpha-synuclein inclusions in a case of familial frontotemporal dementia and progressive aphasia. J Neuropathol Exp Neurol. 2005;64:245–253. doi: 10.1093/jnen/64.3.245. [DOI] [PubMed] [Google Scholar]
- 27.Judkins AR, Forman MS, Uryu K, Hinkle DA, Asbury AK, Lee VM, Trojanowski JQ. Co-occurrence of Parkinson’s disease with progressive supranuclear palsy. Acta Neuropathol. 2002;103:526–530. doi: 10.1007/s00401-001-0483-7. [DOI] [PubMed] [Google Scholar]
- 28.Mori H, Oda M, Komori T, Arai N, Takanashi M, Mizutani T, Hirai S, Mizuno Y. Lewy bodies in progressive supranuclear palsy. Acta Neuropathol. 2002;104:273–278. doi: 10.1007/s00401-002-0555-3. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita S, Sakashita N, Yamashita T, Tawara N, Tasaki M, Kawakami K, Komohara Y, Fujiwara Y, Kamikawa M, Nakagawa T, Hirano T, Maeda Y, Hasegawa M, Takeya M, Ando Y. Concomitant accumulation of alpha-synuclein and TDP-43 in a patient with corticobasal degeneration. J Neurol. 2014;261:2209–2217. doi: 10.1007/s00415-014-7491-8. [DOI] [PubMed] [Google Scholar]
- 30.Smith BR, Santos MB, Marshall MS, Cantuti-Castelvetri L, Lopez-Rosas A, Li G, van Breemen R, Claycomb KI, Gallea JI, Celej MS, Crocker SJ, Givogri MI, Bongarzone ER. Neuronal inclusions of alpha-synuclein contribute to the pathogenesis of Krabbe disease. J Pathol. 2014;232:509–521. doi: 10.1002/path.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 33.Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K. Immunohistochemical comparison of alpha- and beta-synuclein in adult rat central nervous system. Brain Res. 2002;941:118–126. doi: 10.1016/s0006-8993(02)02643-4. [DOI] [PubMed] [Google Scholar]
- 34.Yu S, Li X, Liu G, Han J, Zhang C, Li Y, Xu S, Liu C, Gao Y, Yang H, Ueda K, Chan P. Extensive nuclear localization of alpha-synuclein in normal rat brain neurons revealed by a novel monoclonal antibody. Neuroscience. 2007;145:539–555. doi: 10.1016/j.neuroscience.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 35.Totterdell S, Hanger D, Meredith GE. The ultrastructural distribution of alpha-synuclein-like protein in normal mouse brain. Brain Res. 2004;1004:61–72. doi: 10.1016/j.brainres.2003.10.072. [DOI] [PubMed] [Google Scholar]
- 36.Nakai M, Fujita M, Waragai M, Sugama S, Wei J, Akatsu H, Ohtaka-Maruyama C, Okado H, Hashimoto M. Expression of alpha-synuclein, a presynaptic protein implicated in Parkinson’s disease, in erythropoietic lineage. Biochem Biophys Res Commun. 2007;358:104–110. doi: 10.1016/j.bbrc.2007.04.108. [DOI] [PubMed] [Google Scholar]
- 37.Askanas V, Engel WK, Alvarez RB, McFerrin J, Broccolini A. Novel immunolocalization of alpha-synuclein in human muscle of inclusion-body myositis, regenerating and necrotic muscle fibers, and at neuromuscular junctions. J Neuropathol Exp Neurol. 2000;59:592–598. doi: 10.1093/jnen/59.7.592. [DOI] [PubMed] [Google Scholar]
- 38.Ltic S, Perovic M, Mladenovic A, Raicevic N, Ruzdijic S, Rakic L, Kanazir S. Alpha-synuclein is expressed in different tissues during human fetal development. J Mol Neurosci. 2004;22:199–204. doi: 10.1385/jmn:22:3:199. [DOI] [PubMed] [Google Scholar]
- 39.Tamo W, Imaizumi T, Tanji K, Yoshida H, Mori F, Yoshimoto M, Takahashi H, Fukuda I, Wakabayashi K, Satoh K. Expression of alpha-synuclein, the precursor of non-amyloid beta component of Alzheimer’s disease amyloid, in human cerebral blood vessels. Neurosci Lett. 2002;326:5–8. doi: 10.1016/s0304-3940(02)00297-5. [DOI] [PubMed] [Google Scholar]
- 40.Shin EC, Cho SE, Lee DK, Hur MW, Paik SR, Park JH, Kim J. Expression patterns of alpha-synuclein in human hematopoietic cells and in Drosophila at different developmental stages. Mol Cells. 2000;10:65–70. doi: 10.1007/s10059-000-0065-x. [DOI] [PubMed] [Google Scholar]
- 41.Kim S, Jeon BS, Heo C, Im PS, Ahn TB, Seo JH, Kim HS, Park CH, Choi SH, Cho SH, Lee WJ, Suh YH. Alpha-synuclein induces apoptosis by altered expression in human peripheral lymphocyte in Parkinson’s disease. FASEB J. 2004;18:1615–1617. doi: 10.1096/fj.04-1917fje. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto M, Yoshimoto M, Sisk A, Hsu LJ, Sundsmo M, Kittel A, Saitoh T, Miller A, Masliah E. NACP, a synaptic protein involved in Alzheimer’s disease, is differentially regulated during megakaryocyte differentiation. Biochem Biophys Res Commun. 1997;237:611–616. doi: 10.1006/bbrc.1997.6978. [DOI] [PubMed] [Google Scholar]
- 43.Li QX, Campbell BC, McLean CA, Thyagarajan D, Gai WP, Kapsa RM, Beyreuther K, Masters CL, Culvenor JG. Platelet alpha- and gamma-synucleins in Parkinson’s disease and normal control subjects. J Alzheimers Dis. 2002;4:309–315. doi: 10.3233/jad-2002-4406. [DOI] [PubMed] [Google Scholar]
- 44.Lavedan C, Leroy E, Torres R, Dehejia A, Dutra A, Buchholtz S, Nussbaum RL, Polymeropoulos MH. Genomic organization and expression of the human beta-synuclein gene (SNCB) Genomics. 1998;54:173–175. doi: 10.1006/geno.1998.5556. [DOI] [PubMed] [Google Scholar]
- 45.Nakajo S, Tsukada K, Omata K, Nakamura Y, Nakaya K. A new brain-specific 14-kDa protein is a phosphoprotein. Its complete amino acid sequence and evidence for phosphorylation. Eur J Biochem. 1993;217:1057–1063. doi: 10.1111/j.1432-1033.1993.tb18337.x. [DOI] [PubMed] [Google Scholar]
- 46.Ninkina N, Peters OM, Connor-Robson N, Lytkina O, Sharfeddin E, Buchman VL. Contrasting effects of alpha-synuclein and gamma-synuclein on the phenotype of cysteine string protein alpha (CSPalpha) null mutant mice suggest distinct function of these proteins in neuronal synapses. J Biol Chem. 2012;287:44471–44477. doi: 10.1074/jbc.M112.422402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ninkina NN, Alimova-Kost MV, Paterson JW, Delaney L, Cohen BB, Imreh S, Gnuchev NV, Davies AM, Buchman VL. Organization, expression and polymorphism of the human persyn gene. Hum Mol Genet. 1998;7:1417–1424. doi: 10.1093/hmg/7.9.1417. [DOI] [PubMed] [Google Scholar]
- 48.Ohtake H, Limprasert P, Fan Y, Onodera O, Kakita A, Takahashi H, Bonner LT, Tsuang DW, Murray IV, Lee VM, Trojanowski JQ, Ishikawa A, Idezuka J, Murata M, Toda T, Bird TD, Leverenz JB, Tsuji S, La Spada AR. Beta-synuclein gene alterations in dementia with Lewy bodies. Neurology. 2004;63:805–811. doi: 10.1212/01.wnl.0000139870.14385.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujita M, Sugama S, Sekiyama K, Sekigawa A, Tsukui T, Nakai M, Waragai M, Takenouchi T, Takamatsu Y, Wei J, Rockenstein E, Laspada AR, Masliah E, Inoue S, Hashimoto M. A beta-synuclein mutation linked to dementia produces neurodegeneration when expressed in mouse brain. Nat Commun. 2010;1:110. doi: 10.1038/ncomms1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ninkina N, Peters O, Millership S, Salem H, van der Putten H, Buchman VL. Gamma-synucleinopathy: Neurodegeneration associated with overexpression of the mouse protein. Hum Mol Genet. 2009;18:1779–1794. doi: 10.1093/hmg/ddp090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peters OM, Millership S, Shelkovnikova TA, Soto I, Keeling L, Hann A, Marsh-Armstrong N, Buchman VL, Ninkina N. Selective pattern of motor system damage in gamma-synuclein transgenic mice mirrors the respective pathology in amyotrophic lateral sclerosis. Neurobiol Dis. 2012;48:124–131. doi: 10.1016/j.nbd.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rockenstein E, Hansen LA, Mallory M, Trojanowski JQ, Galasko D, Masliah E. Altered expression of the synuclein family mRNA in Lewy body and Alzheimer’s disease. Brain Res. 2001;914:48–56. doi: 10.1016/s0006-8993(01)02772-x. [DOI] [PubMed] [Google Scholar]
- 53.Myerowitz R, Mizukami H, Richardson KL, Finn LS, Tifft CJ, Proia RL. Global gene expression in a type 2 Gaucher disease brain. Mol Genet Metab. 2004;83:288–296. doi: 10.1016/j.ymgme.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 54.Peters OM, Shelkovnikova T, Highley JR, Cooper-Knock J, Hortobagyi T, Troakes C, Ninkina N, Buchman VL. Gamma-synuclein pathology in amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2015;2:29–37. doi: 10.1002/acn3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kholodilov NG, Neystat M, Oo TF, Lo SE, Larsen KE, Sulzer D, Burke RE. Increased expression of rat synuclein in the substantia nigra pars compacta identified by mRNA differential display in a model of developmental target injury. J Neurochem. 1999;73:2586–2599. doi: 10.1046/j.1471-4159.1999.0732586.x. [DOI] [PubMed] [Google Scholar]
- 56.Petersen K, Olesen OF, Mikkelsen JD. Developmental expression of alpha-synuclein in rat hippocampus and cerebral cortex. Neuroscience. 1999;91:651–659. doi: 10.1016/s0306-4522(98)00596-x. [DOI] [PubMed] [Google Scholar]
- 57.Chu Y, Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson’s disease? Neurobiol Dis. 2007;25:134–149. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 58.Withers GS, George JM, Banker GA, Clayton DF. Delayed localization of synelfin (synuclein, NACP) to presynaptic terminals in cultured rat hippocampal neurons. Brain Res Dev Brain Res. 1997;99:87–94. doi: 10.1016/s0165-3806(96)00210-6. [DOI] [PubMed] [Google Scholar]
- 59.Hsu LJ, Mallory M, Xia Y, Veinbergs I, Hashimoto M, Yoshimoto M, Thal LJ, Saitoh T, Masliah E. Expression pattern of synucleins (non-Abeta component of Alzheimer’s disease amyloid precursor protein/alpha-synuclein) during murine brain development. J Neurochem. 1998;71:338–344. doi: 10.1046/j.1471-4159.1998.71010338.x. [DOI] [PubMed] [Google Scholar]
- 60.Bayer TA, Jakala P, Hartmann T, Egensperger R, Buslei R, Falkai P, Beyreuther K. Neural expression profile of alpha-synuclein in developing human cortex. Neuroreport. 1999;10:2799–2803. doi: 10.1097/00001756-199909090-00019. [DOI] [PubMed] [Google Scholar]
- 61.Galvin JE, Schuck TM, Lee VM, Trojanowski JQ. Differential expression and distribution of alpha-, beta-, and gamma-synuclein in the developing human substantia nigra. Exp Neurol. 2001;168:347–355. doi: 10.1006/exnr.2000.7615. [DOI] [PubMed] [Google Scholar]
- 62.Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, van Der Putten H, Probst A, Kremmer E, Kretzschmar HA, Haass C. Subcellular localization of wild-type and Parkinson’s disease-associated mutant alpha -synuclein in human and transgenic mouse brain. J Neurosci. 2000;20:6365–6373. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zaltieri M, Grigoletto J, Longhena F, Navarria L, Favero G, Castrezzati S, Colivicchi MA, Corte LD, Rezzani R, Pizzi M, Benfenati F, Spillantini MG, Missale C, Spano P, Bellucci A. Alpha-synuclein and synapsin III cooperatively regulate synaptic function in dopamine neurons. J Cell Sci. 2015;128:2231–2243. doi: 10.1242/jcs.157867. [DOI] [PubMed] [Google Scholar]
- 65.Chen RH, Wislet-Gendebien S, Samuel F, Visanji NP, Zhang G, Marsilio D, Langman T, Fraser PE, Tandon A. alpha-Synuclein membrane association is regulated by the Rab3a recycling machinery and presynaptic activity. J Biol Chem. 2013;288:7438–7449. doi: 10.1074/jbc.M112.439497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.George JM. The synucleins. Genome Biol. 2002;3 doi: 10.1186/gb-2001-3-1-reviews3002. REVIEWS3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bussell R, Jr, Eliezer D. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J Mol Biol. 2003;329:763–778. doi: 10.1016/s0022-2836(03)00520-5. [DOI] [PubMed] [Google Scholar]
- 69.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 70.Eliezer D, Kutluay E, Bussell R, Jr, Browne G. Conformational properties of alpha-synuclein in its free and lipid-associated states. J Mol Biol. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- 71.Chandra S, Chen X, Rizo J, Jahn R, Sudhof TC. A broken alpha -helix in folded alpha -Synuclein. J Biol Chem. 2003;278:15313–15318. doi: 10.1074/jbc.M213128200. [DOI] [PubMed] [Google Scholar]
- 72.Bussell R, Jr, Ramlall TF, Eliezer D. Helix periodicity, topology, and dynamics of membrane-associated alpha-synuclein. Protein Sci. 2005;14:862–872. doi: 10.1110/ps.041255905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fusco G, De Simone A, Gopinath T, Vostrikov V, Vendruscolo M, Dobson CM, Veglia G. Direct observation of the three regions in alpha-synuclein that determine its membrane-bound behaviour. Nat Commun. 2014;5:3827. doi: 10.1038/ncomms4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B, Weir D, Thompson C, Szu-Tu C, Trinh J, Aasly JO, Rajput A, Rajput AH, Jon Stoessl A, Farrer MJ. Alpha-synuclein p. H50Q, a novel pathogenic mutation for Parkinson’s disease. Mov Disord. 2013;28:811–813. doi: 10.1002/mds.25421. [DOI] [PubMed] [Google Scholar]
- 75.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 76.Lesage S, Anheim M, Letournel F, Bousset L, Honore A, Rozas N, Pieri L, Madiona K, Durr A, Melki R, Verny C, Brice A. G51D alpha-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol. 2013;73:459–471. doi: 10.1002/ana.23894. [DOI] [PubMed] [Google Scholar]
- 77.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 78.Proukakis C, Dudzik CG, Brier T, MacKay DS, Cooper JM, Millhauser GL, Houlden H, Schapira AH. A novel alpha-synuclein missense mutation in Parkinson disease. Neurology. 2013;80:1062–1064. doi: 10.1212/WNL.0b013e31828727ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 80.Pasanen P, Myllykangas L, Siitonen M, Raunio A, Kaakkola S, Lyytinen J, Tienari PJ, Poyhonen M, Paetau A. Novel alpha-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson’s disease-type pathology. Neurobiol Aging. 2014;35(2180):e2181–e2185. doi: 10.1016/j.neurobiolaging.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 81.Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human alpha-synuclein. J Biol Chem. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 82.Oueslati A, Fournier M, Lashuel HA. Role of post-translational modifications in modulating the structure, function and toxicity of alpha-synuclein: Imlications for Parkinson’s disease pathogenesis and therapies. Prog Brain Res. 2010;183:115–145. doi: 10.1016/S0079-6123(10)83007-9. [DOI] [PubMed] [Google Scholar]
- 83.Fernandez CO, Hoyer W, Zweckstetter M, Jares-Erijman EA, Subramaniam V, Griesinger C, Jovin TM. NMR of alpha-synuclein-polyamine complexes elucidates the mechanism and kinetics of induced aggregation. EMBO J. 2004;23:2039–2046. doi: 10.1038/sj.emboj.7600211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown DR. Interactions between metals and alpha-synuclein–function or artefact? FEBS J. 2007;274:3766–3774. doi: 10.1111/j.1742-4658.2007.05917.x. [DOI] [PubMed] [Google Scholar]
- 85.Paik SR, Shin HJ, Lee JH, Chang CS, Kim J. Copper(II)-induced self-oligomerization of alpha-synuclein. Biochem J. 1999;340(Pt 3):821–828. [PMC free article] [PubMed] [Google Scholar]
- 86.Nielsen MS, Vorum H, Lindersson E, Jensen PH. Ca2+binding to alpha-synuclein regulates ligand binding and oligomerization. J Biol Chem. 2001;276:22680–22684. doi: 10.1074/jbc.M101181200. [DOI] [PubMed] [Google Scholar]
- 87.Bodner CR, Dobson CM, Bax A. Multiple tight phospholipid-binding modes of alpha-synuclein revealed by solution NMR spectroscopy. J Mol Biol. 2009;390:775–790. doi: 10.1016/j.jmb.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sevcsik E, Trexler AJ, Dunn JM, Rhoades E. Allostery in a disordered protein: Oxidative modifications to alha-synuclein act distally to regulate membrane binding. J Am Chem Soc. 2011;133:7152–7158. doi: 10.1021/ja2009554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoyer W, Cherny D, Subramaniam V, Jovin TM. Impact of the acidic C-terminal region comprising amino acids 109-140 on alpha-synuclein aggregation in vitro. Biochemistry. 2004;43:16233–16242. doi: 10.1021/bi048453u. [DOI] [PubMed] [Google Scholar]
- 90.Park SM, Jung HY, Kim TD, Park JH, Yang CH, Kim J. Distinct roles of the N-terminal-binding domain and the C-terminal-solubilizing domain of alpha-synuclein, a molecular chaperone. J Biol Chem. 2002;277:28512–28520. doi: 10.1074/jbc.M111971200. [DOI] [PubMed] [Google Scholar]
- 91.Crowther RA, Jakes R, Spillantini MG, Goedert M. Synthetic filaments assembled from C-terminally truncated alpha-synuclein. FEBS Lett. 1998;436:309–312. doi: 10.1016/s0014-5793(98)01146-6. [DOI] [PubMed] [Google Scholar]
- 92.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 93.Kim J. Evidence that the precursor protein of non-A beta component of Alzheimer’s disease amyloid (NACP) has an extended structure primarily composed of random-coil. Mol Cells. 1997;7:78–83. [PubMed] [Google Scholar]
- 94.Fauvet B, Mbefo MK, Fares MB, Desobry C, Michael S, Ardah MT, Tsika E, Coune P, Prudent M, Lion N, Eliezer D, Moore DJ, Schneider B, Aebischer P, El-Agnaf OM, Masliah E, Lashuel HA. alpha-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287:15345–15364. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burré J, Vivona S, Diao J, Sharma M, Brunger AT, Sudhof TC. Properties of native brain alpha-synuclein. Nature. 2013;498:E4–E6. doi: 10.1038/nature12125. discussion E6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang W, Perovic I, Chittuluru J, Kaganovich A, Nguyen LT, Liao J, Auclair JR, Johnson D, Landeru A, Simorellis AK, Ju S, Cookson MR, Asturias FJ, Agar JN, Webb BN, Kang C, Ringe D, Petsko GA, Pochapsky TC, Hoang QQ. A soluble alpha-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci U S A. 2011;108:17797–17802. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Binolfi A, Theillet FX, Selenko P. Bacterial in-cell NMR of human alpha-synuclein: A disordered monomer by nature? Biochem Soc Trans. 2012;40:950–954. doi: 10.1042/BST20120096. [DOI] [PubMed] [Google Scholar]
- 99.Smaldone G, Diana D, Pollegioni L, Di Gaetano S, Fattorusso R, Pedone E. Insight into conformational modification of alpha-synuclein in the presence of neuronal whole cells and of their isolated membranes. FEBS Lett. 2015;589:798–804. doi: 10.1016/j.febslet.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 100.Burré J, Sharma M, Sudhof TC. alpha-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci U S A. 2014;111:E4274–E4283. doi: 10.1073/pnas.1416598111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luth ES, Bartels T, Dettmer U, Kim NC, Selkoe DJ. Purification of alpha-synuclein from human brain reveals an instability of endogenous multimers as the protein approaches purity. Biochemistry. 2015;54:279–292. doi: 10.1021/bi501188a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Middleton ER, Rhoades E. Effects of curvature and composition on alpha-synuclein binding to lipid vesicles. Biophys J. 2010;99:2279–2288. doi: 10.1016/j.bpj.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jo E, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE. alpha-Synuclein membrane interactions and lipid specificity. J Biol Chem. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 104.Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human alpha-Synuclein and Parkinson’s disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J Biol Chem. 2000;275:34393–34398. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- 105.Kubo S, Nemani VM, Chalkley RJ, Anthony MD, Hattori N, Mizuno Y, Edwards RH, Fortin DL. A combinatorial code for the interaction of alpha-synuclein with membranes. J Biol Chem. 2005;280:31664–31672. doi: 10.1074/jbc.M504894200. [DOI] [PubMed] [Google Scholar]
- 106.Bisaglia M, Schievano E, Caporale A, Peggion E, Mammi S. The 11-mer repeats of human alpha-synuclein in vesicle interactions and lipid composition discrimination: A cooperative role. Biopolymers. 2006;84:310–316. doi: 10.1002/bip.20440. [DOI] [PubMed] [Google Scholar]
- 107.Georgieva ER, Ramlall TF, Borbat PP, Freed JH, Eliezer D. The lipid-binding domain of wild type and mutant alpha-synuclein: Comactness and interconversion between the broken and extended helix forms. J Biol Chem. 2010;285:28261–28274. doi: 10.1074/jbc.M110.157214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trexler AJ, Rhoades E. Alpha-synuclein binds large unilamellar vesicles as an extended helix. Biochemistry. 2009;48:2304–2306. doi: 10.1021/bi900114z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jao CC, Der-Sarkissian A, Chen J, Langen R. Structure of membrane-bound alpha-synuclein studied by site-directed spin labeling. Proc Natl Acad Sci U S A. 2004;101:8331–8336. doi: 10.1073/pnas.0400553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Georgieva ER, Ramlall TF, Borbat PP, Freed JH, Eliezer D. Membrane-bound alpha-synuclein forms an extended helix: Long-distance pulsed ESR measurements using vesicles, bicelles, and rodlike micelles. J Am Chem Soc. 2008;130:12856–12857. doi: 10.1021/ja804517m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jao CC, Hegde BG, Chen J, Haworth IS, Langen R. Structure of membrane-bound alpha-synuclein from site-directed spin labeling and computational refinement. Proc Natl Acad Sci U S A. 2008;105:19666–19671. doi: 10.1073/pnas.0807826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Borbat P, Ramlall TF, Freed JH, Eliezer D. Inter-helix distances in lysophospholipid micelle-bound alpha-synuclein from pulsed ESR measurements. J Am Chem Soc. 2006;128:10004–10005. doi: 10.1021/ja063122l. [DOI] [PubMed] [Google Scholar]
- 113.Drescher M, Veldhuis G, van Rooijen BD, Milikisyants S, Subramaniam V, Huber M. Antiparallel arrangement of the helices of vesicle-bound alpha-synuclein. J Am Chem Soc. 2008;130:7796–7797. doi: 10.1021/ja801594s. [DOI] [PubMed] [Google Scholar]
- 114.Nuscher B, Kamp F, Mehnert T, Odoy S, Haass C, Kahle PJ, Beyer K. Alpha-synuclein has a high affinity for packing defects in a bilayer membrane: A thermodynamics study. J Biol Chem. 2004;279:21966–21975. doi: 10.1074/jbc.M401076200. [DOI] [PubMed] [Google Scholar]
- 115.Kang L, Moriarty GM, Woods LA, Ashcroft AE, Radford SE, Baum J. N-terminal acetylation of alpha-synuclein induces increased transient helical propensity and decreased aggregation rates in the intrinsically disordered monomer. Protein Sci. 2012;21:911–917. doi: 10.1002/pro.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maltsev AS, Ying J, Bax A. Impact of N-terminal acetylation of alpha-synuclein on its random coil and lipid binding properties. Biochemistry. 2012;51:5004–5013. doi: 10.1021/bi300642h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dikiy I, Eliezer D. N-terminal acetylation stabilizes N-terminal helicity in lipid- and micelle-bound alpha-synuclein and increases its affinity for physiological membranes. J Biol Chem. 2014;289:3652–3665. doi: 10.1074/jbc.M113.512459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bartels T, Kim NC, Luth ES, Selkoe DJ. N-alpha-acetylation of alpha-synuclein increases its helical folding propensity, GM1 binding specificity and resistance to aggregation. PLoS One. 2014;9:e103727. doi: 10.1371/journal.pone.0103727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trexler AJ, Rhoades E. N-Terminal acetylation is critical for forming alpha-helical oligomer of alpha-synuclein. Protein Sci. 2012;21:601–605. doi: 10.1002/pro.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 121.McFarland MA, Ellis CE, Markey SP, Nussbaum RL. Proteomics analysis identifies phosphorylation-dependent alpha-synuclein protein interactions. Mol Cell Proteomics. 2008;7:2123–2137. doi: 10.1074/mcp.M800116-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- 123.Paleologou KE, Oueslati A, Shakked G, Rospigliosi CC, Kim HY, Lamberto GR, Fernandez CO, Schmid A, Chegini F, Gai WP, Chiappe D, Moniatte M, Schneider BL, Aebischer P, Eliezer D, Zweckstetter M, Masliah E, Lashuel HA. Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions. J Neurosci. 2010;30:3184–3198. doi: 10.1523/JNEUROSCI.5922-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen L, Periquet M, Wang X, Negro A, McLean PJ, Hyman BT, Feany MB. Tyrosine and serine phosphorylation of alpha-synuclein have opposing effects on neurotoxicity and soluble oligomer formation. J Clin Invest. 2009;119:3257–3265. doi: 10.1172/JCI39088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Paleologou KE, Schmid AW, Rospigliosi CC, Kim HY, Lamberto GR, Fredenburg RA, Lansbury PT, Jr, Fernandez CO, Eliezer D, Zweckstetter M, Lashuel HA. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J Biol Chem. 2008;283:16895–16905. doi: 10.1074/jbc.M800747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nonaka T, Iwatsubo T, Hasegawa M. Ubiquitination of alpha-synuclein. Biochemistry. 2005;44:361–368. doi: 10.1021/bi0485528. [DOI] [PubMed] [Google Scholar]
- 127.Rott R, Szargel R, Haskin J, Shani V, Shainskaya A, Manov I, Liani E, Avraham E, Engelender S. Monoubiquitylation of alpha-synuclein by seven in absentia homolog (SIAH) promotes its aggregation in dopaminergic cells. J Biol Chem. 2008;283:3316–3328. doi: 10.1074/jbc.M704809200. [DOI] [PubMed] [Google Scholar]
- 128.Dorval V, Fraser PE. Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and alpha-synuclein. J Biol Chem. 2006;281:9919–9924. doi: 10.1074/jbc.M510127200. [DOI] [PubMed] [Google Scholar]
- 129.Krumova P, Meulmeester E, Garrido M, Tirard M, Hsiao HH, Bossis G, Urlaub H, Zweckstetter M, Kugler S, Melchior F, Bahr M, Weishaupt JH. Sumoylation inhibits alpha-synuclein aggregation and toxicity. J Cell Biol. 2011;194:49–60. doi: 10.1083/jcb.201010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Munch G, Luth HJ, Wong A, Arendt T, Hirsch E, Ravid R, Riederer P. Crosslinking of alpha-synuclein by advanced glycation endproducts–an early pathophysiological step in Lewy body formation? J Chem Neuroanat. 2000;20:253–257. doi: 10.1016/s0891-0618(00)00096-x. [DOI] [PubMed] [Google Scholar]
- 131.Shaikh S, Nicholson LF. Advanced glycation end products induce in vitro cross-linking of alpha-synuclein and accelerate the process of intracellular inclusion body formation. J Neurosci Res. 2008;86:2071–2082. doi: 10.1002/jnr.21644. [DOI] [PubMed] [Google Scholar]
- 132.Padmaraju V, Bhaskar JJ, Prasada Rao UJ, Salimath PV, Rao KS. Role of advanced glycation on aggregation and DNA binding properties of alpha-synuclein. J Alzheimers Dis. 2011;24(Suppl 2):211–221. doi: 10.3233/JAD-2011-101965. [DOI] [PubMed] [Google Scholar]
- 133.Alfaro JF, Gong CX, Monroe ME, Aldrich JT, Clauss TR, Purvine SO, Wang Z, Camp DG, 2nd, Shabanowitz J, Stanley P, Hart GW, Hunt DF, Yang F, Smith RD. Tandem mass spectrometry identifies many mouse brain O-GlcNAcylated proteins including EGF domain-specific O-GlcNAc transferase targets. Proc Natl Acad Sci U S A. 2012;109:7280–7285. doi: 10.1073/pnas.1200425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marotta NP, Cherwien CA, Abeywardana T, Pratt MR. O-GlcNAc modification prevents peptide-dependent acceleration of alpha-synuclein aggregation. Chembiochem. 2012;13:2665–2670. doi: 10.1002/cbic.201200478. [DOI] [PubMed] [Google Scholar]
- 135.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 136.Hodara R, Norris EH, Giasson BI, Mishizen-Eberz AJ, Lynch DR, Lee VM, Ischiropoulos H. Functional consequences of alpha-synuclein tyrosine nitration: Diminished binding to lipid vesicles and increased fibril formation. J Biol Chem. 2004;279:47746–47753. doi: 10.1074/jbc.M408906200. [DOI] [PubMed] [Google Scholar]
- 137.Yamin G, Uversky VN, Fink AL. Nitration inhibits fibrillation of human alpha-synuclein in vitro by formation of soluble oligomers. FEBS Lett. 2003;542:147–152. doi: 10.1016/s0014-5793(03)00367-3. [DOI] [PubMed] [Google Scholar]
- 138.Li W, West N, Colla E, Pletnikova O, Troncoso JC, Marsh L, Dawson TM, Jakala P, Hartmann T, Price DL, Lee MK. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. Proc Natl Acad Sci U S A. 2005;102:2162–2167. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Murray IV, Giasson BI, Quinn SM, Koppaka V, Axelsen PH, Ischiropoulos H, Trojanowski JQ, Lee VM. Role of alpha-synuclein carboxy-terminus on fibril formation in vitro. Biochemistry. 2003;42:8530–8540. doi: 10.1021/bi027363r. [DOI] [PubMed] [Google Scholar]
- 140.Lokappa SB, Suk JE, Balasubramanian A, Samanta S, Situ AJ, Ulmer TS. Sequence and membrane determinants of the random coil-helix transition of alpha-synuclein. J Mol Biol. 2014;426:2130–2144. doi: 10.1016/j.jmb.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 141.Zhu M, Fink AL. Lipid binding inhibits alpha-synuclein fibril formation. J Biol Chem. 2003;278:16873–16877. doi: 10.1074/jbc.M210136200. [DOI] [PubMed] [Google Scholar]
- 142.Burré J, Sharma M, Sudhof TC. Definition of a molecular pathway mediating alpha-synuclein neurotoxicity. J Neurosci. 2015;35:5221–5232. doi: 10.1523/JNEUROSCI.4650-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jo E, Darabie AA, Han K, Tandon A, Fraser PE, McLaurin J. alpha-Synuclein-synaptosomal membrane interactions: Implications for fibrillogenesis. Eur J Biochem. 2004;271:3180–3189. doi: 10.1111/j.1432-1033.2004.04250.x. [DOI] [PubMed] [Google Scholar]
- 144.Narayanan V, Scarlata S. Membrane binding and self-association of alpha-synucleins. Biochemistry. 2001;40:9927–9934. doi: 10.1021/bi002952n. [DOI] [PubMed] [Google Scholar]
- 145.Lee HJ, Choi C, Lee SJ. Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J Biol Chem. 2002;277:671–678. doi: 10.1074/jbc.M107045200. [DOI] [PubMed] [Google Scholar]
- 146.Wang L, Das U, Scott DA, Tang Y, McLean PJ, Roy S. alpha-synuclein multimers cluster synaptic vesicles and attenuate recycling. Curr Biol. 2014;24:2319–2326. doi: 10.1016/j.cub.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 148.El-Agnaf OM, Jakes R, Curran MD, Middleton D, Ingenito R, Bianchi E, Pessi A, Neill D, Wallace A. Aggregates from mutant and wild-type alpha-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of beta-sheet and amyloid-like filaments. FEBS Lett. 1998;440:71–75. doi: 10.1016/s0014-5793(98)01418-5. [DOI] [PubMed] [Google Scholar]
- 149.Conway KA, Harper JD, Lansbury PT., Jr Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry. 2000;39:2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- 150.Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- 151.Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT., Jr Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322:1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 152.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 153.Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 154.Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: Implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 155.Kahle PJ, Neumann M, Ozmen L, Haass C. Physiology and pathophysiology of alpha-synuclein. Cell culture and transgenic animal models based on a Parkinson’s disease-associated protein. Ann N Y Acad Sci. 2000;920:33–41. doi: 10.1111/j.1749-6632.2000.tb06902.x. [DOI] [PubMed] [Google Scholar]
- 156.Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- 157.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 158.Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Burré J, Sharma M, Sudhof TC. Systematic mutagenesis of alpha-synuclein reveals distinct sequence requirements for physiological and pathological activities. J Neurosci. 2012;32:15227–15242. doi: 10.1523/JNEUROSCI.3545-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gorbatyuk OS, Li S, Nguyen FN, Manfredsson FP, Kondrikova G, Sullivan LF, Meyers C, Chen W, Mandel RJ, Muzyczka N. alpha-Synuclein expression in rat substantia nigra suppresses phospholipase D2 toxicity and nigral neurodegeneration. Mol Ther. 2010;18:1758–1768. doi: 10.1038/mt.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jenco JM, Rawlingson A, Daniels B, Morris AJ. Regulation of phospholipase D2: Selective inhibition of mammalian phospholipase D isoenzymes by alpha- and beta-synucleins. Biochemistry. 1998;37:4901–4909. doi: 10.1021/bi972776r. [DOI] [PubMed] [Google Scholar]
- 162.Payton JE, Perrin RJ, Woods WS, George JM. Structural determinants of PLD2 inhibition by alpha-synuclein. J Mol Biol. 2004;337:1001–1009. doi: 10.1016/j.jmb.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 163.Rappley I, Gitler AD, Selvy PE, LaVoie MJ, Levy BD, Brown HA, Lindquist S, Selkoe DJ. Evidence that alpha-synuclein does not inhibit phospholipase D. Biochemistry. 2009;48:1077–1083. doi: 10.1021/bi801871h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Guo JT, Chen AQ, Kong Q, Zhu H, Ma CM, Qin C. Inhibition of vesicular monoamine transporter-2 activity in alpha-synuclein stably transfected SH-SY5Y cells. Cell Mol Neurobiol. 2008;28:35–47. doi: 10.1007/s10571-007-9227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wersinger C, Rusnak M, Sidhu A. Modulation of the trafficking of the human serotonin transporter by human alpha-synuclein. Eur J Neurosci. 2006;24:55–64. doi: 10.1111/j.1460-9568.2006.04900.x. [DOI] [PubMed] [Google Scholar]
- 166.Butler B, Goodwin S, Saha K, Becker J, Sambo D, Davari P, Khoshbouei H. Dopamine Transporter Activity Is Modulated by alpha-synuclein. J Biol Chem. 2015. [DOI] [PMC free article] [PubMed]
- 167.Swant J, Goodwin JS, North A, Ali AA, Gamble-George J, Chirwa S, Khoshbouei H. alpha-Synuclein stimulates a dopamine transporter-dependent chloride current and modulates the activity of the transporter. J Biol Chem. 2011;286:43933–43943. doi: 10.1074/jbc.M111.241232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Baptista MJ, O’Farrell C, Daya S, Ahmad R, Miller DW, Hardy J, Farrer MJ, Cookson MR. Co-ordinate transcriptional regulation of dopamine synthesis genes by alpha-synuclein in human neuroblastoma cell lines. J Neurochem. 2003;85:957–968. doi: 10.1046/j.1471-4159.2003.01742.x. [DOI] [PubMed] [Google Scholar]
- 170.Yu S, Zuo X, Li Y, Zhang C, Zhou M, Zhang YA, Ueda K, Chan P. Inhibition of tyrosine hydroxylase expression in alpha-synuclein-transfected dopaminergic neuronal cells. Neurosci Lett. 2004;367:34–39. doi: 10.1016/j.neulet.2004.05.118. [DOI] [PubMed] [Google Scholar]
- 171.Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li WW, Yang R, Guo JC, Ren HM, Zha XL, Cheng JS, Cai DF. Localization of alpha-synuclein to mitochondria within midbrain of mice. Neuroreport. 2007;18:1543–1546. doi: 10.1097/WNR.0b013e3282f03db4. [DOI] [PubMed] [Google Scholar]
- 173.Nakamura K, Nemani VM, Wallender EK, Kaehlcke K, Ott M, Edwards RH. Optical reporters for the conformation of alpha-synuclein reveal a specific interaction with mitochondria. J Neurosci. 2008;28:12305–12317. doi: 10.1523/JNEUROSCI.3088-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Thayanidhi N, Helm JR, Nycz DC, Bentley M, Liang Y, Hay JC. Alpha-synuclein delays endoplasmic reticulum (ER)-to-Golgi transport in mammalian cells by antagonizing ER/Golgi SNAREs. Mol Biol Cell. 2010;21:1850–1863. doi: 10.1091/mbc.E09-09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kontopoulos E, Parvin JD, Feany MB. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum Mol Genet. 2006;15:3012–3023. doi: 10.1093/hmg/ddl243. [DOI] [PubMed] [Google Scholar]
- 177.Golovko MY, Faergeman NJ, Cole NB, Castagnet PI, Nussbaum RL, Murphy EJ. Alpha-synuclein gene deletion decreases brain palmitate uptake and alters the palmitate metabolism in the absence of alpha-synuclein palmitate binding. Biochemistry. 2005;44:8251–8259. doi: 10.1021/bi0502137. [DOI] [PubMed] [Google Scholar]
- 178.Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barcelo-Coblijn GC, Nussbaum RL. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein. Mol Cell Biol. 2005;25:10190–10201. doi: 10.1128/MCB.25.22.10190-10201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Barcelo-Coblijn G, Golovko MY, Weinhofer I, Berger J, Murphy EJ. Brain neutral lipids mass is increased in alpha-synuclein gene-ablated mice. J Neurochem. 2007;101:132–141. doi: 10.1111/j.1471-4159.2006.04348.x. [DOI] [PubMed] [Google Scholar]
- 180.Rappley I, Myers DS, Milne SB, Ivanova PT, Lavoie MJ, Brown HA, Selkoe DJ. Lipidomic profiling in mouse brain reveals differences between ages and genders, with smaller changes associated with alpha-synuclein genotype. J Neurochem. 2009;111:15–25. doi: 10.1111/j.1471-4159.2009.06290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sharon R, Goldberg MS, Bar-Josef I, Betensky RA, Shen J, Selkoe DJ. alpha-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins. Proc Natl Acad Sci U S A. 2001;98:9110–9115. doi: 10.1073/pnas.171300598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lucke C, Gantz DL, Klimtchuk E, Hamilton JA. Interactions between fatty acids and alpha-synuclein. J Lipid Res. 2006;47:1714–1724. doi: 10.1194/jlr.M600003-JLR200. [DOI] [PubMed] [Google Scholar]
- 183.Varkey J, Isas JM, Mizuno N, Jensen MB, Bhatia VK, Jao CC, Petrlova J, Voss JC, Stamou DG, Steven AC, Langen R. Membrane curvature induction and tubulation are common features of synucleins and apolipoproteins. J Biol Chem. 2010;285:32486–32493. doi: 10.1074/jbc.M110.139576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Westphal CH, Chandra SS. Monomeric synucleins generate membrane curvature. J Biol Chem. 2013;288:1829–1840. doi: 10.1074/jbc.M112.418871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jiang Z, de Messieres M, Lee JC. Membrane remodeling by alpha-synuclein and effects on amyloid formation. J Am Chem Soc. 2013;135:15970–15973. doi: 10.1021/ja405993r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mizuno N, Varkey J, Kegulian NC, Hegde BG, Cheng N, Langen R, Steven AC. Remodeling of lipid vesicles into cylindrical micelles by alpha-synuclein in an extended alpha-helical conformation. J Biol Chem. 2012;287:29301–29311. doi: 10.1074/jbc.M112.365817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shi Z, Sachs JN, Rhoades E, Baumgart T. Biophysics of alpha-synuclein induced membrane remodelling. Phys Chem Chem Phys. 2015;17:15561–15568. doi: 10.1039/c4cp05883f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Braun AR, Lacy MM, Ducas VC, Rhoades E, Sachs JN. alpha-Synuclein-induced membrane remodeling is driven by binding affinity, partition depth, and interleaflet order asymmetry. J Am Chem Soc. 2014;136:9962–9972. doi: 10.1021/ja5016958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Madine J, Doig AJ, Middleton DA. A study of the regional effects of alpha-synuclein on the organization and stability of phospholipid bilayers. Biochemistry. 2006;45:5783–5792. doi: 10.1021/bi052151q. [DOI] [PubMed] [Google Scholar]
- 190.Adamczyk A, Kacprzak M, Kazmierczak A. Alpha-synuclein decreases arachidonic acid incorporation into rat striatal synaptoneurosomes. Folia Neuropathol. 2007;45:230–235. [PubMed] [Google Scholar]
- 191.Kamp F, Beyer K. Binding of alpha-synuclein affects the lipid packing in bilayers of small vesicles. J Biol Chem. 2006;281:9251–9259. doi: 10.1074/jbc.M512292200. [DOI] [PubMed] [Google Scholar]
- 192.Ouberai MM, Wang J, Swann MJ, Galvagnion C, Guilliams T, Dobson CM, Welland ME. alpha-Synuclein senses lipid packing defects and induces lateral expansion of lipids leading to membrane remodeling. J Biol Chem. 2013;288:20883–20895. doi: 10.1074/jbc.M113.478297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Braun AR, Sevcsik E, Chin P, Rhoades E, Tristram-Nagle S, Sachs JN. alpha-Synuclein induces both positive mean curvature and negative Gaussian curvature in membranes. J Am Chem Soc. 2012;134:2613–2620. doi: 10.1021/ja208316h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K. Parkinson’s disease: Lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 2007;113:421–429. doi: 10.1007/s00401-007-0193-x. [DOI] [PubMed] [Google Scholar]
- 195.Baba T, Kikuchi A, Hirayama K, Nishio Y, Hosokai Y, Kanno S, Hasegawa T, Sugeno N, Konno M, Suzuki K, Takahashi S, Fukuda H, Aoki M, Itoyama Y, Mori E, Takeda A. Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson’s disease: A 3 year longitudinal study. Brain. 2012;135:161–169. doi: 10.1093/brain/awr321. [DOI] [PubMed] [Google Scholar]
- 196.Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson’s disease. Mov Disord. 2013;28:715–724. doi: 10.1002/mds.25187. [DOI] [PubMed] [Google Scholar]
- 197.Fahn S, Sulzer D. Neurodegeneration and neuroprotection in Parkinson disease. NeuroRx. 2004;1:139–154. doi: 10.1602/neurorx.1.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Riederer P, Wuketich S. Time course of nigrostriatal degeneration in parkinson’s disease. A detailed study of influential factors in human brain amine analysis. J Neural Transm. 1976;38:277–301. doi: 10.1007/BF01249445. [DOI] [PubMed] [Google Scholar]
- 199.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 200.Oaks AW, Frankfurt M, Finkelstein DI, Sidhu A. Age-dependent effects of A53T alpha-synuclein on behavior and dopaminergic function. PLoS One. 2013;8:e60378. doi: 10.1371/journal.pone.0060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wersinger C, Sidhu A. Attenuation of dopamine transporter activity by alpha-synuclein. Neurosci Lett. 2003;340:189–192. doi: 10.1016/s0304-3940(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 202.Wersinger C, Prou D, Vernier P, Sidhu A. Modulation of dopamine transporter function by alpha-synuclein is altered by impairment of cell adhesion and by induction of oxidative stress. FASEB J. 2003;17:2151–2153. doi: 10.1096/fj.03-0152fje. [DOI] [PubMed] [Google Scholar]
- 203.Lee FJ, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 2001;15:916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- 204.Li YH, Gao N, Ye YW, Li X, Yu S, Yang H, Ueda K, Chan P. Alpha-synuclein functions as a negative regulator for expression of tyrosine hydroxylase. Acta Neurol Belg. 2011;111:130–135. [PubMed] [Google Scholar]
- 205.Peng X, Tehranian R, Dietrich P, Stefanis L, Perez RG. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci. 2005;118:3523–3530. doi: 10.1242/jcs.02481. [DOI] [PubMed] [Google Scholar]
- 206.Wu B, Liu Q, Duan C, Li Y, Yu S, Chan P, Ueda K, Yang H. Phosphorylation of alpha-synuclein upregulates tyrosine hydroxylase activity in MN9D cells. Acta Histochem. 2011;113:32–35. doi: 10.1016/j.acthis.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 207.Lou H, Montoya SE, Alerte TN, Wang J, Wu J, Peng X, Hong CS, Friedrich EE, Mader SA, Pedersen CJ, Marcus BS, McCormack AL, Di Monte DA, Daubner SC, Perez RG. Serine 129 phosphorylation reduces the ability of alpha-synuclein to regulate tyrosine hydroxylase and protein phosphatase 2A in vitro and in vivo. J Biol Chem. 2010;285:17648–17661. doi: 10.1074/jbc.M110.100867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chadchankar H, Ihalainen J, Tanila H, Yavich L. Decreased reuptake of dopamine in the dorsal striatum in the absence of alpha-synuclein. Brain Res. 2011;1382:37–44. doi: 10.1016/j.brainres.2011.01.064. [DOI] [PubMed] [Google Scholar]
- 209.Al-Wandi A, Ninkina N, Millership S, Williamson SJ, Jones PA, Buchman VL. Absence of alpha-synuclein affects dopamine metabolism and synaptic markers in the striatum of aging mice. Neurobiol Aging. 2010;31:796–804. doi: 10.1016/j.neurobiolaging.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Garcia-Reitboeck P, Anichtchik O, Dalley JW, Ninkina N, Tofaris GK, Buchman VL, Spillantini MG. Endogenous alpha-synuclein influences the number of dopaminergic neurons in mouse substantia nigra. Exp Neurol. 2013;248:541–545. doi: 10.1016/j.expneurol.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Robertson DC, Schmidt O, Ninkina N, Jones PA, Sharkey J, Buchman VL. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J Neurochem. 2004;89:1126–1136. doi: 10.1111/j.1471-4159.2004.02378.x. [DOI] [PubMed] [Google Scholar]
- 212.Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, Hammer RE, Battaglia G, German DC, Castillo PE, Sudhof TC. Double-knockout mice for alpha- and beta-synucleins: Effect on synaptic functions. Proc Natl Acad Sci U S A. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Senior SL, Ninkina N, Deacon R, Bannerman D, Buchman VL, Cragg SJ, Wade-Martins R. Increased striatal dopamine release and hyperdopaminergic-like behaviour in mice lacking both alpha-synuclein and gamma-synuclein. Eur J Neurosci. 2008;27:947–957. doi: 10.1111/j.1460-9568.2008.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ostrerova N, Petrucelli L, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B. alpha-Synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci. 1999;19:5782–5791. doi: 10.1523/JNEUROSCI.19-14-05782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kim TD, Paik SR, Yang CH, Kim J. Structural changes in alpha-synuclein affect its chaperone-like activity. Protein Sci. 2000;9:2489–2496. doi: 10.1110/ps.9.12.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ahn M, Kim S, Kang M, Ryu Y, Kim TD. Chaperone-like activities of alpha-synuclein: Alha-synuclein assists enzyme activities of esterases. Biochem Biophys Res Commun. 2006;346:1142–1149. doi: 10.1016/j.bbrc.2006.05.213. [DOI] [PubMed] [Google Scholar]
- 217.Rekas A, Adda CG, Andrew Aquilina J, Barnham KJ, Sunde M, Galatis D, Williamson NA, Masters CL, Anders RF, Robinson CV, Cappai R, Carver JA. Interaction of the molecular chaperone alphaB-crystallin with alpha-synuclein: Effects on amyloid fibril formation and chaerone activity. J Mol Biol. 2004;340:1167–1183. doi: 10.1016/j.jmb.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 218.Kim TD, Paik SR, Yang CH. Structural and functional implications of C-terminal regions of alpha-synuclein. Biochemistry. 2002;41:13782–13790. doi: 10.1021/bi026284c. [DOI] [PubMed] [Google Scholar]
- 219.Kim TD, Choi E, Rhim H, Paik SR, Yang CH. Alpha-synuclein has structural and functional similarities to small heat shock proteins. Biochem Biophys Res Commun. 2004;324:1352–1359. doi: 10.1016/j.bbrc.2004.09.208. [DOI] [PubMed] [Google Scholar]
- 220.Kanda S, Bishop JF, Eglitis MA, Yang Y, Mouradian MM. Enhanced vulnerability to oxidative stress by alpha-synuclein mutations and C-terminal truncation. Neuroscience. 2000;97:279–284. doi: 10.1016/s0306-4522(00)00077-4. [DOI] [PubMed] [Google Scholar]
- 221.da Costa CA, Ancolio K, Checler F. Wild-type but not Parkinson’s disease-related ala-53 –>Thr mutant alpha -synuclein protects neuronal cells from apoptotic stimuli. J Biol Chem. 2000;275:24065–24069. doi: 10.1074/jbc.M002413200. [DOI] [PubMed] [Google Scholar]
- 222.Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 223.Greten-Harrison B, Polydoro M, Morimoto-Tomita M, Diao L, Williams AM, Nie EH, Makani S, Tian N, Castillo PE, Buchman VL, Chandra SS. alphabetagamma-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc Natl Acad Sci U S A. 2010;107:19573–19578. doi: 10.1073/pnas.1005005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Anwar S, Peters O, Millership S, Ninkina N, Doig N, Connor-Robson N, Threlfell S, Kooner G, Deacon RM, Bannerman DM, Bolam JP, Chandra SS, Cragg SJ, Wade-Martins R, Buchman VL. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J Neurosci. 2011;31:7264–7274. doi: 10.1523/JNEUROSCI.6194-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 226.Pelkonen A, Yavich L. Neuromuscular pathology in mice lacking alpha-synuclein. Neurosci Lett. 2011;487:350–353. doi: 10.1016/j.neulet.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 227.Kokhan VS, Van’kin GI, Ninkina NN, Shelkovnikova TA, Bachurin SO. Impaired spatial and working memory in ageing mice with targeted inactivation of alpha-synuclein gene. Dokl Biol Sci. 2011;441:354–356. doi: 10.1134/S0012496611060081. [DOI] [PubMed] [Google Scholar]
- 228.Kokhan VS, Afanasyeva MA, Van’kin GI. alpha-Synuclein knockout mice have cognitive impairments. Behav Brain Res. 2012;231:226–230. doi: 10.1016/j.bbr.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 229.Watson JB, Hatami A, David H, Masliah E, Roberts K, Evans CE, Levine MS. Alterations in corticostriatal synaptic plasticity in mice overexpressing human alpha-synuclein. Neuroscience. 2009;159:501–513. doi: 10.1016/j.neuroscience.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Steidl JV, Gomez-Isla T, Mariash A, Ashe KH, Boland LM. Altered short-term hippocampal synaptic plasticity in mutant alpha-synuclein transgenic mice. Neuroreport. 2003;14:219–223. doi: 10.1097/00001756-200302100-00012. [DOI] [PubMed] [Google Scholar]
- 231.Liu S, Ninan I, Antonova I, Battaglia F, Trinchese F, Narasanna A, Kolodilov N, Dauer W, Hawkins RD, Arancio O. alpha-Synuclein produces a long-lasting increase in neurotransmitter release. EMBO J. 2004;23:4506–4516. doi: 10.1038/sj.emboj.7600451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gureviciene I, Gurevicius K, Tanila H. Role of alpha-synuclein in synaptic glutamate release. Neurobiol Dis. 2007;28:83–89. doi: 10.1016/j.nbd.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 233.Gureviciene I, Gurevicius K, Tanila H. Aging and alpha-synuclein affect synaptic plasticity in the dentate gyrus. J Neural Transm. 2009;116:13–22. doi: 10.1007/s00702-008-0149-x. [DOI] [PubMed] [Google Scholar]
- 234.Martin ED, Gonzalez-Garcia C, Milan M, Farinas I, Cena V. Stressor-related impairment of synaptic transmission in hippocampal slices from alpha-synuclein knockout mice. Eur J Neurosci. 2004;20:3085–3091. doi: 10.1111/j.1460-9568.2004.03801.x. [DOI] [PubMed] [Google Scholar]
- 235.Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wu N, Joshi PR, Cepeda C, Masliah E, Levine MS. Alpha-synuclein overexpression in mice alters synaptic communication in the corticostriatal pathway. J Neurosci Res. 2010;88:1764–1776. doi: 10.1002/jnr.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Busch DJ, Oliphint PA, Walsh RB, Banks SM, Woods WS, George JM, Morgan JR. Acute increase of alpha-synuclein inhibits synaptic vesicle recycling evoked during intense stimulation. Mol Biol Cell. 2014;25:3926–3941. doi: 10.1091/mbc.E14-02-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Vargas KJ, Makani S, Davis T, Westphal CH, Castillo PE, Chandra SS. Synucleins regulate the kinetics of synaptic vesicle endocytosis. J Neurosci. 2014;34:9364–9376. doi: 10.1523/JNEUROSCI.4787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ben Gedalya T, Loeb V, Israeli E, Altschuler Y, Selkoe DJ, Sharon R. Alpha-synuclein and polyunsaturated fatty acids promote clathrin-mediated endocytosis and synaptic vesicle recycling. Traffic. 2009;10:218–234. doi: 10.1111/j.1600-0854.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of alpha-synuclein. J Neurosci. 2005;25:10913–10921. doi: 10.1523/JNEUROSCI.2922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fortin DL, Nemani VM, Nakamura K, Edwards RH. The behavior of alpha-synuclein in neurons. Mov Disord. 2010;25(Suppl 1):S21–S26. doi: 10.1002/mds.22722. [DOI] [PubMed] [Google Scholar]
- 243.Tao-Cheng JH. Activity-related redistribution of presynaptic proteins at the active zone. Neuroscience. 2006;141:1217–1224. doi: 10.1016/j.neuroscience.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 244.Scott D, Roy S. alpha-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J Neurosci. 2012;32:10129–10135. doi: 10.1523/JNEUROSCI.0535-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lai Y, Kim S, Varkey J, Lou X, Song JK, Diao J, Langen R, Shin YK. Nonaggregated alpha-synuclein influences SNARE-dependent vesicle docking via membrane binding. Biochemistry. 2014;53:3889–3896. doi: 10.1021/bi5002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Diao J, Burré J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, Sudhof TC, Brunger AT. Native alpha-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. Elife. 2013;2:e00592. doi: 10.7554/eLife.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Soper JH, Roy S, Stieber A, Lee E, Wilson RB, Trojanowski JQ, Burd CG, Lee VM. Alpha-synuclein-induced aggregation of cytoplasmic vesicles in Saccharomyces cerevisiae. Mol Biol Cell. 2008;19:1093–1103. doi: 10.1091/mbc.E07-08-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet JC, McCaffery JM, Barlowe C, Lindquist S. The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci U S A. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lee SJ, Jeon H, Kandror KV. Alpha-synuclein is localized in a subpopulation of rat brain synaptic vesicles. Acta Neurobiol Exp (Wars) 2008;68:509–515. doi: 10.55782/ane-2008-1717. [DOI] [PubMed] [Google Scholar]
- 250.Garcia-Reitbock P, Anichtchik O, Bellucci A, Iovino M, Ballini C, Fineberg E, Ghetti B, Della Corte L, Spano P, Tofaris GK, Goedert M, Spillantini MG. SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain. 2010;133:2032–2044. doi: 10.1093/brain/awq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lee HJ, Kang SJ, Lee K, Im H. Human alpha-synuclein modulates vesicle trafficking through its interaction with prenylated Rab acceptor protein 1. Biochem Biophys Res Commun. 2011;412:526–531. doi: 10.1016/j.bbrc.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 252.Gosavi N, Lee HJ, Lee JS, Patel S, Lee SJ. Golgi fragmentation occurs in the cells with prefibrillar alpha-synuclein aggregates and precedes the formation of fibrillar inclusion. J Biol Chem. 2002;277:48984–48992. doi: 10.1074/jbc.M208194200. [DOI] [PubMed] [Google Scholar]
- 253.Choi BK, Choi MG, Kim JY, Yang Y, Lai Y, Kweon DH, Lee NK, Shin YK. Large alpha-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc Natl Acad Sci U S A. 2013;110:4087–4092. doi: 10.1073/pnas.1218424110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 255.Volles MJ, Lansbury PT., Jr Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in Parkinson’s disease. Biochemistry. 2003;42:7871–7878. doi: 10.1021/bi030086j. [DOI] [PubMed] [Google Scholar]
- 256.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Rey NL, Petit GH, Bousset L, Melki R, Brundin P. Transfer of human alpha-synuclein from the olfactory bulb to interconnected brain regions in mice. Acta Neuropathol. 2013;126:555–573. doi: 10.1007/s00401-013-1160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]