Abstract
Patients with chronic obstructive pulmonary disease (COPD) have innate immune dysfunction in the lung largely due to defective macrophage phagocytosis. This deficiency results in periodic bacterial infections that cause acute exacerbations of COPD, a major source of morbidity and mortality. Recent studies indicate that a decrease in Nrf2 (nuclear erythroid–related factor 2) signaling in patients with COPD may hamper their ability to defend against oxidative stress, although the role of Nrf2 in COPD exacerbations has not been determined. Here, we test whether activation of Nrf2 by the phytochemical sulforaphane restores phagocytosis of clinical isolates of nontypeable Haemophilus influenza (NTHI) and Pseudomonas aeruginosa (PA) by alveolar macrophages from patients with COPD. Sulforaphane treatment restored bacteria recognition and phagocytosis in alveolar macrophages from COPD patients. Furthermore, sulforaphane treatment enhanced pulmonary bacterial clearance by alveolar macrophages and reduced inflammation in wild-typemice but not in Nrf2-deficientmice exposed to cigarette smoke for 6 months. Gene expression and promoter analysis revealed that Nrf2 increased phagocytic ability of macrophages by direct transcriptional up-regulation of the scavenger receptor MARCO. Disruption of Nrf2 or MARCO abrogated sulforaphane-mediated bacterial phagocytosis by COPD alveolar macrophages. Our findings demonstrate the importance of Nrf2 and its downstream target MARCO in improving antibacterial defenses and provide a rationale for targeting this pathway, via pharmacological agents such as sulforaphane, to prevent exacerbations of COPD caused by bacterial infection.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is characterized by a progressive decrease in lung function and comprises both chronic bronchitis and emphysema (1). COPD is the fifth leading cause of death worldwide, and tobacco exposure is the primary risk factor for development of COPD in industrialized countries. Patients with COPD have frequent symptomatic exacerbations, which are accompanied by acute and permanent decline in lung function. These exacerbations, which are a major cause of morbidity and mortality (2–4), are primarily due to bacterial and viral infections. The bacterial strain nontypeable Haemophilus influenzae (NTHI) is the most prevalent cause of these infections, but Pseudomonas aeruginosa (PA) is also important in severe COPD (5–7). Cigarette smoking causes disruption of innate immune defenses in the lung and impairs the phagocytic ability of alveolar macrophages, resulting in frequent bacterial infections and inflammation (8–10). Several clinical studies show bacterial colonization in the airways of patients with stable COPD, which is thought to promote persistent inflammation (11). However, infections with newly acquired bacterial strains are largely responsible for acute COPD exacerbations (6, 12). Currently, there are no proven therapies that can inhibit bacterial colonization or prevent infectious COPD exacerbations.
The transcription factor nuclear erythroid–related factor 2 (Nrf2) is a key regulator of cytoprotective proteins including antioxidants, xenobiotic detoxification enzymes, and proteasomal pathways (13, 14). In response to oxidative stress, Nrf2 dissociates from its cytoplasmic inhibitor Keap1, translocates to the nucleus, and transactivates these target genes by binding to the antioxidant response element (ARE). Disruption of Nrf2 causes earlier-onset and more severe emphysema in mice after exposure to cigarette smoke (15, 16). Conversely, pharmacological activation of Nrf2 protects against cigarette smoke–induced emphysema in mice (17). Nrf2 and its transcriptional activity decline with increasing COPD severity in both lung tissue (14, 18, 19) and alveolar macrophages (20). Furthermore, disruption of Nrf2 augments inflammatory responses that cause mortality in mouse models of sepsis (21).
Patients with COPD exhibit defective bacterial phagocytosis by alveolar macrophages and monocyte-derived macrophages (9, 22). Although the underlying molecular mechanisms for this defect phagocytosis are unclear (6, 9), it is thought that oxidative stress plays a role (23, 24). Therefore, we tested the hypothesis that increasing Nrf2-regulated antioxidants in alveolar macrophages from COPD patients would restore phagocytic function by reducing oxidative stress.
RESULTS
We recruited 43 patients with moderate COPD as described in Table 1. Our study participants had no concurrent lung infections or previous diagnosis for any underlying autoimmune disorders.
Table 1.
Patient characteristics.
| Characteristic | Patients with COPD (n = 43) |
|---|---|
| Sex | |
| Male (n) | 23 |
| Female (n) | 20 |
| Age | 63.7 ± 2.3 |
| Pack-year smoking | 42.6 ± 4.9 |
| FEV1 (% predicted) | 50.3 ± 2.6 |
| Current smoker (n) | 13 |
| Former smoker (n) | 30 |
| FEV1 (liter) | 1.29 ± 0.1 |
| FVC (% predicted) | 61.7 ± 3.3 |
| FVC (liter) | 1.98 ± 0.1 |
| FEV1/FVC (%) | 67.4 ± 3.2 |
| Concurrent bacterial infection (n) | 0 |
| Bronchoscopy for research study screening, n (%) | 13 (30.2) |
| Bronchoscopy for lung cancer diagnosis (%) | 46.5 |
| Non–small cell lung cancer (n) | 4 |
| Squamous cell carcinoma (n) | 4 |
| Metastatic breast cancer (n) | 1 |
| Undiagnosed lung nodule (n) | 11 |
| Bronchoscopy for nondiagnostic purpose (%) | 23.3 |
| Tumor excision/destruction (n) | 1 |
| Tracheal stenosis (n) | 2 |
| Stent removal placement (n) | 4 |
| Obstructive sleep apnea | 1 |
| Pre–lung transplant exam | 1 |
| Post–chord transplant evaluation | 1 |
| Underlying autoimmune disorder (n) | 0 |
Values are presented as means ± SEM. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Activation of Nrf2 by sulforaphane increases phagocytic ability of alveolar macrophages from patients with COPD
Sulforaphane is a potent pharmacological activator of Nrf2 and increases endogenous antioxidants (25–28). Nrf2 activity declines in macrophages isolated from COPD patients as a function of disease status, and it was unclear whether sulforaphane treatment could restore Nrf2 activity in these alveolar macrophages. Sulforaphane treatment (10 µM for 16 hours) resulted in more Nrf2 protein as measured by flow cytometry (fig. S1A) and subsequent up-regulation of Nrf2-regulated target genes (NQO1 and GPX2) (fig. S1B) compared to vehicle-treated control cells. These results demonstrate the potency of sulforaphane to increase Nrf2 activity in COPD alveolar macrophages.
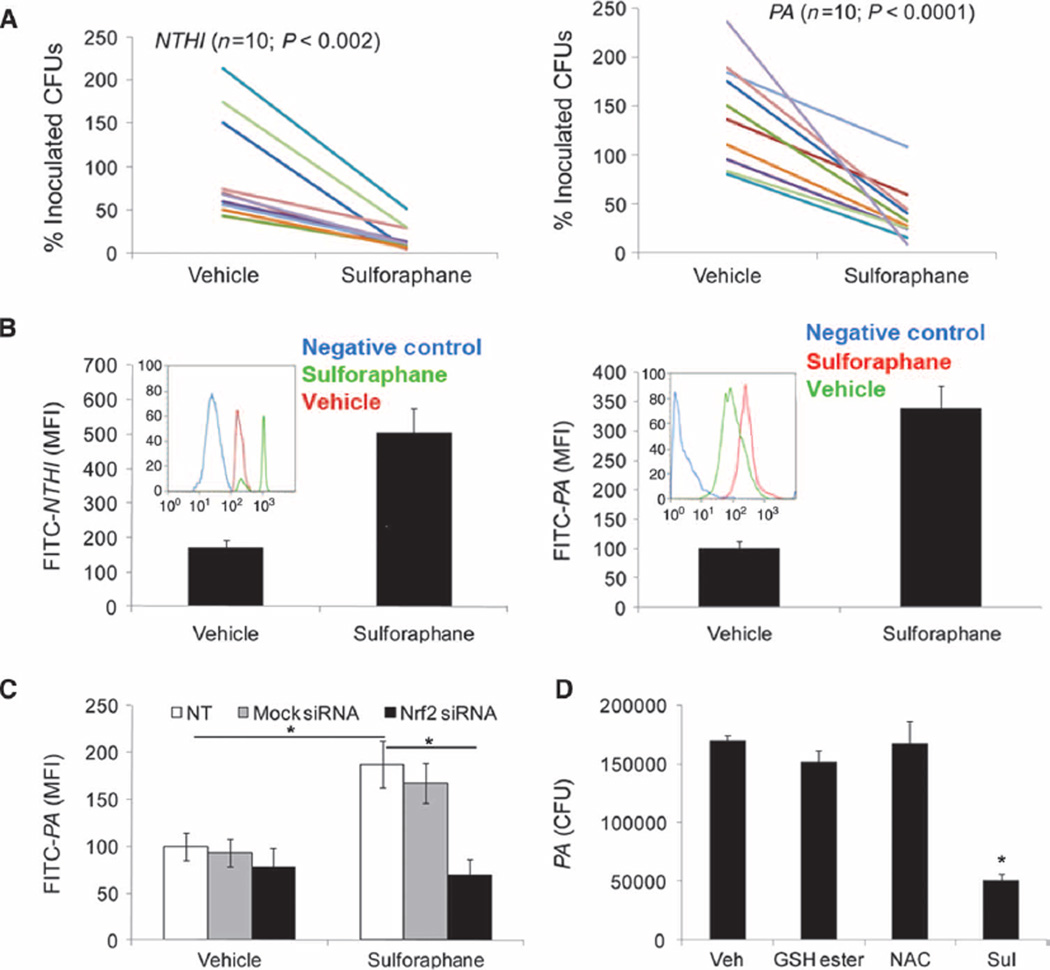
To determine whether sulforaphane enhances bacterial phagocytosis, we isolated alveolar macrophages from 18 patients with COPD via bronchoalveolar lavage (BAL). The purity of the isolated macrophages was >95% as assessed by morphology with Wright-Giemsa stain and confirmed by flow cytometry for the initial clinical samples. Two representative cytograms are presented in fig. S1C. These macrophages were pretreated with sulforaphane (10 µM) for 16 hours and then infected with clinical (COPD) isolates of either PA or NTHI. Sulforaphane treatment markedly enhanced the clearance (40 to 95%) of PA and NTHI from the culture medium by alveolar macrophages from all COPD patients compared to treatment with vehicle. The improvement in bacterial clearance was independent of smoking status as evident from the decrease in bacterial colony-forming units (CFUs) in all samples (Fig. 1A). Sulforaphane treatment also enhanced PA clearance by alveolar macrophages from healthy subjects (fig. S1D). Cell-free medium with sulforaphane alone showed no bactericidal activity toward PA or NTHI, indicating that sulforaphane does not directly cause bacterial killing (fig. S1E). Next, we investigated whether the decrease in bacterial CFUs in the culture medium of alveolar macrophages after sulforaphane treatment was due to increased bacterial phagocytosis. To assess phagocytic ability, we incubated alveolar macrophages with attenuated fluorescein isothiocyanate (FITC)–labeled PA and NTHI, and analyzed phagocytosed bacteria by flow cytometry. Sulforaphane significantly enhanced phagocytosis of PA and/or NTHI by alveolar macrophages compared to vehicle alone by ~300% (Fig. 1B).
Fig. 1.
Sulforaphane-induced activation of Nrf2 improves phagocytosis and clearance of PA and NTHI by COPD alveolar macrophages. (A) Sulforaphane- or vehicle-treated (16 hours) COPD alveolarmacrophageswere incubated with NTHI or PA. Bacterial load in the culture medium was quantified after 4 hours by serial dilution plating. Data are represented as percent of inoculated CFU remaining in the culture medium for individual patients after vehicle or sulforaphane treatment. (B) COPD alveolar macrophages were incubated with attenuated FITC-labeled NTHI or PA after sulforaphane or vehicle treatment, and phagocytosed bacteria were quantified by flow cytometry after 1 hour. Data are representative histograms from n = 5 patients. Insets: Representative flow cytometry histogram from individual patients. (C) COPD alveolar macrophages transfected with Nrf2 siRNA or ssRNA were treated with sulforaphane or vehicle for 16 hours. Subsequently, macrophages were incubated with FITC-labeled PA and phagocytosed bacteria were quantified by flow cytometry after 1 hour. Data are represented as means ± SEM of MFI (mean fluorescence intensity) (n = 3). (D) GSH ester, NAC, and sulforaphane (Sul)– or vehicle-treated alveolar macrophages isolated from patients with COPD were incubated with PA, and bacterial load in culture medium was quantified after 4 hours. Data are represented as CFU means ± SEM (n = 5 patients).
To determine whether the effect of sulforaphane on bacterial phagocytosis was mediated by Nrf2, we transfected alveolar macrophages with Nrf2 small interfering RNA (siRNA) and/or control single-stranded RNA (ssRNA). Nrf2 siRNA transfection caused ~70 to 80% knockdown of the Nrf2 gene and repressed induction of Nrf2-regulated antioxidant genes after sulforaphane treatment when compared to control ssRNA (fig. S1F). Sulforaphane treatment enhanced bacterial phagocytosis of alveolar macrophages transfected with control ssRNA but failed to do so in Nrf2 siRNA–transfected macrophages (Fig. 1C). To examine whether the improvement in bacterial clearance by alveolar macrophages after sulforaphane treatment was dependent on Nrf2-regulated antioxidant function, we treated alveolar macrophages with the exogenous antioxidants N-acetylcysteine (NAC) or glutathione ethyl ester (GSH ester). NAC or GSH ester increased intracellular glutathione concentrations (fig. S1G) but failed to improve bacterial clearance (Fig. 1D) by alveolar macrophages. Together, these data suggest that sulforaphane improves bacterial phagocytosis and clearance by COPD alveolar macrophages by activating Nrf2 through a mechanism that is independent of the intracellular antioxidant glutathione.
Nrf2 mediates transcriptional regulation of the scavenger receptor MARCO
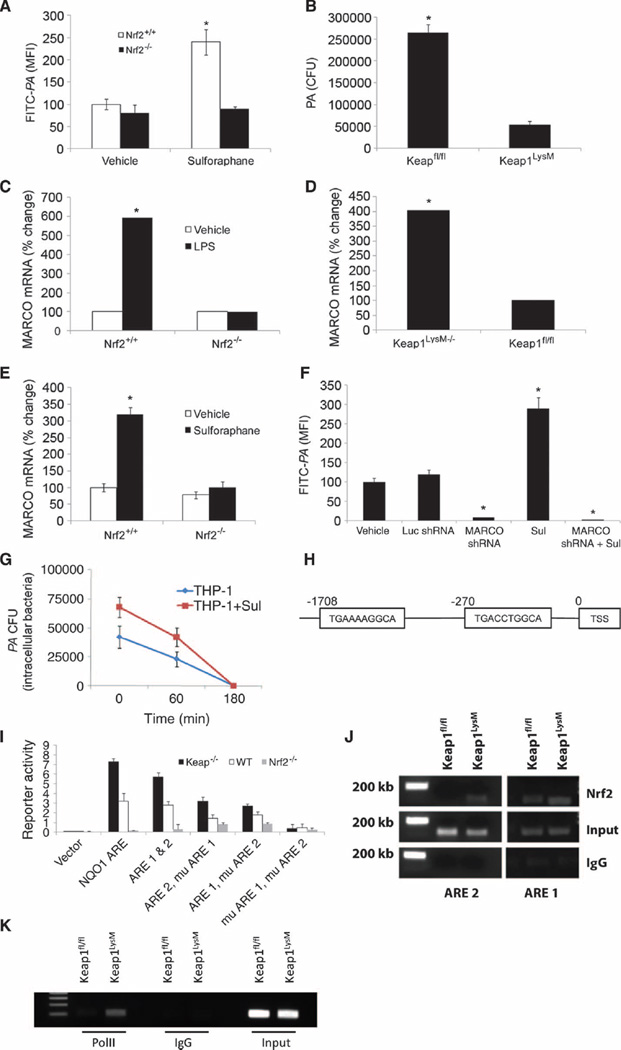
The above data collectively indicate that the activation of Nrf2 improves bacterial phagocytosis and clearance by COPD macrophages; however, the underlying mechanisms are not clear. To address this, we investigated the dependence of bacterial phagocytosis on Nrf2 in macrophages isolated from mice. In vitro sulforaphane treatment significantly increased bacterial phagocytosis of FITC-PA in bone marrow–derived macrophages (BMDMs) of wild-type (Nrf2+/+) but not of Nrf2-deficient (Nrf2−/−) mice (Fig. 2A). Similar results were obtained with alveolar macrophages from Nrf2+/+ and Nrf2−/− mice (fig. S2A). Alveolar macrophages with high endogenous Nrf2 activity, which were isolated from mice that contain a deletion of the Nrf2 inhibitor Keap1 specifically in myeloid cells (Lyzm-Keap1−/− mice), showed greater bacterial uptake when compared to Keap1 flox/flox (Keap1f/f) mice (Fig. 2B). Next, we measured bacterial binding by macrophages in the presence of cytochalasin D. Cytochalasin D specifically inhibits polymerization of actin and prevents phagocytosis but does not prevent extracellular binding of bacteria to macrophages (29). We determined in cytochalasin D–treated macrophages that activation of Nrf2, via either sulforaphane treatment or genetic activation in macrophages obtained from Lyzm-Keap1−/− mice, resulted in greater binding of FITC-PA compared to respective vehicle-treated controls (fig. S2B), suggesting that activation of Nrf2 increases binding of bacteria to macrophages. These two observations confirmed Nrf2-dependent modulation of bacterial binding and phagocytosis.
Fig. 2.
Nrf2 regulates scavenger receptor MARCO expression. (A) BMDMs were derived from Nrf2+/+ and Nrf2−/− mice and treated with vehicle or sulforaphane followed by incubation with FITC-PA. Uptake was quantified by flow cytometry. (B) Alveolar macrophages isolated from Keap1f/f and LysM-Keap1−/− mice were incubated with PA, and bacterial load in culture medium was quantified after 4 hours. Data are represented as CFU means ± SEM. (C) MARCO mRNA expression levels in BMDM isolated from Nrf2+/+ and Nrf2−/− 4 hours after lipopolysaccharide (LPS) or vehicle treatment as measured by microarray analysis. Data are represented as relative fold change compared to vehicle treatment for each genotype. (D) Basal MARCO mRNA expression levels in BMDMs isolated from Keap1f/f and LysM-Keap1−/− as measured by microarray analysis. (E) MARCO mRNA expression levels in BMDMs isolated from Nrf2+/+ and Nrf2−/− 16 hours after sulforaphane or vehicle treatment as measured by qRT-PCR analysis. (F) Phagocytosis of FITC-PA in THP-1 macrophages stably transfected with luciferase (Luc) shRNA or MARCO shRNA and treated with either vehicle or sulforaphane. (G) Intracellular PA (CFUs) in THP-1 macrophage lysates. Lysates were prepared at varying time periods after sulforaphane or vehicle treatment. Data are from an experiment in triplicate. (H) In silico promoter analysis for identification of putative AREs in the 5′ upstream of the transcription start site (TSS) of MARCO gene. (I) Functionality of AREs was evaluated by transfection of luciferase reporter constructs containing ARE1, ARE2; ARE1, mutated (mu) ARE2; ARE2, muARE1; and muARE1, muARE2 into Keap1−/−, Nrf2+/+, and Nrf2−/− MEFs. Data are represented as means ± SEM from three independent experiments. (J) ChIP assay to determine Nrf2 binding to the promoter region of MARCO gene in macrophages derived from LysM-Keap1−/− and Keap1f/f mice. (K) ChIP assay to determine recruitment of RNA PolII (polymerase II) binding to MARCO promoter in macrophages derived from LysM-Keap1−/− and Keap1f/f mice. *P < 0.05.
To determine the mechanisms by which Nrf2 regulates phagocytosis, we used microarray analysis of macrophages exposed to the bacterial cell wall component, lipopolysaccharide (LPS). Due to limitations in harvesting sufficient numbers of alveolar macrophages from mice, we used BMDMs for microarray studies. Microarray analysis and quantitative reverse transcription–polymerase chain reaction (qRT-PCR) validation revealed (i) higher expression (about six-fold) of the class A scavenger receptor MARCO after LPS stimulation in BMDMs of Nrf2+/+ mice compared to vehicle treatment, whereas no induction was observed in Nrf2−/− BMDMs (Fig. 2C), and (ii) higher basal gene expression of MARCO in BMDMs of Lyzm-Keap1−/− mice compared to Keap1f/f mice (Fig. 2D). qRT-PCR analysis also confirmed higher expression of MARCO mRNA in alveolar macrophages of Nrf2+/+ mice but not in Nrf2−/− mice after sulforaphane (Fig. 2E) or bacterial treatment (fig. S2C).
To further address whether sulforaphane-induced expression of MARCO mediates bacterial clearance, we used the human THP-1macrophage cell line. We analyzed bacterial clearance by sulforaphane-treated THP-1 macrophages after exposure with polyinosinic acid [poly(I)]. Poly(I) is a nonselective class A scavenger receptor blocker that inhibits MARCO-mediated bacterial uptake and clearance (29). Poly(I) significantly impaired the ability of sulforaphane to improve bacterial clearance by THP-1 macrophages (fig. S2D). Next, we inhibited MARCO by lentivirus-encoded short hairpin RNA (shRNA) (MARCO shRNA) in THP-1 macrophages and examined phagocytosis of FITC-PA. Endogenous levels of MARCO (fig. S2E) and phagocytic activity (FITC-PA) were both suppressed in THP-1 macrophages infected with MARCO shRNA when compared to control luciferase shRNA (Fig. 2F). Sulforaphane treatment significantly elevated phagocytosis of FITC-PA in THP-1 macrophages transfected with luciferase shRNA but not in MARCO shRNA (Fig. 2F). Together, these findings suggest that Nrf2 regulates bacterial phagocytosis by increasing MARCO in macrophages.
Because reactive oxygen species (ROS) formation is important for bactericidal activity in macrophages, we determined whether up-regulation of Nrf2-dependent antioxidants by sulforaphane interferes with bacterial killing after the bacteria have been internalized by THP-1 macrophages. The CFUs obtained from within macrophages as a function of time are reflective of intracellular killing ability. We found that efficiency for killing intracellular PA was similar in vehicle- and sulforaphane-treated macrophages, although there was a moderate increase in the kinetics of intracellular killing after sulforaphane treatment. These results rule out any detrimental effect of increasedNrf2-regulated antioxidants after sulforaphane treatment in the phagocytosis process (Fig. 2G).
Next, to examine whether the MARCO gene is a transcriptional target of Nrf2, we analyzed the mouse genomic sequence in the 5′ untranslated region (UTR) of MARCO. Genomics software analysis revealed the presence of two AREs, ARE1 and ARE2, which were −270 and −1708 base pairs (bp) upstream of the transcription start site, respectively (Fig. 2H). To determine the functionality of both ARE1 and ARE2, we cloned the full-length promoter and fragments containing the individual AREs into pGL3 basic luciferase vectors. Specific AREs were also subjected to site-directed mutagenesis individually and in combination. Luciferase reporter activity was measured after transfecting the vectors into Keap1−/− (basally high Nrf2 activity), Nrf2+/+, and Nrf2−/− mouse embryonic fibroblasts (MEFs) (30, 31). Due to poor transfection efficiency of macrophages, we used MEFs for the reporter assays. To activate Nrf2, we used Keap1-disrupted cells instead of sulforaphane because it provides specific and high basal Nrf2-dependent transcriptional activity (30–32). Reporter analysis of native constructs in Keap1−/−, Nrf2+/+, andNrf2−/− revealed an Nrf2-dependent increase in reporter activity of the full-length, ARE1, and ARE2 (Fig. 2I). Conversely, the mutated constructs revealed equal functionality of both ARE1 and ARE2, with dual mutation resulting in the complete loss of reporter activity (Fig. 2I). Next, we used macrophages from Lyzm-Keap1−/− and Keap1f/f mice for chromatin immunoprecipitation (ChIP) analysis. ChIP assay showed greater Nrf2 binding to ARE1 and ARE2 as well as binding of RNA polymerase II in the promoter of the MARCO gene in macrophages isolated from Lyzm-Keap1−/− mice compared to Keap1f/f mice, indicating active gene transcription in the former group (Fig. 2, J and K). Together, these results suggest that Nrf2 mediates transcriptional regulation of MARCO.
Sulforaphane enhances bacterial phagocytosis by increasing MARCO in COPD alveolar macrophages
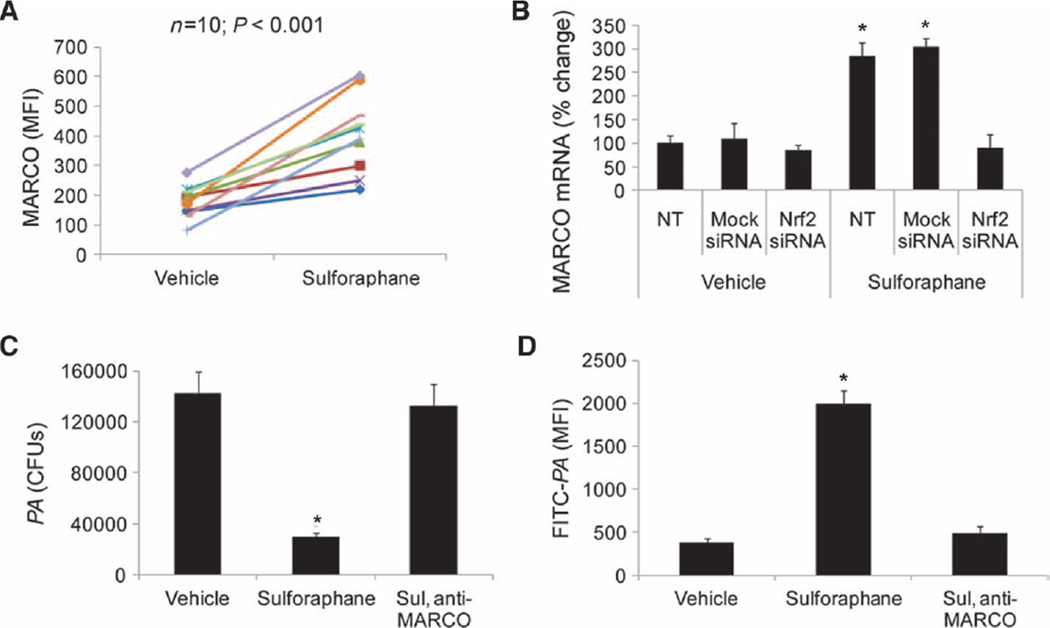
Next, we investigated whether sulforaphane increased the expression of MARCO in alveolar macrophages from patients with COPD. Compared to vehicle treatment, sulforaphane treatment significantly elevated surface expression of MARCO protein, measured by flow cytometry, in alveolar macrophages from patients with COPD (two- to five-fold, P < 0.001) (Fig. 3A). However, sulforaphane failed to up-regulate the expression of MARCO mRNA in alveolar macrophages transfected with Nrf2 siRNA when compared to control ssRNA, indicating Nrf2-dependent regulation of MARCO (Fig. 3B).
Fig. 3.
Sulforaphane improves bacterial phagocytic function in COPD alveolar macrophages by Nrf2-dependent up-regulation of MARCO expression. (A) Surface expression of MARCO in COPD alveolar macrophages after sulforaphane or vehicle treatment by flow cytometry. Data are represented as MFI for individual patient with vehicle or sulforaphane treatment. (B) MARCO mRNA expression in COPD alveolar macrophages transfected with mock siRNA, Nrf2 siRNA, or no treatment (NT, untransfected) before sulforaphane treatment. Data are represented as mean percent change compared to vehicle-treated untransfected cells ± SEM (n = 3). *P < 0.05. (C) Bacteria (PA) remaining in the culture medium after blocking MARCO receptor by anti-MARCO antibody in sulforaphane-treated alveolar macrophages. Data are represented as means ± SEM of CFUs in culture medium from each treatment (n=3subjects). (D) Flow analysis of FITC-PA phagocytosis after blocking MARCO receptor by anti-MARCO antibody in sulforaphane-treated alveolar macrophages. Data are represented as means ± SEM of MFI (n = 3 subjects). *P < 0.05, unless otherwise stated.
To examine the contribution of MARCO in mediating bacterial phagocytosis by alveolar macrophages after sulforaphane treatment, we used an antibody depletion approach. We validated the specificity of anti-MARCO antibody by immunoblot analysis (fig. S3A). Incubation of alveolar macrophages after sulforaphane treatment with anti-MARCO antibody abrogated bacterial clearance (Fig. 3C) and phagocytosis of attenuated FITC-labeled PA (Fig. 3D). To assess the effect of sulforaphane on the phenotype of macrophages, we analyzed CD80/86, MHC-II (major histocompatibility complex class II), CD14, and CD206 by flow cytometry. We noted moderate suppression of receptors CD80/86 and CD206 but no significant difference in the expression of other markers (fig. S3B). Together, sulforaphane restores bacterial phagocytosis and improves clearance in alveolar macrophages by Nrf2-dependent up-regulation of MARCO.
Cigarette smoke exposure impairs phagocytic ability of alveolar macrophages and enhances bacterial CFUs in the lungs, which was augmented in Nrf2−/− mice
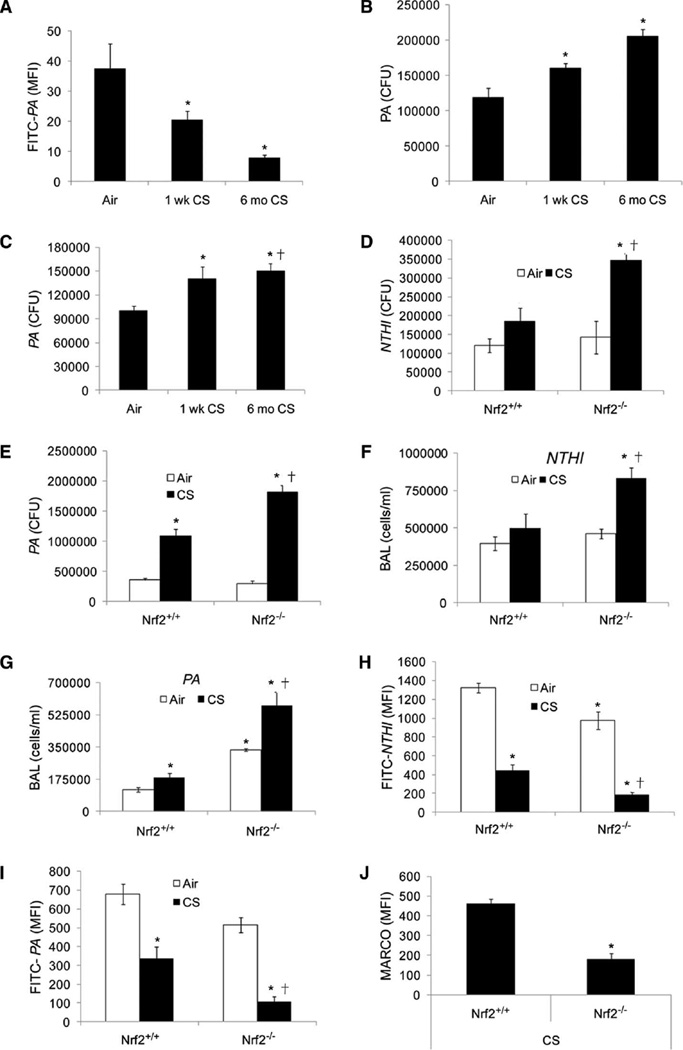
We and others have shown that 6 months of cigarette smoke exposure causes emphysema in mouse models (15, 17). To address the in vivo role of Nrf2 on pulmonary bacterial clearance, we used mouse models exposed to cigarette smoke. We used 6-month (long-term) as well as 1-week (short-term) cigarette smoke exposure mouse models. We found that alveolar macrophages from mice exposed to cigarette smoke for 6 months showed significant impairment in bacterial (PA) uptake and clearance (Fig. 4, A and B) compared to filtered air. Notably, we found that even 1 week of exposure to cigarette smoke induced a significant defect in alveolar macrophage phagocytic ability (Fig. 4, A and B). We found no significant expression of MARCO in alveolar macrophages after cigarette smoke exposure compared to filtered air–exposed mice (fig. S4A). However, sulforaphane treatment increased MARCO expression in alveolar macrophages (fig. S4A) from cigarette smoke– and filtered air–exposed mice and enhanced bacterial phagocytic ability and clearance (fig. S4, B and C).
Fig. 4.
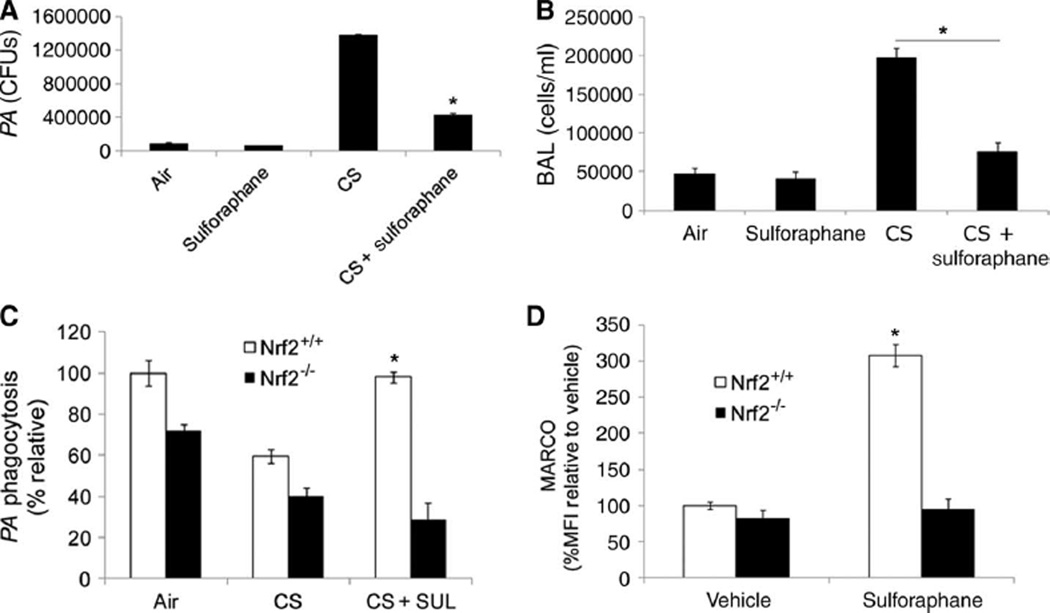
Cigarette smoke exposure impairs bacterial clearance and enhances inflammation in lungs of Nrf2−/− mice when compared to Nrf2+/+ mice. (A) Alveolar macrophages isolated from wild-type mice exposed to filtered air or cigarette smoke (CS) for 1 week or 6 months were incubated with FITC-PA, and uptake was assessed by flow cytometry. Data are represented as means ± SEM of MFI (n = 5 per group). *P < 0.05, compared to air. (B) CFUs in the culture medium of alveolar macrophages isolated from mice exposed to filtered air or cigarette smoke (1 week or 6 months) 4 hours after incubation with PA. Data are represented as means ± SEM of CFUs (n = 5 per group). *P < 0.05, compared to air. (C) Bacterial burden in the lungs of mice exposed to filtered air or cigarette smoke (1 week or 6 months) 4 hours after PA infection. Data are represented as means ± SE of CFUs (n = 5 per group). *P < 0.05, compared to air. (D and E) Bacterial burden in lungs of cigarette smoke (1 week)–or air-exposed Nrf2−/− and Nrf2+/+ mice after 4 hours of NTHI or PA infection. Data are represented as means ± SEM of CFUs (n = 5 per group). *P < 0.05, compared to air-exposed Nrf2+/+; †P < 0.05, compared to cigarette smoke–exposed Nrf2+/+. (F and G) Analysis of inflammatory cells in bronchoalveolar lavage (BAL) fluid of cigarette smoke (1 week)– or air-exposed Nrf2+/+ and Nrf2−/− mice 4 hours after NTHI or PA infection. *P < 0.05, compared to air-exposed Nrf2+/+; †P < 0.05, compared to cigarette smoke–exposed Nrf2+/+. (H and I) Flow cytometric analysis of FITC-labeled PA or NTHI in alveolar macrophages from Nrf2+/+ and Nrf2−/− mice after cigarette smoke (1 week) or air exposure. *P < 0.05, compared to air-exposed Nrf2+/+; †P < 0.05, compared to cigarette smoke–exposed Nrf2+/+. (J) Flow analysis of surface expression of MARCO in alveolar macrophages from Nrf2+/+ and Nrf2−/− mice after cigarette smoke exposure (1 week). Data are represented as means ± SEM of MFI. *P < 0.05, compared to cigarette smoke– exposed Nrf2+/+.
To assess the in vivo role of alveolar macrophages in bacterial clearance after acute respiratory infection, we first sought to discriminate macrophage- and neutrophil-mediated bacterial clearance. Neutrophil depletion by Ly6G-specific antibody administration did not affect bacterial clearance at 4 hours; however, it significantly increased bacterial burden in lungs 24 hours after PA infection (fig. S4D). After PA infection, we found predominantly macrophages until 4 hours; however, later neutrophils infiltrated and became the predominant cell type by 24 hours in BAL fluid (fig. S4E). On the basis of these findings, to assess the role of alveolar macrophages in pulmonary bacterial clearance, we chose to analyze bacterial burden at 4 hours after bacterial infection for future studies. We found that mice exposed to 1 week or 6 months of cigarette smoke exposure showed greater pulmonary bacterial burden than did mice exposed to filtered air (Fig. 4C).
To investigate whether Nrf2 affects bacterial clearance in cigarette smoke–exposed mice, we used Nrf2+/+ and Nrf2−/− mice exposed to smoke for 1 week. After smoke exposure, mice were challenged intranasally with PA or NTHI, and lung bacterial burden and inflammation were analyzed in BAL fluid at 4 hours. Bacterial burden (NTHI or PA) was twice as high in BAL fluid of cigarette smoke–exposed Nrf2−/− mice than in Nrf2+/+ (Fig. 4, D and E). Furthermore, the total number of inflammatory cells was three times higher in BAL of cigarette smoke–exposed Nrf2−/− mice than in Nrf2+/+mice (Fig. 4, F and G). In the air-exposed group, there was no significant difference in bacterial burden between genotypes; however, a significant increase in total inflammatory cells was observed in BAL of PA-infected Nrf2−/− mice when compared to PA-infected Nrf2+/+ mice.
Next, we assessed bacterial phagocytosis ex vivo by incubating alveolar macrophages isolated from cigarette smoke– or air-exposed mice with FITC-PA and/or FITC-NTHI for 1 hour. Cigarette smoke–exposed alveolar macrophages from Nrf2−/− mice showed a greater impairment in phagocytosis of FITC-PA and/or FITC-NTHI than from Nrf2+/+ mice (Fig. 4, H and I). In the air-exposed group, bacterial phagocytosis by alveolar macrophages was moderately higher in Nrf2+/+ mice when compared to Nrf2−/− mice. Consistent with the bacterial phagocytosis results, we found a four times higher surface expression of MARCO in alveolar macrophages from Nrf2+/+ mice than in Nrf2−/− mice after cigarette smoke exposure (Fig. 4J). Together, these results suggest that Nrf2 is critical for up-regulation of MARCO in alveolar macrophages and deficiency of Nrf2 impairs phagocytic ability of alveolar macrophages and reduces pulmonary bacterial clearance.
Nebulizer administration of sulforaphane improves bacterial uptake and inflammation in Nrf2+/+ but not Nrf2−/− mice after cigarette smoke exposure
Finally, we investigated the efficacy of sulforaphane to improve bacterial phagocytosis and clearance in the lungs of mice exposed to cigarette smoke. After 6 months or 1 week of cigarette smoke or air exposure, mice (Nrf2+/+ and Nrf2−/−) were treated with sulforaphane or vehicle (0.5 mg per mouse per day) for 3 consecutive days with an Aeroneb nebulizer (Aerogen Inc.). Mice were challenged intranasally with PA 24 hours after the last dose of sulforaphane, and bacterial burden in BAL fluid was analyzed 4 hours later. Sulforaphane treatment significantly reduced bacterial burden in lungs of Nrf2+/+ mice exposed to 1 week (Fig. 5A) or 6 months (fig. S5A) of cigarette smoke compared to vehicle-treated animals, but did not restore bacterial phagocytic ability in alveolar macrophages isolated from cigarette smoke–exposed Nrf2−/−mice (Fig. 5C). Notably, we observed a significant impairment in lung bacterial clearance even 3 days after cigarette smoke exposure. A decrease in bacterial burden was also observed after sulforaphane treatment in neutrophil-depleted cigarette smoke–exposed mice (fig. S5B). Consistent with the results on bacterial burden, sulforaphane treatment significantly reduced inflammation in the lungs of Nrf2+/+mice (Fig. 5B). Sulforaphane failed to reduce bacterial burden in the lungs of Nrf2−/− mice (fig. S5C). We also examined bacterial phagocytic ability of alveolar macrophages isolated from cigarette smoke–(1 week) or air-exposed mice after sulforaphane or vehicle treatment. As for phagocytic ability, sulforaphane treatment enhanced the expression of MARCO in alveolar macrophages from Nrf2+/+ mice but not in Nrf2−/− mice (Fig. 5D). These results demonstrate the efficacy of sulforaphane in vivo to decrease bacterial burden by improving bacterial phagocytosis and inflammation in an Nrf2-dependent manner in mice exposed chronically to cigarette smoke.
Fig. 5.
Sulforaphane treatment enhances bacterial clearance and reduces inflammation in Nrf2+/+ mice after cigarette smoke exposure. (A) Bacterial burden in lungs of cigarette smoke (1 week) or air-exposed wild-type mice treated with sulforaphane or vehicle 4 hours after PA infection. Data are represented as means ± SEM of CFUs (n = 5 per group). *P < 0.05, compared to cigarette smoke alone. (B) Analysis of inflammatory cells contained in bronchoalveolar lavage (BAL) fluid of cigarette smoke (1 week) or air-exposed, sulforaphane-treated wild-type mice 4 hours after PA infection. Data are represented as means ± SEM (n=5per group). *P < 0.05, compared to cigarette smoke alone. (C) Flow analysis of FITC-labeled PA in alveolar macrophages isolated from sulforaphane (SUL)– or vehicle-treated Nrf2+/+ and Nrf2−/− mice after cigarette smoke (1 week) or air exposure. Data are represented as percent MFI relative to air-exposed Nrf2+/+. (D) Surface expression of MARCO in alveolar macrophages isolated from sulforaphane- or vehicle-treated Nrf2+/+ and Nrf2−/− mice after cigarette smoke (1week) or air exposure as measured by flow cytometry. *P < 0.05, compared to vehicle.
Dietary consumption of sulforaphane-enriched broccoli sprout extract increases MARCO expression
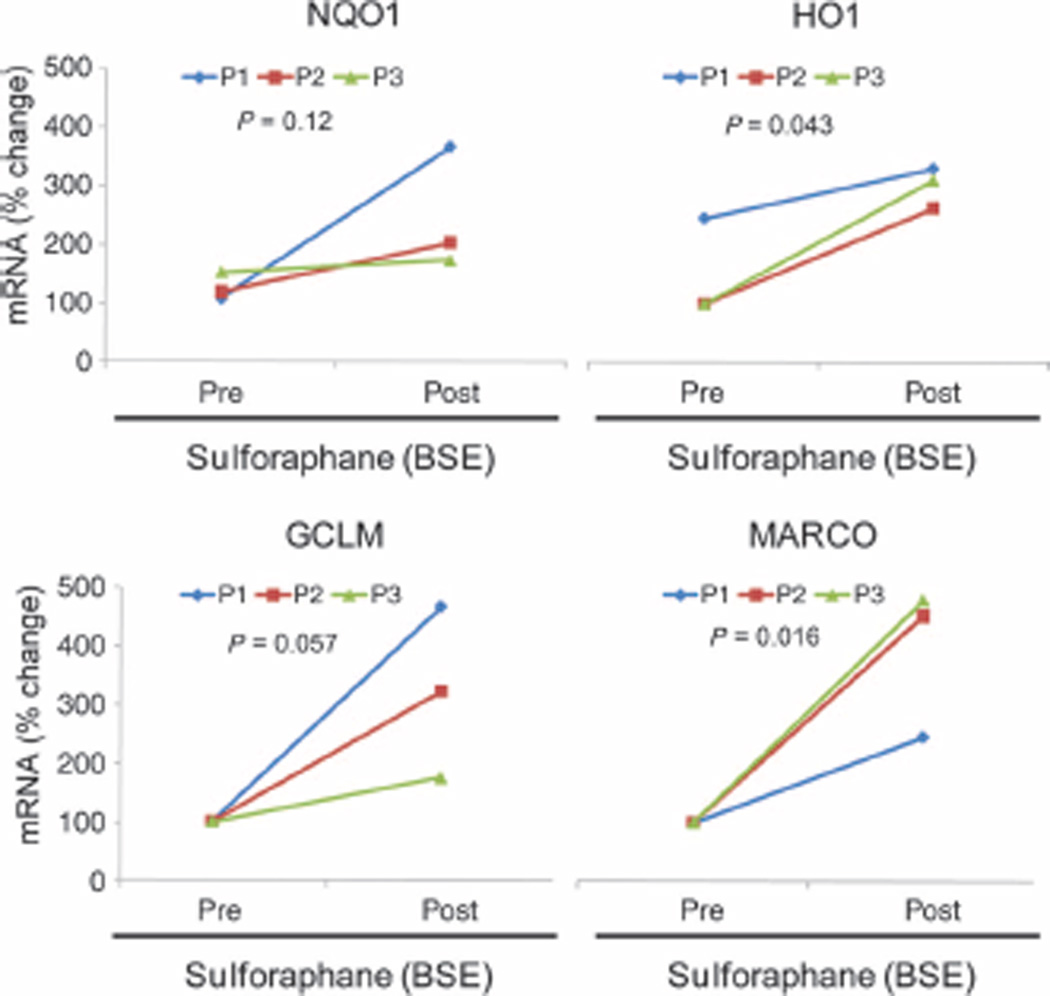
Sulforaphane-enriched broccoli sprout extract (BSE) has been evaluated in human subjects for anticancer properties (26, 33) and shown to increase Nrf2-regulated antioxidants in upper airways (34). To determine whether sulforaphane increases the expression of MARCO in human subjects, we isolated peripheral blood mononuclear cells (PBMCs) from three healthy subjects before and after they consumed BSE for 2 weeks. Sulforaphane significantly increased the expression of MARCO (two- to five-fold) and other Nrf2-regulated antioxidant (NQO1, GCLM, and HO-1) genes in PBMCs of healthy subjects (Fig. 6). These results demonstrate the translational potential of sulforaphane to increase MARCO expression in humans.
Fig. 6.
Dietary administration of sulforaphane-rich broccoli sprout extract (BSE) enhances MARCO expression in PBMCs. mRNA expression of Nrf2 target genes in PBMCs isolated from three healthy subjects at baseline (Pre) and immediately after 2 weeks (Post) of daily consumption of BSE containing 100 µM sulforaphane. Statistical analysis was conducted by a one-way ANOVA.
DISCUSSION
Cigarette smoking is believed to impair lung innate immunity by multiple mechanisms such as mucus hypersecretion, disruption of mucocillary clearance, and inhibition of antimicrobial peptides (9, 22). However, animal and clinical studies have established that impaired bacterial phagocytosis by alveolar macrophages in smokers with or without COPD is the main reason for increased risk of bacterial infection and colonization (35, 36). Here, we show that increasing expression of the scavenger receptor MARCO by targeting Nrf2 with sulforaphane may be an effective approach to improve bacterial phagocytosis and clearance ex vivo by alveolar macrophages of patients with COPD. Furthermore, we illustrate that sulforaphane improves pulmonary antibacterial defenses, specifically increased phagocytic ability, increased bacterial clearance, and decreased acute bacterial infections, in mice exposed to chronic cigarette smoke through Nrf2-dependent MARCO expression in alveolar macrophages.
Alveolar macrophages are the first line of defense against inhaled bacteria and limit airway inflammation during subclinical infection by opportunistic bacteria such as PA or NTHI (37). Macrophages express a repertoire of receptors, specifically scavenger receptors, which help in recognition, binding, and uptake of bacteria. Alveolar macrophages obtained from healthy smokers and smokers with COPD show reduced bacterial phagocytic responses when compared to never smokers (9, 38). However, alveolar macrophages from never smokers with or without COPD exhibit no difference in the phagocytosis of latex beads (23), and there is also no difference in intracellular killing (9). These observations suggest that smoke may affect the recognition or binding of the bacteria to macrophages rather than induce a general defect in phagocytic process (23, 38). The expression of surface receptors involved in phagocytosis, such as CD36, CD51, CD44, CD61, and mannose receptors, was similar between macrophages and peripheral blood monocytes obtained from never smokers and smokers with or without COPD (39). It has been suggested that cigarette smoke may impair bacterial interaction with host alveolar macrophages by altering the expression of Toll-like receptors (TLRs), although data on the role of cigarette smoke in modulating TLR expression are conflicting (6). MARCO is a class A scavenger receptor expressed on macrophages that mediates binding and uptake of Gram-positive and Gram-negative bacteria, oxidized low-density lipoprotein (LDL), and environmental particles but not yeast (40, 41). Ablation of MARCO impairs phagocytosis, whereas overexpression of MARCO enhances the binding of bacteria and phagocytosis (42). A recent report suggests that viral infections inhibit the expression of MARCO in alveolar macrophages via interferon-γ (IFN-γ) signaling, which impedes phagocytic activity and predisposes to bacterial infection (43). The transcription of MARCO is highly elevated on macrophages upon bacterial infection or exposure to bacterial components (44); however, underlying mechanisms are unclear. Our study demonstrates that Nrf2 directly regulates transcriptional expression of MARCO, and we also demonstrate a direct correlation between sulforaphane-induced Nrf2-dependent expression of MARCO and bacterial phagocytic ability by alveolar macrophages isolated from patients with COPD and cigarette smoke–exposed mice. Recent studies suggest that cigarette smoke–induced oxidative activation of RhoA is an etiological factor in defective phagocytosis (24). We found that incubation with antioxidants such as NAC or GSH ester failed to improve the bacterial phagocytic ability of COPD alveolar macrophages, suggesting that inhibition of oxidative stress alone may not be sufficient to restore the phagocytosis. Sulforaphane treatment elevated antioxidants; however, this was not sufficient to restore phagocytosis, as indicated by the inability of sulforaphane to increase bacterial phagocytosis and clearance in COPD alveolar macrophages and human THP-1 macrophages when MARCO was disrupted. These results suggest that Nrf2-dependent regulation of phagocytosis is partly independent of its antioxidant function in normal macrophages. Although Nrf2-dependent antioxidants are not sufficient to restore phagocytosis in COPD macrophages, they may still play a role in modifying phagocytic processes (45).
Peripheral lung tissue and alveolar macrophages from patients with COPD exhibit deficiencies in Nrf2 activity compared to non-COPD tissues (18–20). It is plausible that impaired Nrf2-directed expression of MARCO in alveolar macrophages is one potential mechanism responsible for defective bacterial clearance in COPD. To substantiate the in vivo role of Nrf2-dependent regulation of MARCO in alveolar macrophages, we used cigarette smoke–exposed mouse models. Chronic exposure to cigarette smoke for 6 months causes emphysema in mouse models (15, 17). Consistent with clinical samples, we found that alveolar macrophages from mice exposed to cigarette smoke for 1 week or 6 months displayed significant impairment in bacterial phagocytosis, enhanced bacterial growth, and greater lung inflammation. Consistent with impaired induction of MARCO in alveolar macrophages, Nrf2-deficent mice displayed greater bacterial burden of PA or NTHI and inflammation compared to wild-type mice exposed to cigarette smoke. We found that nebulizer administration of sulforaphane increased bacterial (PA or NTHI) clearance and decreased inflammation in the lungs of Nrf2+/+ mice but not in Nrf2−/− exposed to cigarette smoke for 6 months or 1 week. Ex vivo analysis showed greater expression of MARCO and improved phagocytic ability in alveolar macrophages from mice treated with sulforaphane compared to vehicle. Enhanced inflammation in the lungs of Nrf2−/− mice may be partly due to greater bacterial burden as well as greater inflammatory response due to increased oxidative stress (21).
Currently, pharmacotherapy for COPD is directed toward limiting acute exacerbations. Antibiotics are effective in shortening the duration of exacerbations, but long-term use may predispose to drug-resistant bacterial strains. Thus, there are limited effective therapeutic strategies to counteract bacterial exacerbations in COPD. Sulforaphane is an active ingredient in broccoli sprouts that increases antioxidant, anti-inflammatory, and antibacterial defenses via the activation of Nrf2 (46). Sulforaphane-enriched BSE has been extensively studied in humans for its anticancer properties, has been shown to activate the Nrf2 pathway in upper airways, and is well tolerated by patients (25–28). Our finding that dietary consumption of sulforaphane-enriched BSE increases the expression of MARCO and other Nrf2-regulated antioxidant genes in PBMCs of normal human subjects underscores that the dietary intake of sulforaphane or other phytochemical activators of Nrf2 signaling, by smokers or COPD patients, may improve phagocytic ability of alveolar macrophages and protect from acute infections by opportunistic bacterial strains such as PA or NTHI. A National Heart, Lung, and Blood Institute (NHLBI) multicenter phase II clinical trial has recently been initiated to test whether sulforaphane from BSEs can improve antibacterial defenses in COPD patients.
MATERIALS AND METHODS
Recruitment of subjects
This study was approved by the institutional review board of Johns Hopkins University. Patients with COPD were asked to volunteer for a bronchoscopy for collection of alveolar macrophages by BAL at Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center. These patients had an FEV1/FVC (forced expiratory volume in 1 s/forced vital capacity) of <0.70, an FEV1 of 40 to 80% predicted, and a >10 pack-year smoking history (current and former smokers were included). Demographic data are presented in Table 1.
The Broccoli Sprout Extract (BSE) study was approved by the institutional review board of Johns Hopkins University to test whether sulforaphane protects from airway hyperreactivity in asthma. For our study, we used PBMCs isolated from healthy subjects.
Cell culture
Human alveolar macrophages were purified from individual BAL samples, and purity was determined by nuclear and cytoplasmic DiffQuick staining and verified by flow cytometry. Murine alveolar macrophages and BMDMs were isolated from Nrf2+/+, Nrf2−/−, Keap1f/f, and LysM-Keap1−/− mice. BMDMs were isolated and derived as previously described (47). Mouse embryonic fibroblasts (MEFs) were isolated and cultured as previously described (21). The human THP-1 cell line (American Type Culture Collection) was cultured as per the manufacturer’s instructions.
Bacterial strains
Sputum isolates of nontypeable H. influenza (48) and P. aeruginosa were obtained and grown as previously described (49). For FITC labeling of bacteria, heat-inactivated PA and NTHI (109 CFU/ml) were resuspended in 1 ml of labeling buffer (0.1 M NaHCO3, pH 9.2) and incubated with FITC (Sigma) under constant stirring in the dark at room temperature for 1 hour. Finally, bacteria were washed three times with phosphate-buffered saline (PBS) and dialyzed overnight against PBS. FITC-labeled bacteria were resuspended with PBS at a concentration of 109/ml.
Animals and treatments
Nrf2+/+ and Nrf2−/− C57BL/6J mice were housed under controlled conditions for temperature and humidity using a 12-hour light/dark cycle. At 8 weeks of age, mice were exposed to cigarette smoke (2.5 hours per day for 5 days per week) for 1 week or 6 months as previously described (17). All animal protocols were conducted as approved by the Johns Hopkins University Institutional Animal Care and Use Committee.
Statistical analysis
Results are presented as the means ± SE. Statistical comparisons were performed by paired Student’s t tests. Statistical comparisons of treatment effects in human alveolar macrophages were performed by one way analysis of variance (ANOVA). A value of P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank T. Kensler (Johns Hopkins University, Baltimore, MD) and M. Yamamoto (Tohoku University Graduate School of Medicine, Sendai, Japan) for providing Nrf2−/− mice used in this study. We would like to thank T. Sussan, M. Horton, and S. An (Johns Hopkins University, Baltimore, MD) for helpful review of this manuscript and L. Grove (University at Buffalo, Buffalo, NY) for advice on growing conditions of both clinical isolates.
Funding: This work was supported by NIH grant HL081205 (S.B.); National Heart, Lung, and Blood Institute grant HL10342 (R.H.B.); National Heart, Lung, and Blood Institute Specialized Centers of Clinically Oriented Research grant P50HL084945 (R.W. and S.B.); National Institute of Environmental Health Sciences Disease Investigation Through Specialized Clinically-Oriented Ventures in Environmental Research grant P50ES015903 (S.B.); the Flight Attendant Medical Research Institute (S.B. and R.K.T.); and National Institute of Environmental Health Sciences grants P50ES015903 and ES03819. C.J.H. is supported by National Institute of Environmental Health Sciences Training grant ES07141 and S.S. is supported by the VA Merit Review. We would like to acknowledge the generous support of the Grace Anne Dorney fund for tobacco-related disease research.
Footnotes
SUPPLEMENTARY MATERIAL
www.sciencetranslationalmedicine.org/cgi/content/full/3/78/78ra32/DC1
Materials and Methods
Fig. S1. Sulforaphane stabilizes Nrf2 expression in COPD alveolar macrophages and enhances Nrf2-dependent gene expression.
Fig. S2. Nrf2-dependent increase in bacterial uptake is dependent on scavenger receptor MARCO mediated bacterial binding.
Fig. S3. Determination of antibody specificity and macrophage activation.
Fig. S4. Macrophage response and ex vivo analyses.
Fig. S5. Sulforaphane decreases lung bacterial CFUs in Nrf2+/+ mice.
Fig. S6. Generation and characterization of LysM-Keap1−/−conditional knockout mice.
References
Author contributions: C.J.H. designed and performed the experiments and analysis of data. X.K. performed microarray analysis. R.K.T. and S.B. conceived the study, designed the experiments, and analyzed and interpreted the data. R.K.T., C.J.H., and S.B. wrote the manuscript. S.S. provided the clinical isolates, helped in the methodology, and reviewed the manuscript. R.W., D.F.-K., L.Y., and R.H.B. recruited patients, helped in patient characterization and collection of clinical samples for analysis, and reviewed the manuscript.
Competing interests: S.B., R.K.T., and the Johns Hopkins University hold intellectual property on development of Nrf2-based therapeutics for COPD. Cureveda LLC has licensed this intellectual property. S.B. and R.K.T. have equity in Cureveda, which was co-founded by S.B. and R.K.T. and where they serve on the Scientific Advisory Board. These potential individual and institutional conflicts of interest have been reviewed and managed by the Johns Hopkins University School of Public Health. C.J.H., S.S., X.K., L.Y., R.H.B., D.F.-K., and R.W. declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol. Rev. 2004;56:515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- 2.Wedzicha JA. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2004;1:115–120. doi: 10.1513/pats.2306030. [DOI] [PubMed] [Google Scholar]
- 3.Wedzicha JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respir. Care. 2003;48:1204–1213. [PubMed] [Google Scholar]
- 4.Veeramachaneni SB, Sethi S. Pathogenesis of bacterial exacerbations of COPD. COPD. 2006;3:109–115. doi: 10.1080/15412550600651347. [DOI] [PubMed] [Google Scholar]
- 5.Sethi S. Bacteria in exacerbations of chronic obstructive pulmonary disease: Phenomenon or epiphenomenon? Proc. Am. Thorac. Soc. 2004;1:109–114. doi: 10.1513/pats.2306029. [DOI] [PubMed] [Google Scholar]
- 6.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 7.Sethi S, Sethi R, Eschberger K, Lobbins P, Cai X, Grant BJ, Murphy TF. Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007;176:356–361. doi: 10.1164/rccm.200703-417OC. [DOI] [PubMed] [Google Scholar]
- 8.Barnes PJ. Alveolar macrophages in chronic obstructive pulmonary disease (COPD) Cell. Mol. Biol. 2004;50:OL627–OL637. [PubMed] [Google Scholar]
- 9.Berenson CS, Garlipp MA, Grove LJ, Maloney J, Sethi S. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J. Infect. Dis. 2006;194:1375–1384. doi: 10.1086/508428. [DOI] [PubMed] [Google Scholar]
- 10.Domagala-Kulawik J, Maskey-Warzechowska M, Kraszewska I, Chazan R. The cellular composition and macrophage phenotype in induced sputum in smokers and ex-smokers with COPD. Chest. 2003;123:1054–1059. doi: 10.1378/chest.123.4.1054. [DOI] [PubMed] [Google Scholar]
- 11.White AJ, Gompertz S, Bayley DL, Hill SL, O’Brien C, Unsal I, Stockley RA. Resolution of bronchial inflammation is related to bacterial eradication following treatment of exacerbations of chronic bronchitis. Thorax. 2003;58:680–685. doi: 10.1136/thorax.58.8.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 13.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 14.Malhotra D, Thimmulappa R, Vij N, Navas-Acien A, Sussan T, Merali S, Zhang L, Kelsen SG, Myers A, Wise R, Tuder R, Biswal S. Heightened endoplasmic reticulum stress in the lungs of patients with chronic obstructive pulmonary disease: The role of Nrf2-regulated proteasomal activity. Am. J. Respir. Crit. Care Med. 2009;180:1196–1207. doi: 10.1164/rccm.200903-0324OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke–induced emphysema in mice. J. Clin. Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, Sakamoto T, Shimura M, Yoshida A, Yamamoto M, Sekizawa K. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells. 2005;10:1113–1125. doi: 10.1111/j.1365-2443.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- 17.Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, Sporn MB, Gabrielson KL, Champion HC, Tuder RM, Kensler TW, Biswal S. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc. Natl. Acad. Sci. U.S.A. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am. J. Respir. Crit. Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Goven D, Boutten A, Leçon-Malas V, Marchal-Sommé J, Amara N, Crestani B, Fournier M, Lesèche G, Soler P, Boczkowski J, Bonay M. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax. 2008;63:916–924. doi: 10.1136/thx.2007.091181. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki M, Betsuyaku T, Ito Y, Nagai K, Nasuhara Y, Kaga K, Kondo S, Nishimura M. Down-regulated NF-E2–related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2008;39:673–682. doi: 10.1165/rcmb.2007-0424OC. [DOI] [PubMed] [Google Scholar]
- 21.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sethi S, Mallia P, Johnston SL. New paradigms in the pathogenesis of chronic obstructive pulmonary disease II. Proc. Am. Thorac. Soc. 2009;6:532–534. doi: 10.1513/pats.200905-025DS. [DOI] [PubMed] [Google Scholar]
- 23.Martí-Lliteras P, Regueiro V, Morey P, Hood DW, Saus C, Sauleda J, Agusti AG, Bengoechea JA, Garmendia J. Nontypeable Haemophilus influenzae clearance by alveolar macrophages is impaired by exposure to cigarette smoke. Infect. Immun. 2009;77:4232–4242. doi: 10.1128/IAI.00305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richens TR, Linderman DJ, Horstmann SA, Lambert C, Xiao YQ, Keith RL, Boé DM, Morimoto K, Bowler RP, Day BJ, Janssen WJ, Henson PM, Vandivier RW. Cigarette smoke impairs clearance of apoptotic cells through oxidant-dependent activation of RhoA. Am. J. Respir. Crit. Care Med. 2009;179:1011–1021. doi: 10.1164/rccm.200807-1148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, Ye L, Coady JL, Wang JB, Wu Y, Sun Y, Zhang QN, Zhang BC, Zhu YR, Qian GS, Carmella SG, Hecht SS, Benning L, Gange SJ, Groopman JD, Talalay P. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol. Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: A clinical phase I study. Nutr. Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 28.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 29.DeLoid GM, Sulahian TH, Imrich A, Kobzik L. Heterogeneity in macrophage phagocytosis of Staphylococcus aureus strains: High-throughput scanning cytometry-based analysis. PLoS One. 2009;4:e6209. doi: 10.1371/journal.pone.0006209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, Fujii J, Myers A, Biswal S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic. Biol. Med. 2009;46:443–453. doi: 10.1016/j.freeradbiomed.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh A, Rangasamy T, Thimmulappa RK, Lee H, Osburn WO, Brigelius-Flohé R, Kensler TW, Yamamoto M, Biswal S. Glutathione peroxidase 2, the major cigarette smoke–inducible isoform of GPX in lungs, is regulated by Nrf2. Am. J. Respir. Cell Mol. Biol. 2006;35:639–650. doi: 10.1165/rcmb.2005-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh A, Ling G, Suhasini AN, Zhang P, Yamamoto M, Navas-Acien A, Cosgrove G, Tuder RM, Kensler TW, Watson WH, Biswal S. Nrf2-dependent sulfiredoxin-1 expression protects against cigarette smoke-induced oxidative stress in lungs. Free Radic. Biol. Med. 2009;46:376–386. doi: 10.1016/j.freeradbiomed.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases phase II antioxidant enzymes in the human upper airway. Clin. Immunol. 2009;130:244–251. doi: 10.1016/j.clim.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berenson CS, Wrona CT, Grove LJ, Maloney J, Garlipp MA, Wallace PK, Stewart CC, Sethi S. Impaired alveolar macrophage response to Haemophilus antigens in chronic obstructive lung disease. Am. J. Respir. Crit. Care Med. 2006;174:31–40. doi: 10.1164/rccm.200509-1461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drannik AG, Pouladi MA, Robbins CS, Goncharova SI, Kianpour S, Stämpfli MR. Impact of cigarette smoke on clearance and inflammation after Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 2004;170:1164–1171. doi: 10.1164/rccm.200311-1521OC. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein E, Lippert W, Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J. Clin. Invest. 1974;54:519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor AE, Finney-Hayward TK, Quint JK, Thomas CM, Tudhope SJ, Wedzicha JA, Barnes PJ, Donnelly LE. Defective macrophage phagocytosis of bacteria in COPD. Eur. Respir. J. 2010;35:1039–1047. doi: 10.1183/09031936.00036709. [DOI] [PubMed] [Google Scholar]
- 39.Pons AR, Noguera A, Blanquer D, Sauleda J, Pons J, Agustí AG. Phenotypic characterisation of alveolar macrophages and peripheral bloodmonocytes in COPD. Eur. Respir. J. 2005;25:647–652. doi: 10.1183/09031936.05.00062304. [DOI] [PubMed] [Google Scholar]
- 40.Kanno S, Furuyama A, Hirano S. A murine scavenger receptor MARCO recognizes polystyrene nanoparticles. Toxicol. Sci. 2007;97:398–406. doi: 10.1093/toxsci/kfm050. [DOI] [PubMed] [Google Scholar]
- 41.Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K, Kobzik L. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J. Exp. Med. 2004;200:267–272. doi: 10.1084/jem.20040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elomaa O, Sankala M, Pikkarainen T, Bergmann U, Tuuttila A, Raatikainen-Ahokas A, Sariola H, Tryggvason K. Structure of the human macrophage MARCO receptor and characterization of its bacteria-binding region. J. Biol. Chem. 1998;273:4530–4538. doi: 10.1074/jbc.273.8.4530. [DOI] [PubMed] [Google Scholar]
- 43.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-g during recovery from influenza infection. Nat. Med. 2008;14:558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 44.van der Laan LJ, Kangas M, Döpp EA, Broug-Holub E, Elomaa O, Tryggvason K, Kraal G. Macrophage scavenger receptor MARCO: In vitro and in vivo regulation and involvement in the anti-bacterial host defense. Immunol. Lett. 1997;57:203–208. doi: 10.1016/s0165-2478(97)00077-1. [DOI] [PubMed] [Google Scholar]
- 45.Matsushita N, Komine H, Grolleau-Julius A, Pilon-Thomas S, Mulé JJ. Targeting MARCO can lead to enhanced dendritic cell motility and anti-melanoma activity. Cancer Immunol. Immunother. 2010;59:875–884. doi: 10.1007/s00262-009-0813-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho HY, Imani F, Miller-DeGraff L, Walters D, Melendi GA, Yamamoto M, Polack FP, Kleeberger SR. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am. J. Respir. Crit. Care Med. 2009;179:138–150. doi: 10.1164/rccm.200804-535OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blake DJ, Singh A, Kombairaju P, Malhotra D, Mariani TJ, Tuder RM, Gabrielson E, Biswal S. Deletion of Keap1 in the lung attenuates acute cigarette smoke-induced oxidative stress and inflammation. Am. J. Respir. Cell Mol. Biol. 2010;42:524–536. doi: 10.1165/rcmb.2009-0054OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy TF, Brauer AL, Schiffmacher AT, Sethi S. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2004;170:266–272. doi: 10.1164/rccm.200403-354OC. [DOI] [PubMed] [Google Scholar]
- 49.Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, Cai X, Sethi S. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008;177:853–860. doi: 10.1164/rccm.200709-1413OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.