Abstract
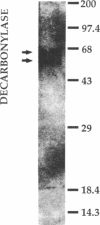
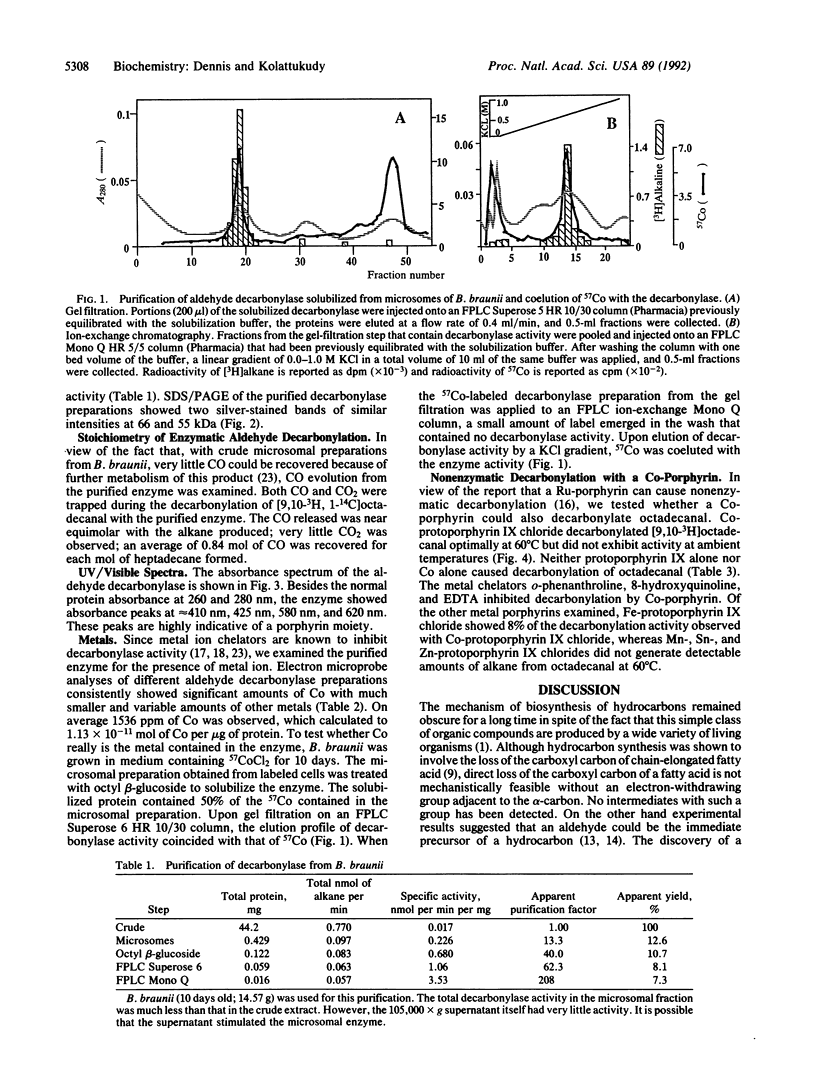
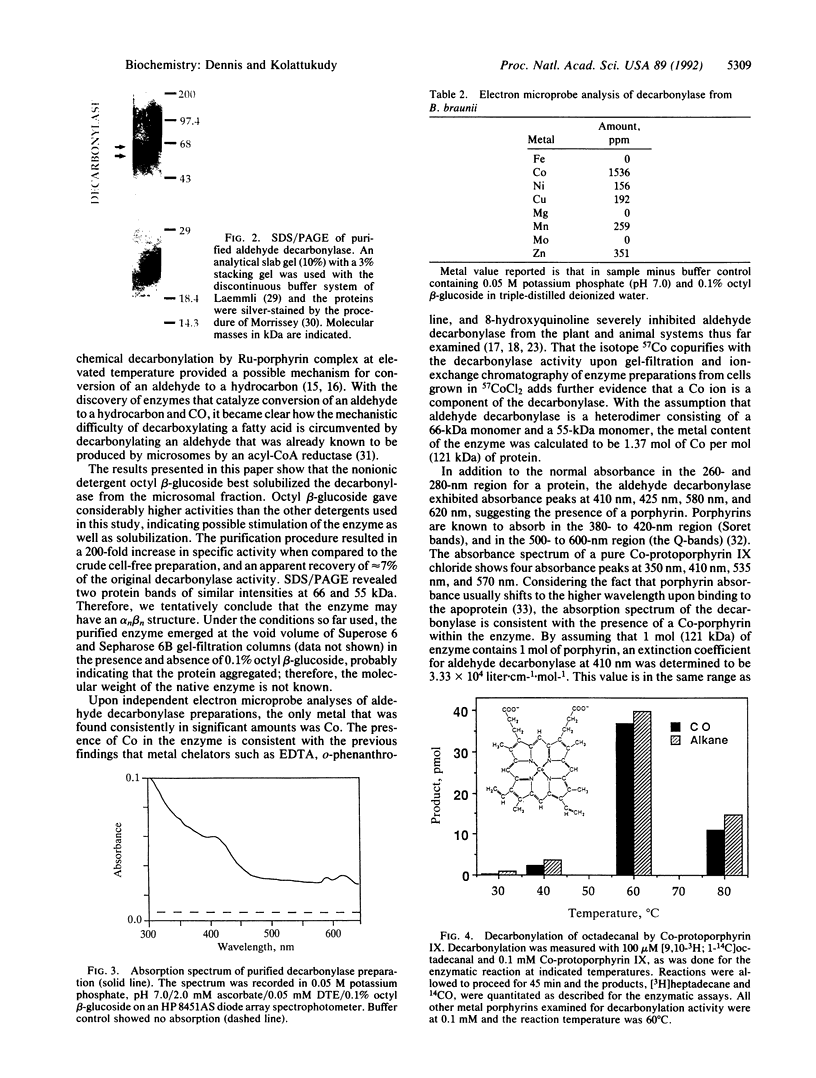
The final step in hydrocarbon biosynthesis involves loss of CO from a fatty aldehyde. This decarbonylation is catalyzed by microsomes from Botyrococcus braunii. Among the several detergents tested for solubilizing the decarbonylase, octyl beta-glucoside (0.1%) was found to be the most effective and released 65% of the enzyme activity in soluble form. FPLC of the solubilized enzyme preparation with Superose 6 followed by ion-exchange FPLC with Mono Q resulted in 200-fold increase in specific activity with 7% recovery. The purified enzyme released nearly 1 mol of CO for each mol of hydrocarbon. SDS/PAGE of the enzyme preparation showed two protein bands of equal intensity at 66 and 55 kDa. The absorption spectrum of the enzyme with bands at 410 nm, 425 nm, 580 nm, and 620 nm suggests the presence of a porphyrin. Electron microprobe analysis revealed that the enzyme contained Co. Purification of the decarbonylase from B. braunii grown in 57CoCl2 showed that 57Co coeluted with the decarbonylase. These results suggest that the enzyme contains Co that might be part of a Co-porphyrin, although a corrin structure cannot be ruled out. Co-protoporphyrin IX itself caused decarbonylation of octadecanal at 60 degrees C, whereas the metal ion or protoporphyrin alone, or several other metal porphyrins, did not cause decarbonylation. These results strongly suggest that biosynthesis of hydrocarbons is effected by a microsomal Co-porphyrin-containing enzyme that catalyzes decarbonylation of aldehydes and, thus, reveal a biological function for Co in plants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal V. P., Kolattukudy P. E. Purification and characterization of a wound-induced omega-hydroxyfatty acid:NADP oxidoreductase from potato tuber disks (Solanum tuberosum L.). Arch Biochem Biophys. 1978 Dec;191(2):452–465. doi: 10.1016/0003-9861(78)90384-3. [DOI] [PubMed] [Google Scholar]
- Agrawal V. P., Stumpf P. K. Characterization and solubilization of an acyl chain elongation system in microsomes of leek epidermal cells. Arch Biochem Biophys. 1985 Jul;240(1):154–165. doi: 10.1016/0003-9861(85)90018-9. [DOI] [PubMed] [Google Scholar]
- Bognar A. L., Paliyath G., Rogers L., Kolattukudy P. E. Biosynthesis of alkanes by particulate and solubilized enzyme preparations from pea leaves (Pisum sativum). Arch Biochem Biophys. 1984 Nov 15;235(1):8–17. doi: 10.1016/0003-9861(84)90249-2. [DOI] [PubMed] [Google Scholar]
- Bourre J. M., Cassagne C., Larrouquere-Regnier S., Darriet D. Occurrence of alkanes in brain myelin. Comparison between normal and quaking mouse. J Neurochem. 1977 Oct;29(4):645–648. doi: 10.1111/j.1471-4159.1977.tb07781.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buckner J. S., Kolattukudy P. E. Specific inhibition of alkane synthesis with accumulation of very long chain compounds by dithioerythritol, dithiothreitol, and mercaptoethanol in Pisum sativum. Arch Biochem Biophys. 1973 May;156(1):34–45. doi: 10.1016/0003-9861(73)90338-x. [DOI] [PubMed] [Google Scholar]
- Cassagne C., Darriet D., Bourre J. M. Evidence of alkane synthesis by the sciatic nerve of the rabbit. FEBS Lett. 1977 Oct 1;82(1):51–54. doi: 10.1016/0014-5793(77)80883-1. [DOI] [PubMed] [Google Scholar]
- Cheesbrough T. M., Kolattukudy P. E. Alkane biosynthesis by decarbonylation of aldehydes catalyzed by a particulate preparation from Pisum sativum. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6613–6617. doi: 10.1073/pnas.81.21.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheesbrough T. M., Kolattukudy P. E. Microsomal preparation from an animal tissue catalyzes release of carbon monoxide from a fatty aldehyde to generate an alkane. J Biol Chem. 1988 Feb 25;263(6):2738–2743. [PubMed] [Google Scholar]
- Dennis M. W., Kolattukudy P. E. Alkane biosynthesis by decarbonylation of aldehyde catalyzed by a microsomal preparation from Botryococcus braunii. Arch Biochem Biophys. 1991 Jun;287(2):268–275. doi: 10.1016/0003-9861(91)90478-2. [DOI] [PubMed] [Google Scholar]
- Hatchikian E. C. A cobalt porphyrin containing protein reducible by hydrogenase isolated from Desulfovibrio desulfuricans (Norway). Biochem Biophys Res Commun. 1981 Nov 30;103(2):521–530. doi: 10.1016/0006-291x(81)90483-6. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E. Biosynthesis of surface lipids. Biosynthesis of long-chain hydrocarbons and waxy esters is discussed. Science. 1968 Feb 2;159(3814):498–505. doi: 10.1126/science.159.3814.498. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E. Biosynthesis of wax in Brassica oleracea. Relation of fatty acids to wax. Biochemistry. 1966 Jul;5(7):2265–2275. doi: 10.1021/bi00871a015. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E., Buckner J. S. Chain elongation of fatty acids by cell-free extracts of epidermis from pea leaves (pisum sativum). Biochem Biophys Res Commun. 1972 Jan 31;46(2):801–807. doi: 10.1016/s0006-291x(72)80212-2. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E., Rogers L., Larson J. D. Enzymatic reduction of fatty acids and alpha-hydroxy fatty acids. Methods Enzymol. 1981;71(Pt 100):263–275. doi: 10.1016/0076-6879(81)71035-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Moura J. J., Moura I., Bruschi M., Le Gall J., Xavier A. V. A cobalt containing protein isolated from Desulfovibrio gigas, a sulfate reducer. Biochem Biophys Res Commun. 1980 Feb 12;92(3):962–970. doi: 10.1016/0006-291x(80)90796-2. [DOI] [PubMed] [Google Scholar]