Abstract
Purpose. To quantitatively evaluate the effects of peeled internal limiting membrane (ILM) area and anatomic outcomes following macular hole surgery using spectral domain optical coherence tomography (SD-OCT). Methods. Forty-one eyes in 37 consecutive patients with idiopathic, Gass stage 3-4 macular hole (MH) were enrolled in this retrospective comparative study. All patients were divided into 2 groups according to anatomic success or failure. Basal MH diameter, peeled ILM area, and MH height were calculated using SD-OCT. Other prognostic parameters, including age, stage, preoperative BCVA, and symptom duration were also assessed. Results. Thirty-two cases were classified as anatomic success, and 9 cases were classified as anatomic failure. Peeled ILM area was significantly wider and MH basal diameter was significantly less in the anatomic success group (p = 0.024 and 0.032, resp.). Other parameters did not demonstrate statistical significance. Conclusion. The findings of the present study show that the peeled ILM area can affect the anatomic outcomes of MH surgery.
1. Introduction
Internal limiting membrane (ILM) peeling is a crucial part of macular hole (MH) surgery [1], and using ILM peeling to remove and treat epiretinal membrane (ERM) improves anatomical outcomes [2]. Histological examinations show that ERM consists of pieces of the ILM [3]. The importance of the ILM in the pathogenesis of MH was also reported by Yoon et al. [4]. Pars plana vitrectomy (PPV) and ILM peeling are used to treat not only MH, but also ERM, diabetic macular edema, and retinal vein occlusion-related macular edema [5].
Optical coherence tomography (OCT) is the gold standard for diagnosing MH and assessing anatomic outcomes after surgery. OCT also provides prognostic information, such as basal MH diameter, MH height, MH minimum diameter, and other indexes of MH formation [6, 7]. Spectral domain optical coherence tomography (SD-OCT) can also assess structural changes in the macular layers, such as the inner and outer segment (IS/OS) and external limiting membrane [8, 9].
Age, basal MH diameter, MH index (MHI), stage, symptom duration, ILM peeling, and preoperative visual acuity affect the anatomic outcomes of MH surgery [10, 11]. However, no studies assess the relationship between peeled ILM area and anatomic outcomes following MH surgery. This study quantitatively evaluates the effects of peeled ILM area on open and surgically closed MHs.
2. Subjects and Methods
Forty-one eyes in 37 consecutive patients with idiopathic, Gass stage 3-4 MH were enrolled in this retrospective comparative study. The participants were classified as anatomic success or anatomic failure.
All MH cases underwent standard, sutureless, 3-port, 23-gauge vitrectomy surgery between March 2012 and March 2014. All surgeries were performed by the same surgeon (Ahmet Taylan Yazici) at Beyoglu Eye Research and Training Hospital. All patients received a complete ophthalmic examination, including measurement of best corrected visual acuity (BCVA) using an ETDRS chart, biomicroscopy of the anterior segment, and dilated fundus examination; all examinations were performed preoperatively on day 1 and week 1 and 1, 3, and 6 months after surgery. Spectral domain optical coherence tomography (SD-OCT) (SPECTRALIS® Heidelberg Engineering, Heidelberg, Germany) was used preoperatively to assess each patient and postoperatively at 1, 3, and 6 months.
Inclusion criteria were stage 3-4 MH according to the Gass classifications [12]. Exclusion criteria included refractive error > −6.00 D, traumatic MH, and history of ocular surgery (except phacoemulsification). Symptom duration was defined as the number of weeks from diagnosis to surgery. All patients provided informed consent prior to surgery, and this study adheres to the Declaration of Helsinki.
2.1. Surgery
All patients underwent standard, sutureless, 3-port, 23-gauge (G) pars plana vitrectomy (PPV) with triamcinolone acetonide- (TA-) assisted posterior vitreous detachment (PVD) (if not already present). The ILM was grasped using ILM forceps and peeled off the retina using 0.2 mL of brilliant blue G dye (Brilliant Peel; Geuder, Heidelberg, Germany). The area of the removed ILM was intended to reach the vascular arcades of the macula. Fluid-air exchange was performed through an extrusion cannula to flatten the hole, which was followed by the injection of 15% perfluoropropane (C3F8) or 20% sulfur hexafluoride (SF6). Patients were postoperatively maintained in the prone position for 5 days. Anatomic success was defined as complete MH closure and the absence of subretinal fluid on SD-OCT. Anatomic failure was defined as open MH after the first surgery.
2.2. SD-OCT
Every patient's SD-OCT parameters were separately analyzed by 2 observers. The initial set of measurements was recorded by the first observer. A second observer—who was blind to the results of the first observer—performed the same measurements in order to assess interobserver reproducibility. The first observer then scanned the same patient again to measure the same parameters and thereby assess intraobserver reliability. To reduce the likelihood of intraobserver bias, >10 minutes was allowed to elapse before the first observer repeated the measurements. The observers were not present in the OCT room during each other's examinations and were unaware of each other's final measurements.
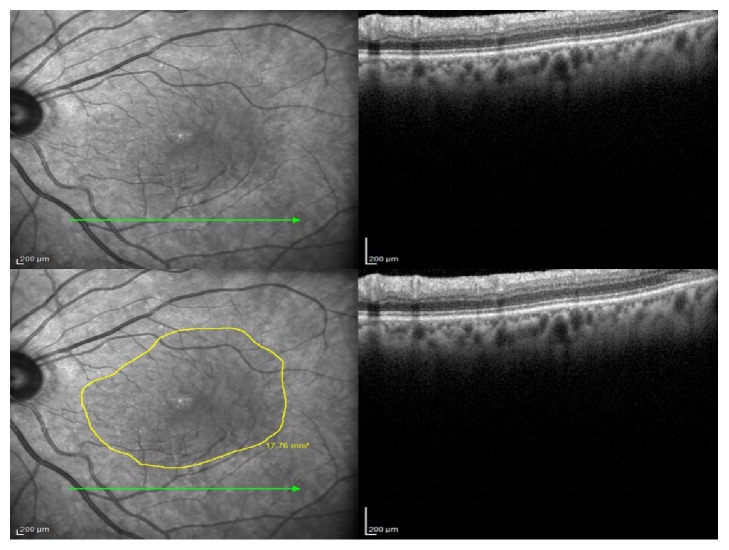
Twenty-five horizontal scans through the fovea were preoperatively and postoperatively performed. Only the lowest section of the retinal macula was scanned to evaluate peeled ILM area. The borders of the peeled and nonpeeled ILM were seen and marked on the OCT scan. The software of the device calculates the area of the peeled ILM in square millimeters (Figure 1). The arithmetic means of by both observers were used in further analyses.
Figure 1.
The borders of the peeled ILM area were marked, and the area was calculated using spectral domain optical coherence tomography.
Basal MH diameter was defined as the diameter at the widest MH cross-section at the retinal pigment epithelium (RPE) [6, 7]. MH height was measured from the RPE to the top of the MH. MHI (hole height/basal hole diameter) was calculated using previously described methods [6]. Anatomic success was defined by complete MH closure and the absence of subretinal fluid on SD-OCT at month 1 postoperatively.
2.3. Statistical Analysis
The statistical analysis was performed using SPSS (Statistical Package for the Social Sciences) (version 16 for Windows; SPSS Inc.). The normality of the data was confirmed using the Kolmogorov-Smirnov test (p > 0.05). One-way ANOVA was used to evaluate homogeneity between groups (p > 0.05). Groups were analyzed using the parametric t-test or nonparametric Mann-Whitney test. Multiple regression analysis was used to determine if there was a significant association between anatomic outcomes and several factors, including Gass stage, basal MH diameter, peeled ILM area, MHI, symptom duration, and preoperative BCVA. BCVA was converted to logMAR (logarithm of the minimal angle of resolution) equivalents for the statistical analysis. In this study, p < 0.05 is considered statistically significant.
3. Results
Thirty-seven patients met our inclusion criteria, and 4 had bilateral MH. The mean follow-up period was 17.4 months (range = 6–30 months). Baseline parameters and patient demographic data are presented in Table 1. Thirty-two cases were included in the anatomic success group, and 9 cases were included in the anatomic failure group. The mean ages of the patients in each group were 67.1 ± 7.3 and 66.3 ± 5.7 years, respectively.
Table 1.
Baseline parameters and demographic data.
| Variable | Anatomical success (Group 1) |
Anatomical failure (Group 2) |
p value |
|---|---|---|---|
| Eyes (N) | 32 | 9 | |
|
| |||
| Gender (N) | |||
| Male | 9 (31.0%) | 2 (25%) | |
| Female | 20 (69.0%) | 6 (75%) | |
|
| |||
| Age, years | 0.762∗ | ||
| Mean ± SD | 67.1 ± 7.3 | 66.3 ± 5.7 | |
| Range | 57–85 | 59–81 | |
|
| |||
| Stage (N) | 0.176∗∗ | ||
| 3 | 11 (34.4%) | 1 (11.1%) | |
| 4 | 21 (65.6%) | 8 (88.9%) | |
∗ t-test.
∗∗Mann-Whitney test.
The clinical characteristics of participants are shown in Table 2. Thirty-four eyes were phakic, and 7 eyes were pseudophakic. Three patients developed significant cataracts during follow-up and underwent phacoemulsification with intraocular lens implantation. No significant difference in lens status was found between groups (p = 0.147). Phacoemulsification with intraocular lens implantation was combined with MH surgery in 2 cases. Therefore, combination surgery did not demonstrate a significant influence (p = 0.332). Perfluoropropane (C3F8) was used in 33 eyes as tamponade, and sulfur hexafluoride (SF6) was used in 8 eyes. There was no significant difference between eyes treated with C3F8 or SF6 in terms of anatomic outcomes (p = 0.616).
Table 2.
Clinical characteristics of participants.
| Variable | Group 1 | Group 2 | p value |
|---|---|---|---|
| Preoperative BCVA, logMAR | 0.936∗ | ||
| Mean ± SD | 0.87 ± 0.36 | 0.80 ± 0.25 | |
| Range | 0.4–1.8 | 0.52–1.3 | |
|
| |||
| Symptom duration, weeks | 0.738∗ | ||
| Mean ± SD | 18.9 ± 12.8 | 17.22 ± 14.65 | |
| Range | 4–64 | 4–40 | |
|
| |||
| Lens status, N | 0.147∗∗ | ||
| Phakic | 28 (87.5%) | 6 (66.7%) | |
| Pseudophakic | 4 (12.5%) | 3 (33.3%) | |
|
| |||
| Tamponade, N | 0.616∗∗ | ||
| C3F8 | 27 (84.4%) | 6 (66.7%) | |
| SF6 | 5 (15.6%) | 3 (33.3%) | |
|
| |||
| Surgery, N | 0.332∗∗ | ||
| PPV | 31 (96.9%) | 8 (88.9%) | |
| Combined PPV + phaco | 1 (3.1%) | 1 (11.1%) | |
|
| |||
| MH basal diameter, μm | 0.032∗∗ | ||
| Mean ± SD | 963.2 ± 325.1 | 1426.0 ± 621.3 | |
| Range | 302–1625 | 760–2627 | |
|
| |||
| MHI | 0.347∗ | ||
| Mean ± SD | 0.53 ± 0.25 | 0.45 ± 0.10 | |
| Range | 0.28–1.55 | 0.30–0.68 | |
|
| |||
| Peeled ILM area, mm2 | 0.024∗ | ||
| Mean ± SD | 16.51 ± 6.15 | 12.8 ± 4.0 | |
| Range | 3.90–30.0 | 6.89–17.67 | |
Bold values are significant at p < 0.05. BCVA, best corrected visual acuity; ILM, internal limiting membrane; MH, macular hole; MHI, macular hole index; PPV, pars plana vitrectomy; phaco, phacoemulsification; μm, micrometer; mm2, millimeter square.
∗ t-test.
∗∗Mann-Whitney test.
Mean preoperative BCVA was 0.85 ± 0.33 logMAR, which postoperatively improved to 0.66 ± 0.37 logMAR (p = 0.001) (0.87 ± 0.36 versus 0.80 ± 0.25 logMAR in patients classified as anatomic success or failure, resp., however, there was no significant difference between groups (p = 0.936)). Symptom duration was 18.9 ± 12.8 versus 17.22 ± 14.65 weeks in patients classified as anatomic success or failure, respectively. Therefore, symptom duration did not demonstrate a significant difference between groups (p = 0.738).
Mean peeled ILM area was 16.51 ± 6.15 mm2 (range = 3.90–30.0 mm2) and 12.8 ± 4.0 mm2 (range = 6.89–17.67 mm2) in patients classified as anatomic success or failure. There was a statistically significant difference between groups in terms of peeled ILM area (p = 0.024). Mean basal MH diameter was 963.2 ± 325.1 μm (range = 302–1625 μm) and 1426.0 ± 621.3 μm (range = 760–2627 μm) in anatomic success and failure patients, respectively. Basal MH diameter was also significantly different between groups (p = 0.032). Furthermore, there was a significant association between anatomic outcomes and 2 factors—basal MH diameter and peeled ILM area (p = 0.001 and 0.009, resp.)—according to the multiple regression analysis (shown in Table 3).
Table 3.
Multiple regression model of variables associated with anatomical outcome.
| Variables | 95% confidence intervals | p value |
|---|---|---|
| MH basal diameter | 0.545–0.940 | 0.001 |
| MHI | 0.246–0.668 | 0.137 |
| Peeled ILM area | 0.111–0.467 | 0.009 |
| Stage | 0.335–0.409 | 0.461 |
| Symptom duration | 0.129–0.601 | 0.559 |
| Preoperative BCVA | 0.358–0.763 | 0.076 |
Bold values are significant at p < 0.01. BCVA, best corrected visual acuity; ILM, internal limiting membrane; MH, macular hole; MHI, macular hole index.
The primary and final anatomic success rates were 78% (32 of 41 cases) and 92.7% (38 of 41 cases), respectively. Overall, 9 cases remained open (anatomic failure) after the first surgery, and second surgery was recommended for 8 cases. One case that had not been recommended for second surgery developed wide RPE atrophy and would not have benefited from surgery. Among the open MHs, 2 patients could not postoperatively maintain the prone position for 5 days and subsequently refused additional surgery.
4. Discussion
Over the past 20 years, ILM peeling has played a crucial role in the surgical treatment of a variety of retinal disorders, including epiretinal membrane, MH, diabetic macular edema, and retinal vein occlusions. The available evidence supports using ILM peeling as the treatment of choice for patients with idiopathic stages 2–4 MH [13]. ILM removal relieves the forces around the fovea, including those that are tangential and axial; however, there is no general consensus regarding the extent of the ILM area that should be peeled [5]. In this retrospective study, we found that larger peeled areas demonstrated better anatomic outcomes.
Many factors affect anatomic outcomes, and age, Gass stage, basal MH diameter, MHI, preoperative BCVA, and symptom duration are some prognostic criteria for MH surgery [10, 11]. All could also affect anatomic outcomes. These parameters—including basal MH diameter, MHI, peeled ILM area, Gass stage, symptom duration, and preoperative BCVA—were assessed by our multiple regression analysis, but only MH basal diameter and peeled ILM area were found to be statistically significant.
Balducci et al. reported early and late changes in retinal nerve fiber layer thickness (RNFLT) after ILM peeling for idiopathic macular hole or epiretinal membrane [14]. RNFLT increased at 1 month after surgery, returned to preoperative levels by 3 months, and was lower than basal at 6 months after surgery. Balducci et al. proposed that reduced RNFLT at 6 months after surgery could indicate damage caused by ILM peeling. In addition, according to a retrospective study that used microperimetry, Tadayoni et al. reported that decreased retinal sensitivity was associated with paracentral absolute and relative microscotomas in 8 of 16 eyes following ILM peeling and MH surgery due to large macular holes (>400 mm) [15]. Some authors have proposed that ILM peeling causes the loss of Müller cell footplates and affects retinal function. Terasaki et al. reported delayed implicit time and reduced b-wave amplitude on focal electroretinography (ERG) soon after ILM peeling [16]. Steven et al. reported that ILM peeling may result in retinal weakening via Müller cell damage, which causes structural breakdowns and finally paracentral retinal hole formation. Steven et al., Mason III et al., and Rubinstein et al. have all separately reported the increased risk of secondary paracentral retinal hole formation after ILM peeling [17–19]. On the contrary, Che et al. evaluated 134 eyes in 130 IMH patients who received PPV in combination with ILM peeling (2 disk diameters). Thirteen eyes underwent a second surgery that involved enlarging the peeled ILM area to the vascular arcades of the posterior fundus. MH closure was successfully achieved in 8 of 13 eyes (61.5%) [20].
The surgeon may perform many manipulations to enlarge the peeled ILM area. The retina nerve fiber layers can hemorrhage and iatrogenic retinal holes may develop, and these hemorrhages may result in visual field defects and other retinal alterations. Accordingly, many surgeons do not widen the peeled area, and a smaller peeled ILM results in less of Müller cells loss, stronger retinal structure, lower risk of visual field defects, and paracentral retinal hole formation. On the other hand, small peeled ILM demonstrates worsened anatomic outcomes.
There is tangential traction in the etiology of macular hole formation that is induced by vitreous shrinkage, as observed and reported by Gaudric et al. [21]. We propose that wider ILM peeling relieves this traction more efficiently, therefore resulting in better anatomic outcomes. Here, the mean area of peeled ILM in anatomically successful patients was 16.51 mm2, whereas patients with anatomic failure demonstrated a mean area of 12.8 mm2. It is difficult to determine a good cut-off value for the peeled area that confirms the best anatomic outcomes. The surgeon should peel the ILM to as much close to the vascular arcades of the macula as possible.
The limitations of the present study include the relatively small numbers of patients, its retrospective design, and the fact that the peeled ILM borders were only assessed using SD-OCT. Therefore, the peeled area could have been inaccurately measured. Using preoperative ILM markings could improve ILM assessment. Also we did not histologically examine the peeled ILM. A strength of this study is the quantitative assessment of the peeled ILM using SD-OCT. In conclusion, we propose that peeled ILM area is important in MH surgery and that it can affect anatomic outcomes.
Acknowledgments
This retrospective study was not supported by any of the companies. These data have not been previously published. This retrospective study was accomplished in Beyoglu Eye Training and Research Hospital.
Competing Interests
None of the other authors have financial or proprietary interests in any mentioned material or method.
References
- 1.Brooks H. L. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology. 2000;107(10):1939–1949. doi: 10.1016/s0161-6420(00)00331-6. [DOI] [PubMed] [Google Scholar]
- 2.Liesenhoff O., Messmer E. M., Pulur A., Kampik A. Treatment of full-thickness idiopathic macular holes. Ophthalmologe. 1996;93(6):655–659. doi: 10.1007/s003470050053. [DOI] [PubMed] [Google Scholar]
- 3.Messmer E. M., Heidenkummer H.-P., Kampik A. Ultrastructure of epiretinal membranes associated with macular holes. Graefe's Archive for Clinical and Experimental Ophthalmology. 1998;236(4):248–254. doi: 10.1007/s004170050072. [DOI] [PubMed] [Google Scholar]
- 4.Yoon H. S., Brooks H. L., Capone A., L'Hernault N. L., Grossniklaus H. E. Ultrastructural features of tissue removed during idiopathic macular hole surgery. American Journal of Ophthalmology. 1996;122(1):67–75. doi: 10.1016/s0002-9394(14)71965-8. [DOI] [PubMed] [Google Scholar]
- 5.Almony A., Nudleman E., Shah G. K., et al. Techniques, rationale, and outcomes of internal limiting membrane peeling. Retina. 2012;32(5):877–891. doi: 10.1097/IAE.0b013e318227ab39. [DOI] [PubMed] [Google Scholar]
- 6.Kusuhara S., Teraoka Escaño M. F., Fujii S., et al. Prediction of postoperative visual outcome based on hole configuration by optical coherence tomography in eyes with idiopathic macular holes. American Journal of Ophthalmology. 2004;138(5):709–716. doi: 10.1016/j.ajo.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 7.Uemoto R., Yamamoto S., Aoki T., Tsukahara I., Yamamoto T., Takeuchi S. Macular configuration determined by Optical coherence tomography after idiopathic macular hole surgery with or without internal limiting membrane peeling. British Journal of Ophthalmology. 2002;86(11):1240–1242. doi: 10.1136/bjo.86.11.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh J., Smiddy W. E., Flynn H. W., Jr., Gregori G., Lujan B. Photoreceptor inner/outer segment defect imaging by spectral domain OCT and visual prognosis after macular hole surgery. Investigative Ophthalmology and Visual Science. 2010;51(3):1651–1658. doi: 10.1167/iovs.09-4420. [DOI] [PubMed] [Google Scholar]
- 9.Ooka E., Mitamura Y., Baba T., Kitahashi M., Oshitari T., Yamamoto S. Foveal microstructure on spectral-domain optical coherence tomographic images and visual function after macular hole surgery. American Journal of Ophthalmology. 2011;152(2):283–290. doi: 10.1016/j.ajo.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Gupta B., Laidlaw D. A. H., Williamson T. H., Shah S. P., Wong R., Wren S. Predicting visual success in macular hole surgery. British Journal of Ophthalmology. 2009;93(11):1488–1491. doi: 10.1136/bjo.2008.153189. [DOI] [PubMed] [Google Scholar]
- 11.Ullrich S., Haritoglou C., Gass C., Schaumberger M., Ulbig M. W., Kampik A. Macular hole size as a prognostic factor in macular hole surgery. British Journal of Ophthalmology. 2002;86(4):390–393. doi: 10.1136/bjo.86.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gass J. D. M. Reappraisal of biomicroscopic classification of stages of development of a macular hole. American Journal of Ophthalmology. 1995;119(6):752–759. doi: 10.1016/S0002-9394(14)72781-3. [DOI] [PubMed] [Google Scholar]
- 13.Spiteri Cornish K., Lois N., Scott N. W., et al. Vitrectomy with internal limiting membrane peeling versus no peeling for idiopathic full-thickness macular hole. Ophthalmology. 2014;121(3):649–655. doi: 10.1016/j.ophtha.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Balducci N., Morara M., Veronese C., Torrazza C., Pichi F., Ciardella A. P. Retinal nerve fiber layer thickness modification after internal limiting membrane peeling. Retina. 2014;34(4):655–663. doi: 10.1097/IAE.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 15.Tadayoni R., Svorenova I., Erginay A., Gaudric A., Massin P. Decreased retinal sensitivity after internal limiting membrane peeling for macular hole surgery. British Journal of Ophthalmology. 2012;96(12):1513–1516. doi: 10.1136/bjophthalmol-2012-302035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terasaki H., Miyake Y., Nomura R., et al. Focal macular ERGs in eyes after removal of macular ILM during macular hole surgery. Investigative Ophthalmology and Visual Science. 2001;42(1):229–234. [PubMed] [Google Scholar]
- 17.Steven P., Laqua H., Wong D., Hoerauf H. Secondary paracentral retinal holes following internal limiting membrane removal. British Journal of Ophthalmology. 2006;90(3):293–295. doi: 10.1136/bjo.2005.078188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason J. O., III, Feist R. M., Albert M. A., Jr. Eccentric macular holes after vitrectomy with peeling of epimacular proliferation. Retina. 2007;27(1):45–48. doi: 10.1097/01.iae.0000256661.56617.69. [DOI] [PubMed] [Google Scholar]
- 19.Rubinstein A., Bates R., Benjamin L., Shaikh A. Iatrogenic eccentric full thickness macular holes following vitrectomy with ILM peeling for idiopathic macular holes. Eye. 2005;19(12):1333–1335. doi: 10.1038/sj.eye.6701771. [DOI] [PubMed] [Google Scholar]
- 20.Che X., He F., Lu L., et al. Evaluation of secondary surgery to enlarge the peeling of the internal limiting membrane following the failed surgery of idiopathic macular holes. Experimental and Therapeutic Medicine. 2014;7(3):742–746. doi: 10.3892/etm.2014.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaudric A., Haouchine B., Massin P., Paques M., Blain P., Erginay A. Macular hole formation: new data provided by optical coherence tomography. Archives of Ophthalmology. 1999;117(6):744–751. doi: 10.1001/archopht.117.6.744. [DOI] [PubMed] [Google Scholar]