Abstract
Haloperidol is a classic antipsychotic drug known for its propensity to cause extrapyramidal symptoms due to blockade of dopamine D2 receptors in the striatum. Interest in medicinal uses of cannabis is growing. Cannabis sativa has been suggested as a possible adjunctive in treatment of Parkinson's disease. The present study aimed to investigate the effect of repeated administration of an extract of Cannabis sativa on catalepsy and brain oxidative stress induced by haloperidol administration in mice. Cannabis extract was given by subcutaneous route at 5, 10 or 20 mg/kg (expressed as Δ9-tetrahydrocannabinol) once daily for 18 days and the effect on haloperidol (1 mg/kg, i.p.)-induced catalepsy was examined at selected time intervals using the bar test. Mice were euthanized 18 days after starting cannabis injection when biochemical assays were carried out. Malondialdehyde (MDA), reduced glutathione (GSH) and nitric oxide (the concentrations of nitrite/nitrate) were determined in brain and liver. In saline-treated mice, no catalepsy was observed at doses of cannabis up to 20 mg/kg. Mice treated with haloperidol at the dose of 1 mg/kg, exhibited significant cataleptic response. Mice treated with cannabis and haloperidol showed significant decrease in catalepsy duration, compared with the haloperidol only treated group. This decrease in catalepsy duration was evident on days 1-12 after starting cannabis injection. Later the effect of cannabis was not apparent. The administration of only cannabis (10 or 20 mg/kg) decreased brain MDA by 17.5 and 21.8 %, respectively. The level of nitric oxide decreased by 18 % after cannabis at 20 mg/kg. Glucose in brain decreased by 20.1 % after 20 mg/kg of cannabis extract. The administration of only haloperidol increased MDA (22.2 %), decreased GSH (25.7 %) and increased brain nitric oxide by 44.1 %. The administration of cannabis (10 or 20 mg/kg) to haloperidol-treated mice resulted in a significant decrease in brain MDA and nitric oxide as well as a significant increase in GSH and glucose compared with the haloperidol-control group. Cannabis had no significant effects on liver MDA, GSH, nitric oxide in saline or haloperidol-treated mice. It is concluded that cannabis improves catalepsy induced by haloperidol though the effect is not maintained on repeated cannabis administration. Cannabis alters the oxidative status of the brain in favor of reducing lipid peroxidation, but reduces brain glucose, which would impair brain energetics.
Keywords: Cannabis sativa extract, haloperidol, catalepsy, mice
Introduction
Cannabis preparations are the most widely used illicit drugs worldwide. They are derived from the female plant of Cannabis sativa Linn (Family Cannabidaceae). Marijuana is prepared from the dried flowering tops and leaves; hashish consists of dried cannabis resin and compressed flowers. The primary psychoactive constituent is Δ9-tetrahydrocannabinol (THC) (Gaoni and Mechoulam, 1964[17]; Ashton, 2001[1]). Other plant cannabinoids include Δ8-THC, cannabinol, cannabidiol and cannabichromene. These and other cannabinoids have additive, synergistic or antagonistic effects with THC and may modify its actions when herbal cannabis is smoked (Russo and McPartland, 2003[54]; DeLong et al., 2010[9]; Long et al., 2010[37]). Cannabinoids exert their effects by acting on specific receptors. Two subtypes of cannabinoid receptors have been identified so far; CB1 and CB2 receptors. The former is expressed strongly in the basal ganglia, cerebellum, olfactory areas, cortex, and hippocampus, which accounts for the well-known effects of cannabis on cognition, memory reward, motor coordination and short-term-memory processing. CB1 is also expressed at high concentrations in the dorsal primary afferent spinal-cord regions, which are important in pain pathways. In contrast, CB2 receptor is expressed mainly in peripheral tissues and is predominantly associated with the immune system (Pertwee and Ross, 2002[47]; Pertwee, 2005[43], 2008[44]).
Interest in medicinal uses of cannabis is growing. THC extracts (i.e. Sativex) or THC derivatives such as nabilone and dronabinol are used in the clinic for the treatment of several pathological conditions like chemotherapy-induced nausea and vomiting, multiple sclerosis and glaucoma (Gerra et al., 2010[18]). Cannabinoids might also prove useful as anticancer agents (Ligresti et al., 2006[36]; Vara et al., 2011[67]) and in neurodegenerative diseases (Iuvone et al., 2009[29]; Basavarajappa et al., 2009[2]). In multiple sclerosis patients, symptomatic improvement, particularly for muscle stiffness and spasms, neuropathic pain and sleep and bladder disturbance has been reported when using oromucosal spray of whole plant extract containing equal proportions of Δ9-tetrahydrocannabinol and cannabidiol (Sativex) (Lakhan and Rowland, 2009[35]; Sastre-Garriga et al., 2011[56]; Zajicek and Apostu, 2011[75]). Some form of benefit has been also described in a significant proportion of Parkinson's disease patients taking cannabis (Venderová et al., 2004[69]). Central cannabinoid receptors are densely located in the output nuclei of the basal ganglia (globus pallidus, substantia nigra pars reticulata), suggesting their involvement in the regulation of motor activity (Müller-Vahl et al., 1999[41]). Cannabinoids may prove useful in Parkinson's disease by inhibiting the excitotoxic neurotransmitter glutamate and counteracting oxidative damage to dopaminergic neurons. The inhibitory effect of cannabinoids on reactive oxygen species, glutamate and tumour necrosis factor also suggested likely neuroprotective properties for cannabis (Croxford, 2003[8]). Cannabis extracts or their psychoactive constituent Δ9-THC, have been reported, however, to cause catalepsy in rodents. The latter is a state of postural immobility (akinesia) with muscular rigidity which can model human Parkinson's disease. Haloperidol is a classic antipsychotic used in the treatment of schizphrenia that is well known for its propensity to cause extrapyramidal symptoms, effects that are mediated by a blockade of striatal D2 receptors (Jibson and Tandon, 1998[30]). Catalepsy caused in rodents by haloperidol is a widely accepted model of the Parkinson's-like side effects caused by typical antipsychotics in humans (Hoffman and Donovan, 1995[27]). Catalepsy occurs when more than 80 % of D2 receptors are occupied by the drug (Wadenberg et al., 2001[71]).
In light of the above data, the present study was designed to (1) investigate the ability of a cannabis extract with known Δ9-THC content to alter catalepsy produced by the D2 receptor antagonist haloperidol in mice; (2) examine the effects of cannabis administration on antioxidant reserve and oxidative stress markers in brain under these experimental conditions. A total extract from Cannabis sativa was used based on the fact that the effect of the whole plant which is abused by humans differs from that of THC in view of its content of other cannabinoids, terpenoids and flavonoids (Russo and McPartland, 2003[54]).
Materials and Methods
Animals
Swiss male albino mice 20-22 g of body weight (age: 5-6 weeks) were used. Mice were obtained from animal house colony of the National Research Centre (Cairo, Egypt). Mice were housed under standardized conditions with free access to standard laboratory food and water. Animal procedures were performed in accordance with the Ethics Committee of the National Research Centre and followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985). Equal groups of 6 mice each were used in all experiments.
Plant material and extraction
Cannabis sativa L. plant was supplied by the Ministry of Justice- Egypt. Extract of Cannabis sativa L. was obtained by chloroform treatment, and contained 10 % of Δ9-THC as determined by (GC mass) gas-chromatography.
Dry extract of Cannabis sativa
Cannabis sativa extract was prepared from the dried flowering tops and leaves of the plant. The method of extraction followed that described by Turner and Mahlberg (1984[64]) with modification. In brief, 10 g of dried cannabis was ground with a mortar and pestle. Decarboxylation of the plant material was achieved by placing the sample in a glass test tube (30 mL) and covering it with aluminum foil. The test tubes were placed in boiling water bath (100 ºC) for 2 h. Ten milliliters of analytical grade chloroform was added and allowed to react for 1 h. The dried cannabis was extracted three times and fractions were combined, filtered over filter paper and collected in a 100 mL volumetric flask. The filtrate was evaporated under a gentle stream of nitrogen (on ice and protected from light and stored at 4 ºC and protected from light in an aluminum-covered container, which provided the dry extract as residue.
Preparation of diluted extract for injection
The residue (dry extract) is suspended in 2 mL of 96 % ethanol and the total volume in the volumetric flask increased to 100 mL by adding distilled water. The extract was injected subcutaneously at doses of 5, 10 and 20 mg/kg (expressed as Δ9-THC). The injection volume was 0.1, 0.2 and 0.3 ml/mouse, respectively. Tetrahydrocannabinol (THC) content was quantified using “GC mass spec”. The dry extract contained 10 % of the Δ9-THC. Cannabinoids are enzymatically biosynthesised in the plant as their corresponding carboxylic acid forms (Taura et al., 2007). Neutral cannabinoids are formed via decarboxylation (loss of CO2) of the acidic cannabinoids during exposure to light, heat (e.g. smoking), or as a result of prolonged storage (Thakur et al., 2005[60]) (Figure 1(Fig. 1)).
Figure 1. The decarboxylation of the acidic cannabinoids: tetrahydrocannabinolic acid (THCA) to delta 9-tetrahydrocannabinol (Δ 9THC).
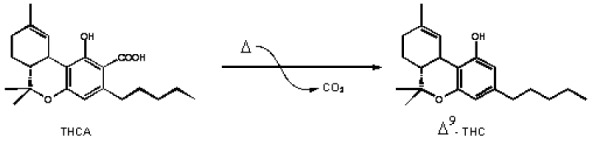
The decarboxylation is carried out by heat in water bath at 100 oC for 2 h. The 1H-NMR proves that there is no signal corresponding to the carboxylic acid (COOH) around 12-13 ppm.
Study design
The following groups were studied:
Saline normal mice
5 mg/kg cannabis-treated mice
10 mg/kg cannabis-treated mice
20 mg/kg cannabis-treated mice
5 mg/kg cannabis-treated mice + haloperidol 1 mg/kg
10 mg/kg cannabis-treated mice + haloperidol 1 mg/kg
20 mg/kg cannabis-treated mice + haloperidol 1 mg/kg
Catalepsy
Cannabis extract was given by subcutaneous route at 5, 10 or 20 mg/kg (expressed as Δ9-THC) (together with haloperidol 1 mg/kg, i.p.) once daily for 18 days. Mice thereafter were tested for the presence of catalepsy at selected time intervals. Catalepsy, defined as a reduced ability to initiate movement and a failure to correct abnormal posture, was measured by means of the bar test. To test for catalepsy, mice were positioned so that their hindquarters were on the bench and their forelimbs rested on a 1 cm diameter horizontal bar, 4 cm above the bench. This procedure was conducted 30 min after drug administration. The length of time the mice maintained this position was recorded by stopwatch to a maximum of 180 s (mean of three consecutive trials; inter-trial interval: 1 min). Mice were judged to be cataleptic if they maintained this position for 30 s or more.
Biochemical analysis
Mice were euthanized 24 days after starting cannabis injection by decapitation under ether anaesthesia, brains and livers were excised, washed with ice-cold saline solution (0.9 % NaCl), weighed and stored at -80 ºC for the biochemical analyses. The liver was homogenized with 0.1 M phosphate buffer saline at pH 7.4, to give a final concentration of 10 % w/v for the biochemical assays.
Determination of lipid peroxidation
Lipid peroxidation was assayed by measuring the level of malondialdehyde (MDA) in the brain and liver tissues. Malondialdehyde was determined by measuring thiobarbituric reactive species using the method of Ruiz-Larrea et al. (1994[52]), in which the thiobarbituric acid reactive substances react with thiobarbituric acid to produce a red colored complex having peak absorbance at 532 nm.
Determination of reduced glutathione
Reduced glutathione (GSH) was determined by Ellman's method (1959[10]). The procedure is based on the reduction of Ellman´s reagent by -SH groups of GSH to form 2-nitro-s-mercaptobenzoic acid, the nitromercaptobenzoic acid anion has an intense yellow color which can be determined spectrophotometrically.
Determination of nitric oxide
Nitric oxide measured as nitrite was determined by using Griess reagent, according to the method of Moshage et al. (1995[40]) where nitrite, stable end product of nitric oxide radical, is mostly used as indicator for the production of nitric oxide
Determination of brain glucose
Brain tissue glucose content was determined according to the method of Trinder (1969[62]). Glucose in the presence of glucose oxidase is converted to peroxide and gluconic acid. The produced hydrogen peroxide reacts with phenol and 4-amino-antipyrine in the presence of peroxidase to yield a colored quinonemine, which is measured spectrophotometrically.
Analysis of data
Drug effects on haloperidol-induced behaviors and biochemical changes were expressed as the mean SEM. Data were analyzed with a one-way ANOVA followed by post hoc comparisons using Duncan's multiple range test.
Results
Catalepsy
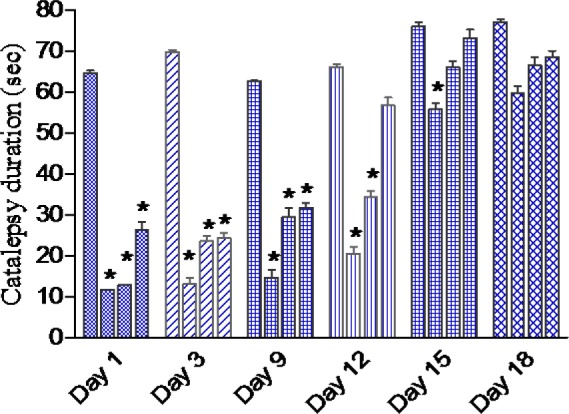
Results are shown in Figure 2(Fig. 2). Cannabis given to saline treated mice failed to induce catalepsy. Haloperidol administered i.p. at the dose of 1 mg/kg produced a significant cataleptic response. Starting on the first day of cannabis administration till the 9th day, the duration of haloperidol-induced catalepsy was significantly reduced by cannabis in a dose-dependent manner. On the 12th day of drug injection, the duration of catalepsy was decreased by cannabis at 5 and 10 mg/kg. On the 15th day of drug injection, the duration of catalepsy was decreased by cannabis at 5 mg/kg.
Figure 2. Effect of daily administration of Cannabis sativa extract on the duration of catalepsy (sec) induced by haloperidol administration over 18 days in mice. Data showed the duration of catalepsy on day 1, 3, 9, 15 and 18 of treatment. Columns 1-4 represent haloperidol control, haloperidol + cannabis 5, 10 or 20 mg/kg, respectively. Asterisks indicate significant change from the haloperidol control group. Cannabis given to saline treated mice failed to induce catalepsy (not shown).
Biochemical Results
Effect of cannabis on brain oxidative stress
The administration of cannabis at 10 or 20 mg/kg to saline treated mice significantly decreased brain MDA by 17.5 and 21.8 %, respectively (21.2 ± 1.0 and 20.1 ± 0.8 vs 25.7 ± 1.3 nmol/g, p<0.05) (Figure 3(Fig. 3)). GSH increased by 18.0 % after cannabis given at 20 mg/kg (3.9 ± 0.16 vs 3.5 ± 0.15 µmol/g, p<0.05) (Figure 4(Fig. 4)), while nitric oxide decreased by 18.3 % (21.0 ± 1.0 vs 25.6 ± 1.5 µmol/g, p<0.05) after cannabis (20 mg/kg) injection compared with the saline control group (Figure 5(Fig. 5)).
Figure 3. Effect of Cannabis sativa extract on brain malondialdehyde (nmol/ g. tissue) in saline- or haloperidol-treated mice. Asterisks indicate significant change from the saline treated group. The plus (+) sign indicates significant change from the haloperidol control group.
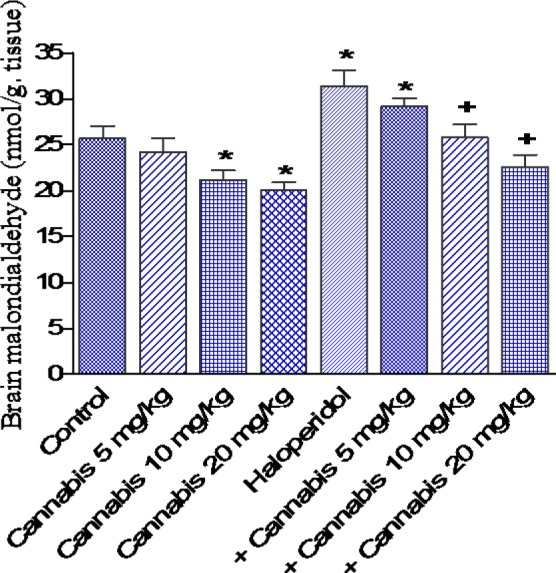
Figure 4. Effect of Cannabis sativa extract on brain reduced glutathione (µmol/ g. tissue) in saline- or haloperidol-treated mice. Asterisks indicate significant change from the saline treated group. The plus (+) sign indicates significant change from the haloperidol control group.
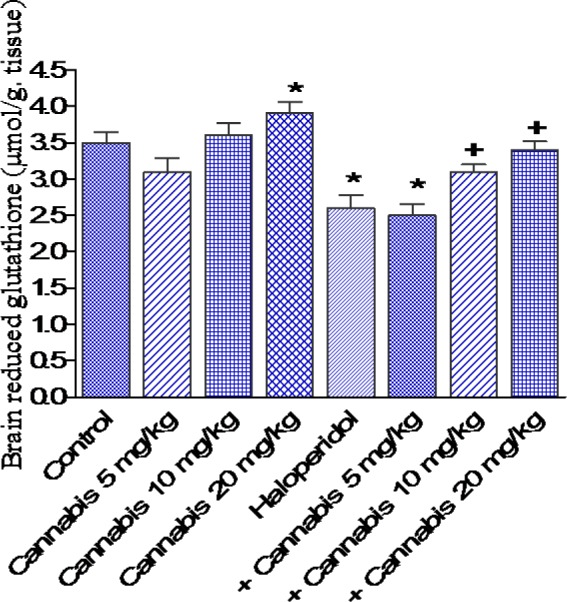
Figure 5. Effect of Cannabis sativa extract on brain nitric oxide (µmol/g tissue) in saline- or haloperidol-treated mice. Asterisks indicate significant change from the saline treated group. The plus (+) sign indicates significant change from the haloperidol control group.
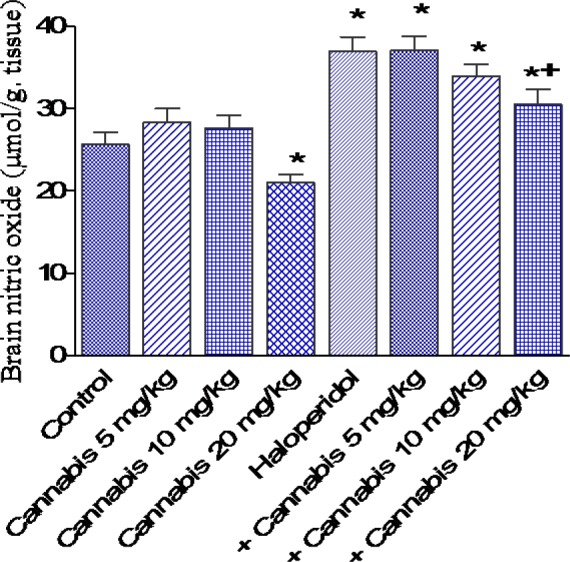
The administration of haloperidol (1 mg/kg, i.p.) significantly increased MDA by 22.2 % compared with the saline control group (31.4 ± 1.7 vs 25.7 ± 1.3 nmol/g, p<0.05) (Figure 3(Fig. 3)). Meanwhile, GSH decreased by 25.7 % (2.6 ± 0.18 vs 3.5 ± 0.15 µmol/g, p<0.05) (Figure 4(Fig. 4)) and nitric oxide increased by 44.1 % (36.9 ± 1.8 vs 25.6 ± 1.5 µmol/g, p<0.05) by haloperidol treatment (Figure 5(Fig. 5)). In haloperidol-treated mice, however, MDA decreased by 17.8 and 28 % after cannabis administration at doses of 10 or 20 mg/kg, respectively (25.8 ± 1.4 and 22.6 ± 1.3 vs 31.4 ± 1.7 nmol/g) (Figure 3(Fig. 3)). In addition, the administration of cannabis at 10 or 20 mg/kg resulted in 19.2 and 30.8 % increase in GSH, respectively (3.1 ± 0.10 and 3 .4 ± 0.12 vs 2.6 ± 0.18 µmol/g, p<0.05) (Figure 4(Fig. 4)). Meanwhile, the level of nitric oxide decreased by 17.3 % (30.5 ± 1.8 vs 36.9 ± 1.8, p<0.05) after the administration of 20 mg/kg of cannabis (Figure 5(Fig. 5)).
Effect of cannabis on brain glucose
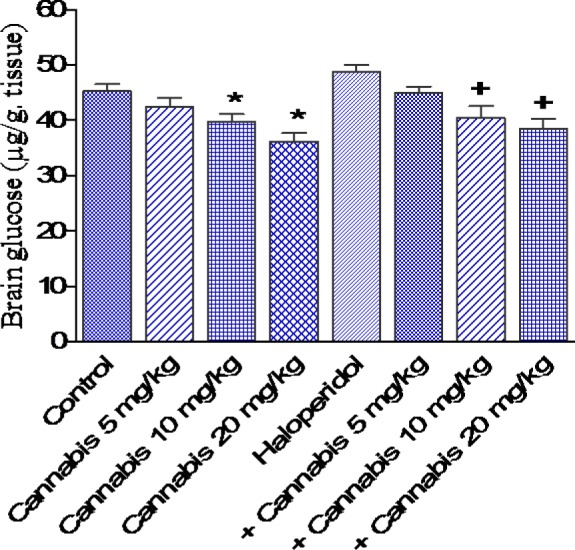
In saline-treated mice, cannabis administration at 10 or 20 mg/kg resulted in significant decrease in brain glucose by 12.4 and 20.1 %, respectively compared with the saline control group (39.7 ± 1.4 and 36.2 ± 1.5 vs 45.3 ± 1.2 g/g, p<0.05). Haloperidol treatment had no significant effect on brain glucose. In haloperidol-treated mice, however, cannabis at 10 or 20 mg/kg, significantly decreased glucose by 17 and 21 %, respectively as compared with the haloperidol only treated group (40.5 ± 2.1 and 38.6 ± 1.8 vs 48.8 ± 1.3 g/g, p<0.05)(Figure 6(Fig. 6)).
Figure 6. Effect of Cannabis sativa extract on brain glucose (µg/g tissue) in saline- or haloperidol-treated mice. Asterisks indicate significant change from the saline treated group. The plus (+) sign indicates significant change from the haloperidol control group.
Effect of cannabis on liver oxidative stress
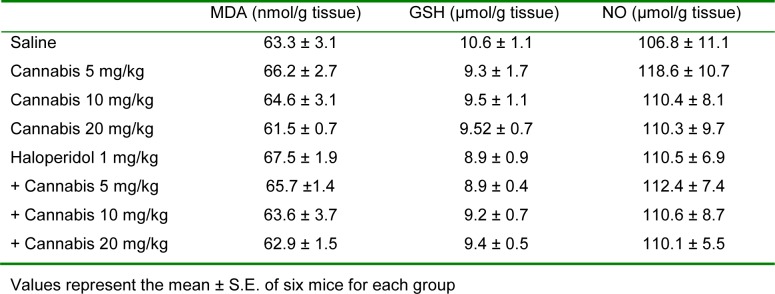
Liver MDA, GSH or nitric oxide was not significantly altered by the administration of only cannabis, haloperidol or cannabis-haloperidol (Table 1(Tab. 1)).
Table 1. Effect of Cannabis sativa extract on liver malondialdehyde (MDA), reduced glutathione (GSH), nitric oxide (NO) and glucose in saline- and haloperidol-treated mice.
Discussion
This study investigated the effect of cannabis extract with known Δ9-THC content on haloperidol-induced catalepsy and on oxidative stress in brain of mice. The present findings indicate that the single and repeated daily administration of the extract at doses corresponding to 5, 10 and 20 mg Δ9-THC/kg by itself failed to induce catalepsy in mice. Haloperidol, an antipsychotic used in humans, is well known to cause extrapyramidal symptoms, due to blockade of dopamine D2 receptors in the striatum. Haloperidol administered subcutaneously at 1 mg/kg induced significant catalepsy. Cannabis initially decreased catalepsy induced by haloperidol. On repeated administration, the effect of cannabis was not apparent. These observations contradict other studies that reported a cataleptogenic response in rodents following the single administration of cannabis extracts or their psychoactive constituent Δ9-THC. In rats, catalepsy has been reported following intravenous (12.5 mg/kg), intraperitoneal or subcutaneous (100 mg/kg) administrations of cannabis extract (Pertwee, 1972[45]) as well as marijuana (200 mg) smoke inhalation (Varvel et al., 2005[68]). Rats or mice administered Δ9-THC intravenously (Kataoka et al., 1987[32]; Kinoshita et al., 1994[33]) or intraperitoneally (5-20 mg/kg) (Pertwee and Greentree, 1998[46]) also exhibited cataleptogenic response. Catalepsy caused by Δ9-THC Catalepsy caused by Δ9-THC or cannabis extracts was attenuated by 6-hydroxydopamine, lesions of locus coeruleus, non-competitive N-methyl-D-aspartate (NMDA) antagonist (Kataoka et al., 1987[32]), flurazepam or chlordiazepoxide pretreatment (Pertwee and Greentree, 1998[46]), atropine (3 mg/kg, sc) (Ferri et al., 1981[12]), serotonergic drugs or antiglutamatergic drugs (Sano et al., 2008[55]). Other researchers have reported that haloperidol, but not clozapine, induced intense rat catalepsy when co-administered with Δ9-tetrahydrocannabinol (Marchese et al., 2003[38]).
The failure of cannabis extract in the present study to cause catalepsy or to enhance that due to haloperidol is likely to be due to the different methods of extraction employed in different studies, leading to the extraction of Δ9-THC together with significant proportion of other cannabinoids such as cannabinol, cannabidiol, cannabichromene, cannabipinol, cannabidivarin and others, being present or other yet unidentified constituents that modulated or antagonized the cataleptogenic effect of Δ9-THC. Other possibility is that large doses of Δ9-THC are required to elicit a cataleptogenic response. Other cannabinoids have additive, synergistic or antagonistic effects with Δ9-THC and may modify its actions when herbal cannabis is smoked (Russo and McPartland, 2003[54]; Hayakawa et al., 2008[25]). In support of this notion studies showing that the action of cannabis is quite different from that of pure Δ9-THC. For example, while both a standardized cannabis extract and Δ9-THC inhibited spasticity in a mouse model of multiple sclerosis to comparable level, the former caused a more rapid onset of muscle relaxation. In the chronic relapsing experimental autoimmune encephalomyelitis mouse model of multiple sclerosis, chronic administration of Δ9-THC-rich extract (though not cannabidiol-rich extract or combined treatment with Δ9-THC- and cannabidiol-rich extracts) resulted in a significant reduction of neurological deficits (Buccellato et al., 2011[5]). Δ9-THC and cannabidiol, a non-psychoactive component of cannabis had clearly distinct effects on neural activation with the former having anxiogenic effects in contrast to cannabidiol-induced anxiolytic effects (Fusar-Poli et al., 2009[16], 2010[15]; Bhattacharyya et al., 2009[3]). Cannabis extract, but not Δ9-THC, revealed a significant reduction of right-hand tapping frequencies that was also found in schizophrenia (Roser et al., 2009[51]). In vitro, standard cannabis extract as well as Δ9-THC-free extract but not Δ9-THC exerted anticonvulsant activity (Wilkinson et al., 2003[73]). Other researchers observed that either Δ9-THC or to lesser extent cannabidiol induced a catatonic response in mice. When both compounds were injected simultaneously (2.5, 5 or 10 mg/kg of each), however, a clear inhibition of the catatonic effect of Δ9-THC was seen (Karniol and Carlini, 1973[31]). Cannabidiol blocked certain effects of Δ9-THC: catatonia in mice, corneal areflexia in rabbits, increased defecation and decreased ambulation in rats in the open field after chronic administration and aggressiveness in rats after REM-sleep deprivation. In contrast, cannabidiol potentiated Δ9-THC analgesia in mice and the impairment of rope climbing in rats (Russo and Guy, 2006[53]). Δ9-THC is subject to first-pass metabolism by the liver. The metabolism of Δ9-THC is by hydroxylation and oxidation catalyzed by the cytochrome P450 enzyme (CYP) system. The major two metabolites of Δ9-THC are 11-hydroxy-Δ9-THC and 11-nor-9-carboxy-Δ9-THC. The former is a compound that is more psychoactive than THC itself (Hazekamp and Grotenhermen, 2010[26]). CYP2C9 and CYP3A4 are the major enzymes involved in the 11-hydroxylation and the 8-(or the 7-) hydroxylation, respectively, of the cannabinoids by human hepatic microsomes (Yamamoto et al., 2003[74]; Watanabe et al., 2007[72]). The main enzymes responsible for the formation of 11-OH-THC are CYP2C and the ratio of oxidized metabolites formed is dependent on population of CYPs present in hepatic microsomes of the experimental animals (Yamamoto et al., 2003[74]). The in vivo liver metabolism of Δ9-THC and cannabidiol differed in the mouse and rat (Harvey et al., 1977[24]). Discrepancies between Δ9-THC effects shown here and data from the literature may be also due to the species used, i.e. mice which differ from rats in their specificity and capacity to metabolize THC.
In the rat, akinetic catalepsy induced by haloperidol can model human Parkinson's disease which occurs when more than 80 % of D2 receptors are occupied by the drug (Wadenberg et al., 2001[71]; Natesan et al., 2006[42]). Catalepsy due to haloperidol can be blocked by D2 dopamine receptor agonists (Moratalla et al., 1996[39]), adenosine receptor antagonists (Trevitt et al., 2009[61]), by alpha2 receptor antagonists (Imaki et al., 2009[28]) or by 5-HT1A agonists (Prinssen et al., 2002[49]). The current observations that cannabis did not enhance motor impairment due to D2 receptor blockade by haloperidol, also suggest that cannabis preparations are unlikely to worsen Parkinsonian symptoms. There is evidence that cannabis might in fact increase dopamine release in human striatum (Voruganti et al., 2001[70]; Bossong et al., 2009[4]) or in rodent brain (Cheer et al., 2004[7]). Δ9-THC, the endocannabinoid anandamide as well as the synthetic cannabinoid receptor agonist WIN 55,212-2 inhibited the enzyme monoamine oxidase (MAO) which catalyzes inactivation of monoamines, including neurotransmitters, such as dopamine, norepinephrine, and serotonin (Fišar, 2010[13]).This in addition to an antioxidant effect (Hampson et al., 1998[22], 2000[23]) might underlie their symptomatic effect in patients with Parkinson's disease (Venderová et al., 2004[69]) as well as their neuroprotective effects reported in vitro as well as in experimental animals (van der Stelt, 2001[65]; Gilbert et al., 2007[20]). Moreover, in the 1-methyl-1,2,3,6-tetrahydropyridine (MPTP)-marmoset model for Parkinson's disease, Δ9-THC improved activity and hand-eye coordination (van Vliet et al., 2008[66]). The effect of cannabis on catalepsy in the present study can be ascribed to a dopamine releasing effect by the extract. It was noted that the lower dose of the extract was more effective in decreasing the duration of catalepsy. This occurs despite no effect on oxidative stress. It is likely that at the higher doses of cannabis, other neurotransmitter systems are affected eg., effect on 5-HT(1A) and or alpha(2)-adrenoceptors (Zanelati et al., 2010[76]; Cascio et al., 2010[6]; Fišar, 2010[13]; Kleijn et al., 2011[34]).
In the striatum, acetylcholine (ACh) is another neurotransmitter that plays an important role in the genesis of Parkinsonian symptoms and anticholinergic drugs are used in early stages and to decrease Parkinson's tremor (Schrag et al., 1999[57]; Tarsy, 2006[58]). Inconsistent results have been reported however, as regards the effect of cannabinoids on acetylcholine release in the striatum. For example, Δ9-THC 5 mg/kg and cannabiodiol 20 mg/kg produced a significant decrease in ACh turnover in the striatum (Revuelta et al., 1978[50]), while Δ9-THC (30 mg/kg) caused a significant elevation of ACh in cortex, hippocampus, striatum, mid brain and medulla-pons (Tripathi et al., 1987[63]). Other researchers, however, reported inhibition by Δ8-THC and Δ9-THC of the synthesis of 3H-ACh in cortex, hypothalamus, and striatum rat brain (Friedman et al., 1976[14]). Synthetic cannabinoids were also found to inhibit ACh release in the rat hippocampus (Gessa et al., 1997[19]).
The effect of cannabis sativa on brain oxidative stress is important in view of the evidence linking increased oxidative stress to a number of neurodegenerative diseases including Parkinson's disease. The present studies suggest that cannabis extract alters the oxidative balance in the brain. Unexpectedly, the administration of cannabis for one month decreased malondialdehyde, a marker of lipid peroxidation (Gutteridge, 1995[21]). Reduced glutathione, an important antioxidant defense system is increased, especially with the highest dose of cannabis examined. Nitric oxide in brain also decreased by cannabis administration. One notable finding, however, was the decreased brain glucose by cannabis administration, thereby impairing cerebral energy metabolism. The antioxidant effects of cannabis in brain must thus be weighed against a decrease in brain energetics (glucose decrease possibly underlying their cognitive impairing effects observed in humans as well as in rodents). Meanwhile and in contrast to the effect of repeated cannabis extract on oxidative stress in brain, the extract did no affect lipid peroxidation or reduced glutathione levels in the liver tissue in saline- or haloperidol-treated mice.
The present findings also indicated increased oxidative stress in the brain by haloperidol administration, which is in accordance with other researchers (Polydoro et al., 2004[48]). There was increased nitric oxide, decreased glutathione and increased lipid peroxidation. These changes were ameliorated by the administration of cannabis extract. These results can be explained by the fact that cannabis extract is not only Δ9-THC, but it contained other cannabinoids, some of them were found to exert neuroprotective effects. The decrease in brain lipid peroxidation by the cannabis extract can be ascribed to the other cannabinoids or flavenoids eg. flavocannabiside or flavosativaside it contains. In rat cortical neuron cultures exposed to toxic levels of glutamate, Δ9-THC and cannabidiol reduced NMDA, AMPA and Kainate receptor-mediated neurotoxicities by cannabinoid receptor-independent mechanism of action (Hampson et al., 2000[23]). Moreover, systemic administration of a cannabinoid modulator that enhances anandamide availability exerted antiparkinsonian effects in 6-hydroxydopamine-lesioned rats through stimulation of 5-HT1B receptor function (Fernandez-Espejo et al., 2004[11]).
In summary, short term cannabis administration was effective in reducing the cataleptic response to haloperidol. On repeated cannabis administration, this effect however was markedly reduced. The biochemical alterations (increased nitric oxide decreased glutathione and increased lipid peroxidation) induced in brain by haloperidol was ameliorated by the administration of cannabis extract.
Acknowledgement
For the supply of Cannabis sativa plant, the authors are indebted to the Ministry of Justice of the Arab Republic of Egypt.
References
- 1.Ashton CH. Pharmacology and effects of cannabis: A brief review. Br J Psychiat. 2001;178:101–6. doi: 10.1192/bjp.178.2.101. [DOI] [PubMed] [Google Scholar]
- 2.Basavarajappa BS, Nixon RA, Arancio O. Endocannabinoid system: emerging role from neurodevelopment to neurodegeneration. Mini Rev Med Chem. 2009;9:448–462. doi: 10.2174/138955709787847921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O'Carroll C, et al. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry. 2009;66:442–451. doi: 10.1001/archgenpsychiatry.2009.17. [DOI] [PubMed] [Google Scholar]
- 4.Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, et al. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34:759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- 5.Buccellato E, Carretta D, Utan A, Cavina C, Speroni E, Grassi G, et al. Acute and chronic cannabinoid extracts administration affects motor function in a CREAE model of multiple sclerosis. J Ethnopharmacol. 2011;133:1033–1038. doi: 10.1016/j.jep.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 6.Cascio MG, Gauson LA, Stevenson LA, Ross RA, Pertwee RG. Evidence that the plant cannabinoid cannabigerol is a highly potent alpha2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br J Pharmacol. 2010;159:129–141. doi: 10.1111/j.1476-5381.2009.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croxford JL. Therapeutic potential of cannabinoids in CNS disease. CNS Drugs. 2003;17:179–202. doi: 10.2165/00023210-200317030-00004. [DOI] [PubMed] [Google Scholar]
- 9.DeLong GT, Wolf CE, Poklis A, Lichtman AH. Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by D(9)-tetrahydrocannabinol. Drug Alcohol Depend. 2010;112:126–133. doi: 10.1016/j.drugalcdep.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Espejo E, Caraballo I, Rodriguez de Fonseca F, Ferrer B, El Banoua F, Flores JA, et al. Experimental parkinsonism alters anandamide precursor synthesis, and functional deficits are improved by AM404: a modulator of endocannabinoid function. Neuropsychopharmacology. 2004;29:1134–1142. doi: 10.1038/sj.npp.1300407. [DOI] [PubMed] [Google Scholar]
- 12.Ferri S, Costa G, Murari G, Panico AM, Rapisarda E, Speroni E, et al. Investigations on behavioral effects of an extract of Cannabis sativa L. in the rat. Psychopharmacology. 1981;75:144–147. doi: 10.1007/BF00432176. [DOI] [PubMed] [Google Scholar]
- 13.Fišar Z. Inhibition of monoamine oxidase activity by cannabinoids. NSArch Pharmacol. 2010;381:563–72. doi: 10.1007/s00210-010-0517-6. [DOI] [PubMed] [Google Scholar]
- 14.Friedman E, Hanin I, Gershon S. Effect of tetrahydrocannabinols on 3H-acetylcholine biosynthesis in various rat brain slices. J Pharmacol Exp Ther. 1976;196:339–345. [PubMed] [Google Scholar]
- 15.Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S, et al. Modulation of effective connectivity during emotional processing by Delta 9-tetrahydrocannabinol and cannabidiol. Int J Neuropsychopharmacol. 2010;13:421–432. doi: 10.1017/S1461145709990617. [DOI] [PubMed] [Google Scholar]
- 16.Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, et al. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66:95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- 17.Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–7. [Google Scholar]
- 18.Gerra G, Zaimovic A, Gerra ML, Ciccocioppo R, Cippitelli A, Serpelloni G, et al. Pharmacology and toxicology of Cannabis derivatives and endocannabinoid agonists. Recent Pat CNS Drug Discov. 2010;5:46–52. doi: 10.2174/157488910789753521. [DOI] [PubMed] [Google Scholar]
- 19.Gessa GL, Mascia MS, Casu MA, Carta G. Inhibition of hippocampal acetylcholine release by cannabinoids: reversal by SR 141716A. Eur J Pharmacol. 1997;327:R1–R2. doi: 10.1016/s0014-2999(97)89683-5. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert GL, Kim HJ, Waataja JJ, Thayer SA. Δ9-Tetrahydrocannabinol protects hippocampal neurons from excitotoxicity. Brain Res. 2007;1128:61–69. doi: 10.1016/j.brainres.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Gutteridge JMC. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–1828. [PubMed] [Google Scholar]
- 22.Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci USA. 1998;95:8268–73. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampson AJ, Grimaldi M, Lolic M, Wink D, Rosenthal R, Axelrod J. Neuroprotective antioxidants from marijuana. Ann N Y Acad Sci. 2000;899:274–282. [PubMed] [Google Scholar]
- 24.Harvey DJ, Martin BR, Paton WDM. In vivo metabolism of cannabinol by the mouse and rat and a comparison with the metabolism of Δ1-tetrahydrocannabinol and cannabidiol. Biol Mass Spectrom. 1977;4:364–70. doi: 10.1002/bms.1200040608. [DOI] [PubMed] [Google Scholar]
- 25.Hayakawa K, Mishima K, Hazekawa M, Sano K, Irie K, Orito K, et al. Cannabidiol potentiates pharmacological effects of Delta(9)-tetrahydrocannabinol via CB(1) receptor-dependent mechanism. Brain Res. 2008;1188:157–164. doi: 10.1016/j.brainres.2007.09.090. [DOI] [PubMed] [Google Scholar]
- 26.Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids. 2005-2009. Cannabinoids. 2010;5(special issue):1–21. [Google Scholar]
- 27.Hoffman DC, Donovan H. Catalepsy as a rodent model for detecting antipsychotic drugs with extrapyramidal side effect liability. Psychopharmacology. 1995;120:128–133. doi: 10.1007/BF02246184. [DOI] [PubMed] [Google Scholar]
- 28.Imaki J, Mae Y, Shimizu S, Ohno Y. Therapeutic potential of alpha2 adrenoceptor antagonism for antipsychotic-induced extrapyramidal motor disorders. Neurosci Lett. 2009;454:143–147. doi: 10.1016/j.neulet.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Iuvone T, Esposito G, De Filippis D, Scuderi C, Steardo L. Cannabidiol: a promising drug for neurodegenerative disorders? CNS Neurosci Ther. 2009;15:65–75. doi: 10.1111/j.1755-5949.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jibson MD, Tandon R. New atypical antipsychotic medications. J Psychiatr Res. 1998;32:215–28. doi: 10.1016/s0022-3956(98)00023-5. [DOI] [PubMed] [Google Scholar]
- 31.Karniol G, Carlini EA. Pharmacological interaction between cannabidiol and delta 9-tetrahydrocannabinol. Psychopharmacologia. 1973;33:53–70. doi: 10.1007/BF00428793. [DOI] [PubMed] [Google Scholar]
- 32.Kataoka Y, Ohta H, Fujiwara M, Oishi R, Ueki S. Noradrenergic involvement in catalepsy induced by Δ9-tetrahydrocannabinol. Neuropharmacology. 1987;26:55–60. doi: 10.1016/0028-3908(87)90044-x. [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita H, Hasegawa T, Katsumata Y, Kameyama T, Yamamoto I, Nabeshima T. Effect of dizocilpine (MK-801) on the catalepsy induced by Δ9-tetrahydrocannabinol in mice. J Neural Transm [Gen Sect] 1994;95:137–143. doi: 10.1007/BF01276432. [DOI] [PubMed] [Google Scholar]
- 34.Kleijn J, Cremers TI, Hofland CM, Westerink BH. CB-1 receptors modulate the effect of the selective serotonin reuptake inhibitor, citalopram on extracellular serotonin levels in the rat prefrontal cortex. Neurosci Res. 2011;70:334–337. doi: 10.1016/j.neures.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Lakhan SE, Rowland M. Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: a systematic review. BMC Neurology. 2009;9:59. doi: 10.1186/1471-2377-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318:1375–1387. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- 37.Long LE, Chesworth R, Huang XF, McGregor IS, Arnold JC, Karl T. A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int J Neuropsychopharmacol. 2010;13:861–876. doi: 10.1017/S1461145709990605. [DOI] [PubMed] [Google Scholar]
- 38.Marchese G, Casti P, Ruiu S, Saba PL, Sanna A, Casu GL, et al. Haloperidol, but not clozapine, produces dramatic catalepsy in D9-THC-treated rats: possible clinical implications. Br J Pharmacol. 2003;140:520–6. doi: 10.1038/sj.bjp.0705478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moratalla R, Xu M, Tonegawa S, Graybiel AM. Cellular responses to psychomotor stimulant and neuroleptic drugs are abnormal in mice lacking the D1 dopamine receptor. Proc Natl Acad Sci USA. 1996;93:14928–14933. doi: 10.1073/pnas.93.25.14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moshage H, Kok B, Huizenga JR. Nitrite and nitrate determination in plasma: A critical evaluation. Clin Chem. 1995;41:892–896. [PubMed] [Google Scholar]
- 41.Müller-Vahl KR, Kolbe H, Schneider U, Emrich HM. Cannabis in movement disorders. Forsch Komplementarmed. 1999;6(Suppl 3):23–27. doi: 10.1159/000057153. [DOI] [PubMed] [Google Scholar]
- 42.Natesan S, Reckless GE, Nobrega JN, et al. Dissociation between in vivo occupancy and functional antagonism of dopamine D2 receptors: comparing aripiprazole to other antipsychotics in animal models. Neuropsychopharmacology. 2006;31:1854–1863. doi: 10.1038/sj.npp.1300983. [DOI] [PubMed] [Google Scholar]
- 43.Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005;168:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- 44.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pertwee RG. The ring test: a quantitative method for assessing the 'cataleptic' effect of cannabis in mice. Br J Pharmac. 1972;46:753–763. doi: 10.1111/j.1476-5381.1972.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pertwee RG, Greentree SG. Delta-9-tetrahydrocannabinol-induced catalepsy in mice is enhanced by pretreatment with flurazepam or chlordiazepoxide. Neuropharmacology. 1998;27:485–491. doi: 10.1016/0028-3908(88)90130-x. [DOI] [PubMed] [Google Scholar]
- 47.Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids. 2002;66:101–21. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- 48.Polydoro M, Schröder N, Lima MN, Caldana F, Laranja DC, Bromberg E, et al. Haloperidol- and clozapine-induced oxidative stress in the rat brain. Pharmacol Biochem Behav. 2004;78:751–756. doi: 10.1016/j.pbb.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 49.Prinssen EP, Colpaert FC, Koek W. 5-HT1A receptor activation and anti-cataleptic effects: high-efficacy agonists maximally inhibit haloperidol-induced catalepsy. Eur J Pharmacol. 2002;453:217–221. doi: 10.1016/s0014-2999(02)02430-5. [DOI] [PubMed] [Google Scholar]
- 50.Revuelta AV, Moroni F, Cheney DL, Costa E. Effect of cannabinoids on the turnover rate of acetylcholine in rat hippocampus, striatum and cortex. NS Arch Pharmacol. 1978;304:107–110. doi: 10.1007/BF00495546. [DOI] [PubMed] [Google Scholar]
- 51.Roser P, Gallinat J, Weinberg G, Juckel G, Gorynia I, Stadelmann AM. Psychomotor performance in relation to acute oral administration of Delta9-tetrahydrocannabinol and standardized cannabis extract in healthy human subjects. Eur Arch Psychiatry Clin Neurosci. 2009;259:284–292. doi: 10.1007/s00406-009-0868-5. [DOI] [PubMed] [Google Scholar]
- 52.Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, de Groot H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids. 1994;59:383–388. doi: 10.1016/0039-128x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 53.Russo E, Guy GW. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Medical Hypotheses. 2006;66:234–46. doi: 10.1016/j.mehy.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 54.Russo EB, McPartland JM. Cannabis is more than simply D9-tetrahydrocannabinol. Psychopharmacology. 2003;165:431–432. doi: 10.1007/s00213-002-1348-z. [DOI] [PubMed] [Google Scholar]
- 55.Sano K, Mishima K, Koushi E, Orito K, Egashira N, Irie K, et al. Delta 9-tetrahydrocannabinol-induced catalepsy-like immobilization is mediated by decreased 5-HT neurotransmission in the nucleus accumbens due to the action of glutamate-containing neurons. Neuroscience. 2008;151:320–8. doi: 10.1016/j.neuroscience.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 56.Sastre-Garriga J, Vila C, Clissold S, Montalban X. THC and CBD oromucosal spray (Sativex A®) in the management of spasticity associated with multiple sclerosis. Expert Rev Neurother. 2011;11:627–637. doi: 10.1586/ern.11.47. [DOI] [PubMed] [Google Scholar]
- 57.Schrag A, Schelosky L, Scholz U, Poewe W. Reduction of Parkinsonian signs in patients with Parkinson's disease by dopaminergic versus anticholinergic single-dose challenges. Mov Disord. 1999;14:252–255. doi: 10.1002/1531-8257(199903)14:2<252::aid-mds1009>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 58.Tarsy D. Initial treatment of Parkinson’s disease. Curr Treat Options Neurol. 2006;8:224–235. doi: 10.1007/s11940-006-0013-y. [DOI] [PubMed] [Google Scholar]
- 59.Taura F, Sirikantaramas S, Shoyama Y, Shoyama Y, Morimoto S. Phytocannabinoids in Cannabis sativa: recent studies on biosynthetic enzymes. Chem Biodiv. 2007;4:1649–1663. doi: 10.1002/cbdv.200790145. [DOI] [PubMed] [Google Scholar]
- 60.Thakur GA, Duclos RI, Makriyannis A. Natural cannabinoids: templates for drug discovery. Life Sci. 2005;78:454–66. doi: 10.1016/j.lfs.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 61.Trevitt J, Vallance C, Harris A, Goode T. Adenosine antagonists reverse the cataleptic effects of haloperidol: implications for the treatment of Parkinson's disease. Pharmacol Biochem Behav. 2009;92:521–527. doi: 10.1016/j.pbb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24–25. [Google Scholar]
- 63.Tripathi HL, Vocci FJ, Brase DA, Dewey WL. Effects of cannabinoids on levels of acetylcholine and choline and on turnover rate of acetylcholine in various regions of the mouse brain. Alcohol Drug Res. 1987;7:525–532. [PubMed] [Google Scholar]
- 64.Turner JC, Mahlberg PG. Separation of acid and neutral cannabinoids in Cannabis sativa L. using HPLC. In: Agurell S, Dewey WL, Willete RE, editors. The cannabinoids: chemical, pharmacologic and therapeutic aspects. Orlando, Fla: Academic Press Inc; 1984. pp. 79–88. [Google Scholar]
- 65.van der Stelt M, Veldhuis WB, Bär PR, Veldink GA, Vliegenthart JF, Nicolay K. Neuroprotection by Delta 9-tetrahydrocannabinol, the main active compound in marijuana, against ouabain-induced in vivo excitotoxicity. J Neurosci. 2001;21:6475–6479. doi: 10.1523/JNEUROSCI.21-17-06475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Vliet SA, Vanwersch RA, Jongsma MJ, Olivier B, Philippens IH. Therapeutic effects of Delta 9-THC and modafinil in a marmoset Parkinson model. Eur Neuropsychopharmacol. 2008;18:383–389. doi: 10.1016/j.euroneuro.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 67.Vara D, Salazar M, Olea-Herrero N, Guzmán M, Velasco G, Díaz-Laviada I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011;18:1099–1111. doi: 10.1038/cdd.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varvel SA, Bridgen DT, Tao Q, Thomas BF, Martin BR, Lichtman AH. Δ9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J Pharmacol Exp Ther. 2005;314:329–37. doi: 10.1124/jpet.104.080739. [DOI] [PubMed] [Google Scholar]
- 69.Venderová K, Růzicka E, Vorísek V, Visnovský P. Survey on cannabis use in Parkinson's disease: subjective improvement of motor symptoms. Mov Disord. 2004;19:1102–1106. doi: 10.1002/mds.20111. [DOI] [PubMed] [Google Scholar]
- 70.Voruganti LN, Slomka P, Zabel P, Mattar A, Awad AG. Cannabis induced dopamine release: an in-vivo SPECT study. Psychiatry Res. 2001;107:173–177. doi: 10.1016/s0925-4927(01)00104-4. [DOI] [PubMed] [Google Scholar]
- 71.Wadenberg ML, Soliman A, VanderSpek SC, Kapur S. Dopamine D2 receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effects. Neuropsychopharmacology. 2001;25:633–641. doi: 10.1016/S0893-133X(01)00261-5. [DOI] [PubMed] [Google Scholar]
- 72.Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci. 2007;80:1415–9. doi: 10.1016/j.lfs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 73.Wilkinson JD, Whalley BJ, Baker D, Pryce G, Constanti A, Gibbons S, et al. Medicinal cannabis: is delta9-tetrahydrocannabinol necessary for all its effects? J Pharm Pharmacol. 2003;55:1687–94. doi: 10.1211/0022357022304. [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto I, Watanabe K, Matsunaga T, Kimura T, Funahashi T, Yoshimura H. Pharmacology and toxicology of major constituents of marijuana-On the metabolic activation of cannabinoids and its mechanism. J Toxicol. 2003;22:577–89. [Google Scholar]
- 75.Zajicek JP, Apostu VI. Role of cannabinoids in multiple sclerosis. CNS Drugs. 2011;25:187–201. doi: 10.2165/11539000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 76.Zanelati TV, Biojone C, Moreira FA, Guimarães FS, Joca SRL. Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br J Pharmacol. 2010;159:122–128. doi: 10.1111/j.1476-5381.2009.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]