Abstract
A number of essential oils derived from plants are claimed to have several medicinal functions, including anti-cancer and anti-inflammation effects. However, the chemical composition and biological activities of flower-derived components have not been sufficiently characterized. Therefore, we investigated the composition of essential oils from Hallabong flower [(Citrus unshiu Marcov × Citrus sinensis Osbeck) × Citrus reticulata Blanco] and their anti-inflammatory effects. Hydro-distilled essential oils (HEOs) were analyzed using gas chromatography-mass spectrometry (GC-MS). In total, 21 components were identified, representing more than 98 % of the oils, with sabinene (34.75 %), linalool (14.77 %), β-ocimene (11.07 %), 4-terpineol (9.63 %), l-limonene (5.88 %), and γ-terpinene (4.67 %) as the main components. In the present study, we also investigated the anti-inflammatory effects of HEOs on lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage cells. HEOs were found to inhibit nitric oxide (NO) and prostaglandin E2 (PGE2) production and to suppress the LPS-induced expression of cyclooxygenase-2 (COX-2) protein. In addition, HEOs downregulated the production of the inflammatory cytokines, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β (IC50 values are 0.05 %, 0.02 %, and 0.01 %, respectively). On the basis of these results, we suggest that HEOs can be considered potential anti-inflammatory candidates for therapeutic use in humans.
Keywords: chemical composition, citrus flower, essential oil, inflammation, Hallabong
Introduction
Essential oils are volatile, natural, complex mixtures comprising many unique compounds, primarily monoterpenes, sesquiterpenes, and their corresponding oxygenated derivatives. These compounds are secondary metabolites of aromatic plants and are characterized by strong scents and flavors (Bajpai et al., 2012[4], 2013[5]). Each of these compounds contributes to the beneficial or adverse effects of essential oils. Therefore, an understanding of the composition of an essential oil enables improved and focused applications (Dorman and Deans, 2000[11]; Haddouchi et al., 2013[16]). Essential oils extracted from several plant organs, including buds, flowers, leaves, stems, twigs, seeds, fruits, roots, wood, or bark, are valuable natural products and are used as raw materials for many products, including food flavorings, feed additives, flavoring agents used in the cigarette industry, and in the compounding of cosmetics and perfumes. Furthermore, essential oils have become an integral part of everyday life. They are used in air fresheners and deodorizers as well as in all branches of medicines such as pharmacy, balneology, massage, and homeopathy. Additionally, essential oils are good natural sources of substances with commercial potential, and are used as starting materials for chemical syntheses (Baser and Buchbauer, 2010[6]; Teixeira et al., 2013[22]). This importance has recently attracted the attention of many scientists and encouraged them to screen plants to study the biological activities of their oils and their application to chemical and pharmacological investigations, as well as therapeutic applications. Indeed, some essential oils appear to exhibit unique medicinal properties that have been claimed to cure some organ dysfunctions or systemic disorders (Gomes et al., 2013[13]). Furthermore, the use of essential oils is becoming popular as therapeutic treatments for diseases, such as non-steroidal anti-inflammatory drugs (NSAIDs) and natural preservatives, since consumers are more conscious about the health problems caused by several synthetic agents (Gómez-Estaca et al., 2010[14]; Teixeira et al., 2013[22]). Synthetic agents that are added to food and cosmetic items are considered to be devoid of potential adverse effects, and are classified as safe for human use. However, there have been problems concerning the safety of some chemicals, including the possibility of allergic reactions in humans and carcinogenicity observed in rodents after administration of steroids (Teixeira et al., 2013[22]). Therefore, natural substances isolated from plants (for example, essential oils) are considered to be promising sources of compounds with health benefits in humans (Djabou et al., 2013[10]). It is anticipated that characterization of these compounds will reveal new information about the applications of plant-based compounds and a new perspective on the potential use of these natural products (Tang et al., 2013[21]). For this reason, many recent studies have explored the chemical composition and biological activities of essential oils. In particular, essential oils derived from Citrus species are of medicinal and commercial importance, and are used in preservatives.
Citrus is a genus of flowering plants in the Rutaceae family, and its fruits are important owing to their nutritional and industrial applications. The genus consists of about 1,000 species, all of which produce a characteristic and distinct flavor used in foods, perfumery, and cosmetics. According to the Dongui Bogam, a book of traditional Korean medicine and one of the classics of oriental medicine today whose name literally means “Mirror of Eastern Medicine,” citrus fruits, in particular, have been used for the treatment of gastroenteric maladies, asthma, and loss of appetite. The most abundant volatile compound in the peel of citrus fruits, including those of the species Citrus unshiu, C. sunki, C. obovoidea, C. natsudaidai, and Fortunella japonica var. margarita, is limonene, a monoterpene hydrocarbon (Baik et al., 2008[3]; Kim et al., 2008[20]; Yang et al., 2010[23]). However, little information is available on the volatile composition and biological potential of the flowers of citrus species. Hallabong [(C. unshiu Marcov × C. sinensis Osbeck) × C. reticulata Blanco] is a new hybrid citrus crop on Jeju Island and has been regarded as a citrus fruit with potential commercial value because it is attractive and has a good taste and a sweet aroma (Choi, 2003[8]).
This variety was developed in Japan in 1972 and then introduced on Jeju Island, Republic of Korea. The variety was named 'Hallabong' after Hallasan Mountain located on Jeju Island, its primary region of growth since 1998 (Han et al., 2010[17]).
Previous studies reported that essential oils of Citrus species have many pharmacologic activities including anti-inflammatory, antioxidant, anti-obesity, and anti-allergic properties (Asnaashari et al., 2010[2]; Han et al., 2010[17]; Yang et al., 2010[23]; Ellouze et al., 2012[12]; Graziano et al., 2012[15]). However, the chemical composition and biological activities of these essential oils, such as anti-inflammatory effects of the essential oils derived from the flower parts, have not been described. Therefore, in this study, the essential oils of C. hallabong flower (HEOs), native to Jeju Island, Korea, were investigated for their chemical composition and anti-inflammatory activity. To the best of our knowledge, we are the first to report that HEOs show anti-inflammatory activity.
Materials and Methods
Isolation of essential oil
Hallabong flowers were collected from Hallim (a region in the western part of Jeju Island, Korea) in August 2011. Essential oils were extracted from the flowers according to the method described by Yoon et al. (2010[24]), with minor modifications. Briefly, the dried Hallabong flowers (1.2 kg) were ground and hydro-distilled for 6 h by using a modified Clevenger-type apparatus. The essential oil obtained on the top of the aqueous distillate was separated and dried over anhydrous sodium sulfate. The oil obtained was stored in a hermetically sealed glass ampoule at 4 °C until further analysis. The essential oil yield (0.034 %) was calculated on the basis of the volume of oil collected and the weight of fresh Hallabong flowers.
Gas chromatography-mass spectrometry analysis
Gas chromatography-mass spectrometry (GC-MS) analysis of the volatile components of HEOs were carried out using an Agilent 7890A gas chromatograph (Agilent Technologies, Palo Alto, CA) with DB-17 (30 m × 0.25 mm × 0.25 μm film thickness) equipped with a 5975C inert XL MSD (Agilent Technologies, Palo Alto, CA). Electron-impact ionization of mass spectrometry was performed at 70 eV. The HEOs were dissolved in n-hexane and injected into the column. The temperature was first set at 40 °C (5 min) and increased to 230 °C (5 min) at a rate of 5 °C/min. The carrier gas was helium, and it was used at a flow rate of 1.5 mL/min. The HEO compounds were identified by comparing their retention times and mass spectra with those of standards, the Wiley 2001 library data of the GC-MS system, and previously published data (Adams, 2001[1]). Alkanes (C8-C22) were used as reference points in the calculation of relative retention indices.
Cell culture
The murine RAW 264.7 macrophage cell line was obtained from the Korean Cell Line Bank (Seoul, Korea) and cultured in Dulbecco's modified Eagle's medium (DMEM; GIBCO Inc, NY, USA) supplemented with 10 % fetal bovine serum (FBS; GIBCO Inc, NY, USA), penicillin (100 units/mL), and streptomycin (100 μg/mL) at 37 °C under 5 % CO2 in a humidified incubator. The cells were sub-cultured every 2 to 3 d at 1:5 split ratios.
Lactate dehydrogenase assay for cell viability
Cell viability was determined by the lactate dehydrogenase (LDH) activity, by measuring the production of NADH during the conversion of lactate to pyruvate. LDH activity was determined using an LDH cytotoxicity detection kit (Promega, Madison, WI, USA). Briefly, the murine RAW 264.7 macrophage cells were cultured in 96-well plates for 18 h, followed by treatment with 1 μg/mL of LPS in the presence of various concentrations of HEOs. The reaction mixture of the cytotoxicity detection kit was mixed and incubated with an equal volume of a cell-free culture medium at room temperature for 30 min in the dark. Then, 1/2 volume of 1N HCl was added to each sample to stop the enzymatic reaction. The optical density of the reaction mixture was then measured by using an enzyme-linked immunosorbent assay (ELISA) and a plate reader (PowerWave X340, Bio-tech Instruments, Inc., Vermont, USA) at 490 nm. The percentage of cytotoxicity was determined relative to that in the control group.
Quantification of nitrite production using the Griess assay
Nitric oxide (NO) production was measured based on nitrite accumulation in the culture medium, by a colorimetric reaction with the Griess reagent. Briefly, murine RAW 264.7 macrophage cells (5 × 105 cells/mL) were pre-incubated for 18 h and then treated with 1 μg/mL of LPS plus varying concentrations of HEOs for 24 h. Following incubation, 100 μL of culture supernatants were mixed with an equal volume of Griess reagent (1 % sulfanilamide and 0.1 % N-[1-naphthyl]-ethylenediamine dihydrochloride in 5 % phosphoric acid) and incubated for 10 min in the dark. Nitrite concentration was determined using ELISA by measuring the absorbance at 540 nm with a plate reader (PowerWave X340, Bio-tech Instruments). NaNO2 was used to generate a standard curve. All measurements were performed in triplicate and expressed as micromolar concentrations of NO.
Measurement of prostaglandin E2 and cytokines
The amount of prostaglandin E2 (PGE2), tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 produced by mouse macrophages was measured in RAW 264.7 cell culture supernatant. RAW 264.7 cells were plated at a density of 1.0 × 106 cells/well in a 48-well cell culture plate with 500 μL of culture medium and incubated for 18 h. The cells were then treated with a specified concentration of HEOs plus 1 μg/mL of LPS and incubated for an additional 24 h. The production of PGE2, TNF-α, IL-1β, and IL-6 was measured using ELISA and a plate reader (PowerWave X340, Bio-tech Instruments) according to the manufacturer's instructions.
Western blot analysis
Whole cell lysates were extracted using PRO-PREP Protein Extraction Solution (iNtRON Biotechnology, Seongnam-Si, Korea) according to the manufacturer's instructions. Thirty micrograms of protein from each sample was separated using 10 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were electro-transferred onto polyvinylidene fluoride (PVDF) membrane (BIO-RAD, HC, USA). Next, the membrane was incubated for 24 h with TTBS (25 mM Tris-HCl, 150 mM NaCl, and 0.2 % Tween-20) containing 5 % non-fat dry milk, and then probed with COX-2 antibody at room temperature for 1 h. The membrane was washed 3 times with TTBS and incubated for 30 min with a secondary horseradish peroxidase (HRP)-conjugated antibody at room temperature. Finally, immunoreactive bands were detected by incubating the samples with HRP-conjugated secondary antibodies and visualized using WEST-ZOL plus Western Blot Detection System (iNtRON Biotechnology).
Statistical analysis
All data are expressed as the means ± S.D. Significant differences between the groups were determined using the Student's t-test. P-values of less than 0.05 were considered statistically significant.
Results and Discussion
Currently, there are several concerns about the impact of synthetic compounds used as therapeutic agents on the environment and human health. For this reason, eco-friendly essential oils that are highly efficacious may provide potential alternatives to the synthetic agents used, because these essential oils are rich in bioactive chemicals and are commonly used as fragrances and flavoring agents in foods and beverages. Essential oils generally possess two main advantages: they do not pose long-term genotoxic risks, and they prevent the development of drug resistance. Moreover, some essential oils possess potent anti-inflammatory and anti-mutagenic activities that are possibly related to their biological activity. Thus, in the future, these essential oils and their constituents can be considered in clinical practice, for clinical evaluations, and as adjuvant therapies (Hyun et al., 2010[18]). In our on-going screening program designed to identify the biological potential of essential oils, we investigated the composition of HEOs and their anti-inflammatory effects. The ultimate aim of this study was to determine whether HEOs inhibit NO production in LPS-stimulated macrophages.
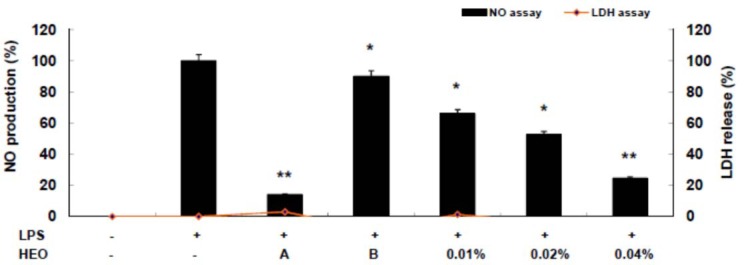
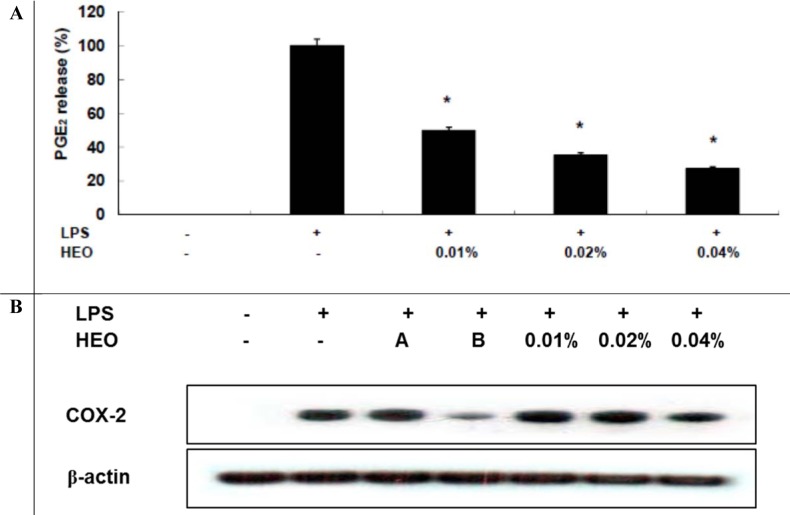
The HEO yield was 0.034 %. Table 1(Tab. 1) shows the general chemical profile, percent content, and retention indices of the constituents of HEOs. In total, 21 compounds constituting 98.41 % of HEOs were identified on the basis of their mass spectra, which were compared to those in the computer library. Only the volatile constituents that matched more than 90 % of the oils were characterized. The main constituents of HEOs were sabinene (34.75 %), linalool (14.77 %), β-ocimene (11.07 %), 4-terpineol (9.63 %), l-limonene (5.88 %), and γ-terpinene (4.67 %). Inflammation, characterized by classical symptoms, including pain, heat, redness, swelling, and loss of function, is a critical aspect of a host's response to infection and injury. It is essential for a host's self-defense against harmful stimuli, such as pathogens, damaged cells, or irritants. However, if uncontrolled, the inflammatory response can prove harmful, contributing to the pathogenesis of many inflammatory disorders, including arthritis, asthma, multiple sclerosis, inflammatory bowel disease, and atherosclerosis (Kim et al., 2010[19]; Cha et al., 2013[7]). Inflammatory responses are also involved in the production of a variety of pro-inflammatory mediators and cytokines including NO, PGE2, TNF-α, IL-1β, and IL-6, which are regarded as crucial anti-inflammatory targets (Choi et al., 2010[9]; Cha et al., 2013[7]). Therefore, in the present study, we investigated HEO-mediated inhibition of LPS-induced production of inflammatory mediators and cytokines in LPS-activated RAW 264.7 macrophages. The effects of HEO concentration (0.01 to 0.04 %) on cell viability were assessed using an LDH assay, where no significant change in cell viability was observed in response to these concentrations (Figure 1(Fig. 1)). Owing to the fact that NO is a major inflammatory mediator, we first examined the effects of HEOs on NO production in LPS-activated RAW 264.7 macrophages. To quantify NO production, RAW 264.7 cells were pre-incubated with HEO for 18 h, after which they were stimulated with 1 µg/mL LPS for 24 h. Nitrite levels in the culture media were determined. At concentrations of 0.01 %, 0.02 %, and 0.04 %, HEOs decreased the production of NO in LPS-treated macrophages by 24.54 %, 52.55 %, and 66 %, respectively, compared to the NO levels in LPS-treated macrophages that were not exposed to HEOs. Dexamethasone (20 μM), a NO inhibitor and positive control, also markedly reduced NO levels in RAW 264.7 macrophages. In contrast, NS-398 (20 µM) did not affect NO production. We next examined the effects of HEOs on PGE2 production and COX-2 protein expression following LPS stimulation of RAW 264.7 cells. When macrophages were stimulated with LPS (1 μg/ mL) for 24 h, PGE2 levels increased in the culture medium. As shown in Figure 2(Fig. 2), HEOs suppressed LPS-induced PGE2 production in a dose-dependent manner, with IC50 values below 0.01 %. As shown in Figure 2B(Fig. 2), we did not detect COX-2 proteins in unstimulated RAW 264.7 cells. On the contrary, a significant increase in the level of COX-2 proteins was observed in response to LPS stimulation. In addition, HEO treatment resulted in dose-dependent inhibition of LPS-stimulated COX-2 protein production. On the other hand, HEOs did not affect the expression of β-actin, a housekeeping gene. These results were consistent with the inhibitory effects of HEOs on PGE2 production in LPS-stimulated RAW 264.7 cells. In general, these results suggest that the inhibitory effects of HEOs on LPS-stimulated PGE2 production are attributable to the suppression of COX-2 protein expression.
Table 1. Chemical composition of the essential oils from Hallabong flower.
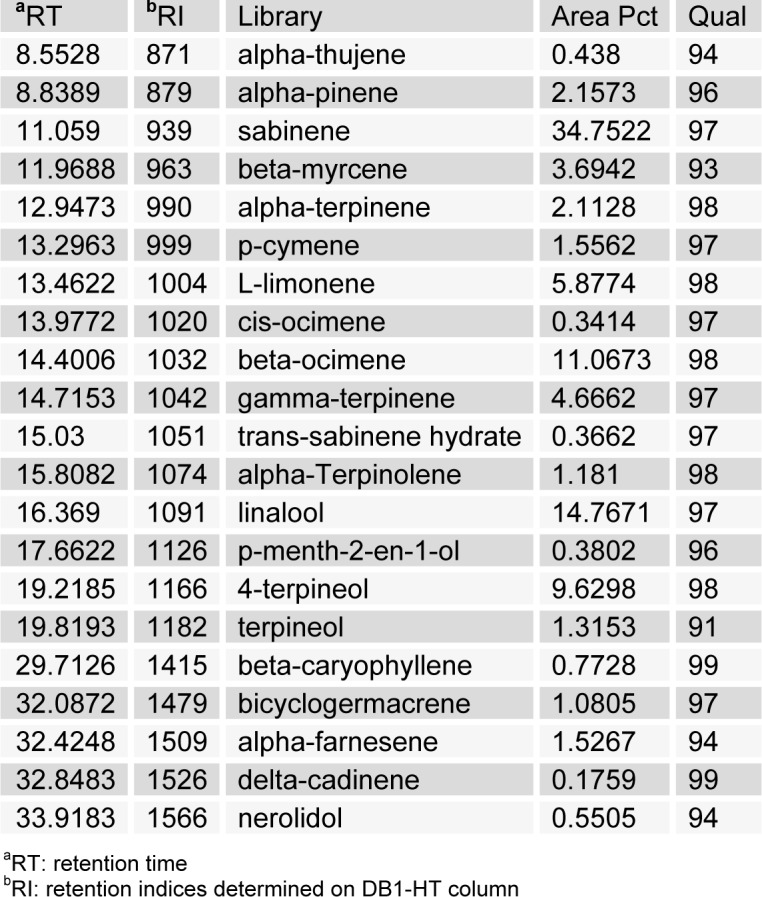
Figure 1. Inhibitory effects of HEOs on nitric oxide production and cell viability in RAW 264.7 cells. We measured nitric oxide content in the culture medium of cells stimulated with LPS (1 µg/mL) for 24 h in the presence of HEOs (0.01, 0.02 or 0.04 %), dexamethasone (20 µM), and NS-398 (20 µM). Cytotoxicity was determined using the LDH method. Values are the mean ± SEM of three independent experiments. *p < 0.05; **p < 0.01.
Figure 2. Inhibitory effects of HEOs on PGE2 production and COX-2 protein expression in RAW 264.7 cells. RAW 264.7 cells (1.0 × 106 cells/mL) were pre-incubated for 18 h, and PGE2 production determined after stimulation for 24 h with LPS (1 µg/mL) in the presence of HEOs (0.01, 0.02 or 0.04 %) and A) dexamethasone (20 µM) and NS-398 (20 µM). The concentration of PGE2 in supernatants was determined by ELISA. Immunoblotting was used to determine the COX-2 protein levels.
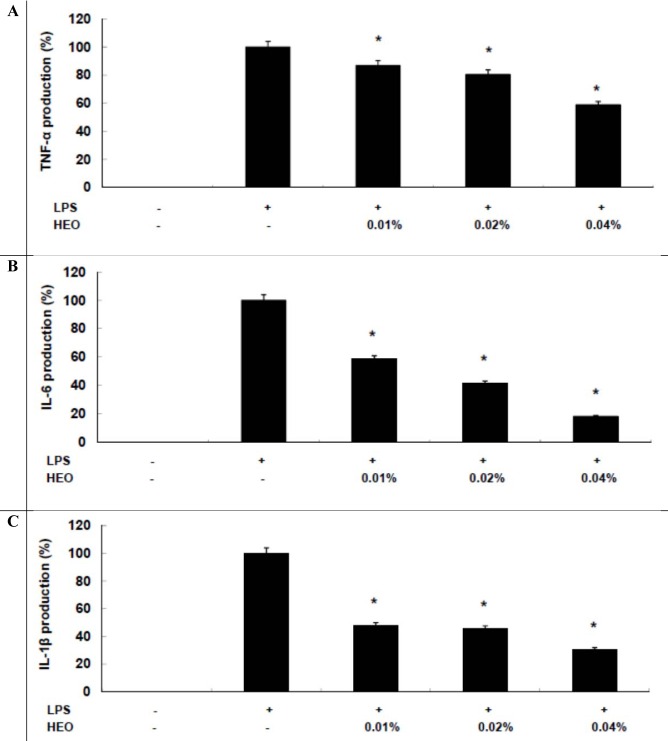
To analyze the anti-inflammatory mechanism mediated by HEOs, we investigated HEOs' effects on LPS-induced proinflammatory cytokine production, including that of TNF-α, IL-1β, and IL-6. Cytokine production was quantified using an enzyme immunoassay (EIA). It was found that HEOs reduced TNF-α, IL-6, and IL-1β production in a concentration-dependent manner (Figures 3A-C(Fig. 3), respectively). These results suggest that HEOs exert their anti-inflammatory effects via inhibition of TNF-α, IL-1β, and IL-6 production, which has important implications for the development of therapeutic strategies for chronic inflammation. In conclusion, the results of the present study provide the first evidence that HEOs inhibit LPS-induced NO and PGE2 production in macrophages. These inhibitory effects of HEOs were found to be associated with proinflammatory cytokines (TNF-α, IL-1β, and IL-6), suggesting a possible approach for the treatment of inflammatory diseases. In future studies, we will investigate the molecular mechanisms underlying the anti-inflammatory effect of HEOs, such as NF-κB and/or MAPKs signaling pathways, and the ability of HEOs to stimulate the immune system in an in vivo model system.
Figure 3. Inhibitory effects of HEOs on TNF-α, IL-1β, and IL-6 production in RAW 264.7 cells. Cells (1.5 × 105 cells/mL) were stimulated by LPS (1 µg/mL) for 24 h in the presence of HEOs (0.01, 0.02, or 0.04 %). Supernatants were collected, and the concentrations of TNF-α, IL-1β, and IL-6 were determined by ELISA. Values are the mean ± SEM of three independent experiments. *p < 0.05; **p < 0.01.
Acknowledgements
This research was supported by a grant from the Ministry of Trade, Industry and Energy, Republic of Korea through the Korea Industrial Complex Corp.
References
- 1.Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. Carol Stream, Ill.: Allured Publ. Corp.; 2001. [Google Scholar]
- 2.Asnaashari S, Delazar A, Habibi B, Vasfi R, Nahar L, Hamedeyazdan S, et al. Essential oil from Citrus aurantifolia prevents ketotifen-induced weight-gain in mice. Phytother Res. 2010;24:1893–1897. doi: 10.1002/ptr.3227. [DOI] [PubMed] [Google Scholar]
- 3.Baik JS, Kim SS, Lee JA, Oh TH, Kim JY, Lee NH, et al. Chemical composition and biological activities of essential oils extracted from Korean endemic citrus species. J Microbiol Biotechnol. 2008;18:74–79. [PubMed] [Google Scholar]
- 4.Bajpai VK, Baek KH, Kang SC. Control of Salmonella in foods by using essential oils: a review. Food Res Int. 2012;45:722–734. [Google Scholar]
- 5.Bajpai VK, Sharma A, Baek KH. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control. 2013;32:582–590. [Google Scholar]
- 6.Baser KHC, Buchbauer G. Handbook of essential oils: science, technology, and applications. Boca Raton, FL: CRC Press; 2010. [Google Scholar]
- 7.Cha JY, Jung JY, Jung JY, Lee JR, Cho IJ, Ku SK, et al. Inhibitory effects of traditional herbal formula pyungwi-san on inflammatory response in vitro and in vivo. Evid Based Complement Alternat Med. 2013;2013:630198. doi: 10.1155/2013/630198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi HS. Character impact odorants of Citrus Hallabong [(C. unshiu Marcov x C. sinensis Osbeck) x C. reticulata Blanco] cold-pressed peel oil. J Agric Food Chem. 2003;51:2687–2692. doi: 10.1021/jf021069o. [DOI] [PubMed] [Google Scholar]
- 9.Choi JH, Park YN, Li Y, Jin MH, Lee J, Lee Y, et al. Flowers of Inula japonica attenuate inflammatory responses. Immune Netw. 2010;10:145–152. doi: 10.4110/in.2010.10.5.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djabou N, Lorenzi V, Guinoiseau E, Andreani S, Giuliani MC, Desjobert JM, et al. Phytochemical composition of Corsican Teucrium essential oils and antibacterial activity against foodborne or toxi-infectious pathogens. Food Control. 2013;30:354–363. [Google Scholar]
- 11.Dorman HJ, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 12.Ellouze I, Abderrabba M, Sabaou N, Mathieu F, Lebrihi A, Bouajila J. Season's variation impact on Citrus aurantium leaves essential oil: chemical composition and biological activities. J Food Sci. 2012;77:T173–T180. doi: 10.1111/j.1750-3841.2012.02846.x. [DOI] [PubMed] [Google Scholar]
- 13.Gomes MRF, Schuh RS, Jacques ALB, Augustin OA, Bordignon SAL, Dias DO, et al. Citotoxic activity evaluation of essential oils and nanoemulsions of Drimys angustifolia and D. brasiliensis on human glioblastoma (U-138 MG) and human bladder carcinoma (T24) cell lines in vitro. Braz J Pharmacogn. 2013;23:259–257. [Google Scholar]
- 14.Gómez-Estaca J, López de Lacey A, López-Caballero ME, Gómez-Guillén MC, Montero P. Biodegradable gelatin-chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010;27:889–896. doi: 10.1016/j.fm.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Graziano AC, Cardile V, Crascì L, Caggia S, Dugo P, Bonina F, et al. Protective effects of an extract from Citrus bergamia against inflammatory injury in interferon-γ and histamine exposed human keratinocytes. Life Sci. 2012;90:968–974. doi: 10.1016/j.lfs.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 16.Haddouchi F, Chaouche TM, Zaouali Y, Ksouri R, Attou A, Benmansour A. Chemical composition and antimicrobial activity of the essential oils from four Ruta species growing in Algeria. Food Chem. 2013;141:253–258. doi: 10.1016/j.foodchem.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Han S, Kim HM, Lee JM, Mok SY, Lee S. Isolation and identification of polymethoxyflavones from the hybrid Citrus, hallabong. J Agric Food Chem. 2010;58:9488–9491. doi: 10.1021/jf102730b. [DOI] [PubMed] [Google Scholar]
- 18.Hyun CG, Kim GC, Hwang KT, Lee NH. Recent scientific advances on some essential oils in skin care. Household Personal Care TODAY. 2010;5:16–18. [Google Scholar]
- 19.Kim KN, Heo SJ, Yoon WJ, Kang SM, Ahn G, Yi TH, et al. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur J Pharmacol. 2010;649:369–375. doi: 10.1016/j.ejphar.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Kim SS, Baik JS, Oh TH, Yoon WJ, Lee NH, Hyun CG. Biological activities of Korean Citrus obovoidea and Citrus natsudaidai essential oils against acne-inducing bacteria. Biosci Biotechnol Biochem. 2008;72:2507–2513. doi: 10.1271/bbb.70388. [DOI] [PubMed] [Google Scholar]
- 21.Tang TF, Liu XM, Ling M, Lai F, Zhang L, Zhou YH, et al. Constituents of the essential oil and fatty acid from Malania oleifera. Ind Crop Prod. 2013;43:1–5. [Google Scholar]
- 22.Teixeira B, Marques A, Ramos C, Neng NR, Nogueira JMF, Saraiva JA, et al. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind Crop Prod. 2013;43:587–595. [Google Scholar]
- 23.Yang EJ, Kim SS, Moon JY, Oh TH, Baik JS, Lee NH, et al. Inhibitory effects of Fortunella japonica var. margarita and Citrus sunki essential oils on nitric oxide production and skin pathogens. Acta Microbiol Immunol Hung. 2010;57:15–27. doi: 10.1556/AMicr.57.2010.1.2. [DOI] [PubMed] [Google Scholar]
- 24.Yoon WJ, Moon JY, Song G, Lee YK, Han MS, Lee JS, et al. Artemisia fukudo essential oil attenuates LPS-induced inflammation by suppressing NF-kappaB and MAPK activation in RAW 264.7 macrophages. Food Chem Toxicol. 2010;48:1222–1229. doi: 10.1016/j.fct.2010.02.014. [DOI] [PubMed] [Google Scholar]