Abstract
The aim of the present study was to investigate the effects of crocin - a natural antioxidant derived from saffron - on cardiac reperfusion-induced arrhythmia and antioxidant systems such as catalase and superoxide dismutase (SOD) enzyme activities, glutathione (GSH) and malondialdehyde (MDA, as a marker of lipid peroxidation) levels. Rats in 4 experimental groups were administered crocin (20 mg/kg/day) or vehicle (i.p.) for 21 days with or without cardiac ischemia-reperfusion (IR). At the end of this period, hearts of anaesthetized animals in IR and “Cr + IR” groups were subjected to 10 min occlusion of the left anterior descending coronary artery and thereafter reperfused for 30 min. The results suggest that crocin is partially capable of suppressing reperfusion-induced arrhythmias. Compared to control group, ischemic-reperfusion injury significantly decreased SOD activity and GSH level and increased MDA level of heart muscle. “Cr + IR” group showed remarkably increased catalase activity in heart tissue (28.7 ± 6.6 vs. 23.6 ± 4.1 U/mg protein, P < 0.05) compared to the IR group. The level of cardiac tissue SOD activity in the “Cr + IR” group animals did not decline significantly compared to rats that were administered crocin alone with no ischemia. The results suggest a protective role of crocin on cardiac reperfusion arrhythmias which may at least partially be related to stability or even amplification of antioxidant systems. Crocin may potentially be useful for treatment or prevention of arrhythmias in patients with ischemic heart disease and this issue remains to be investigated in future clinical studies.
Keywords: arrhythmias, crocin, reperfusion, ischemia, catalase, SOD
Introduction
Reperfusion-induced arrhythmias are among the common causes of sudden cardiac death and can be produced both in experimental animals and humans (Bernier et al., 1986[6]; Foadoddini et al., 2010[12]). Therefore, investigating new methods for minimizing sudden cardiac death caused by reperfusion arrhythmias has very important clinical relevance.
Reactive oxygen species (ROS), which are increased after a few minutes of reperfusion, are one of the most important factors in pathophysiology of cardiac ischemic-reperfusion (IR) injury. Various studies have reported the beneficial effects of different antioxidants as agents for reducing heart IR injury (Dhallaa et al., 2000[10]). For example it has been reported that antioxidants might be useful as adjuvants in controlling reperfusion-induced arrhythmias following therapeutic thrombolysis (Hicks et al., 2007[14]).
Crocus sativus L., known as saffron, is a small perennial plant from the Iridaceae family. The main active constituent of saffron is picrocrocin and its derivatives including safranal, flavonoid derivatives and crocins (Boskabady et al., 2008[7]). Crocin (a water soluble carotenoid) is the most abundant antioxidant among active constituents of C. sativus (Goyal et al., 2010[13]). It has been documented that crocin has protective effects in brain (Zheng et al., 2007[39]), skeletal muscle (Hosseinzadeh et al., 2009[15]) and kidney ischemia-reperfusion injury models via its antioxidant properties (Hosseinzadeh et al., 2005[16]). Twice a day administration of 50 milligrams of saffron - dissolved in 100 ml of milk - to human patients with coronary artery disease leads to significant decrease in lipoprotein oxidation susceptibility; which indicates the potential of saffron as an antioxidant (Srivastava et al., 2010[31]). A lowering effect on blood pressure and relaxant effects on vascular smooth muscle were also described for this plant (Boskabady et al., 2008[7]).
Based on the documented protective role of antioxidant agents against cardiac arrhythmias and the potential antioxidant property of crocin, it is hypothesized in this study that crocin may reduce reperfusion-induced arrhythmias. Thus, the present study was planned to examine the effects of crocin on reperfusion-induced arrhythmias and antioxidant systems in an in vivo ischemic reperfusion (IR) injury model.
Materials and Methods
Animals
Male Wistar rats weighing 200 to 250 g were housed under standard conditions with free access to food and water. The investigation was performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Cohen et al., 1970[9]), and was approved by the Animal Ethics Committee of Baqiyatallah University.
Experimental groups
The rats were assigned randomly to four groups: IR (n = 18), “Cr + IR” (n = 18), Control (n = 10) and Crocin (n = 10) groups. In the IR and control groups, rats received normal saline (1cc/kg, i.p) once a day for 3 weeks. In “Cr + IR” and crocin groups, rats were treated with crocin (Sigma, Japan) (20 mg/kg, i.p) once a day for 3 weeks. The dosage of crocin was determined according to other studies (Zheng et al., 2007[39]; Goyal et al., 2010[13]). On the 21st day, 1 h after administering the above treatments, the rats in the IR and “Cr + IR” groups were subjected to 10 min of left anterior descending coronary artery (LAD) occlusion followed by 30 min reperfusion. In the crocin and control groups, all steps were similar to IR groups except for the occlusion of LAD to induce ischemia.
Surgical procedure
Coronary artery occlusion and reperfusion was performed according to the method described by Foadoddini et al. (2010[12]) and Baharvand et al. (2010[5]).
In brief, rats were anaesthetized with sodium pentobarbital (Sigma, St. Louis, MO) (30 mg/kg, ip) and ketamine (Alfasan, Holland) (40 mg/kg, ip). Rectal temperature was continuously monitored and maintained at 37 ± 0.5 °C. The neck was opened with a ventral midline incision and a tracheotomy was performed. The rats were artificially ventilated with room air enriched with oxygen at a rate of 80 per min and tidal volume of 1 ml 100gـ1 to maintain blood PO2, PCO2 and pH in the normal physiological ranges. A standard limb lead II electrocardiogram was monitored and recorded throughout the experiment. A catheter was inserted into the left carotid artery for monitoring of blood pressure. A left thoracotomy was performed in the fourth intercostal space, and pericardium was opened to expose the heart. A 6-0 silk suture was passed around the left anterior coronary artery (LAD) and the ends of it were threaded through a small polyethylene tube to form a snare.
Following a stabilization period of 20 min, the artery was occluded for 10 min by clamping the snare against the surface of the heart. Ischemia was confirmed by regional cyanosis and ST elevation in the electrocardiogram (ECG). Reperfusion was established by releasing the snare for 30 min. It is generally believed that reperfusion after 3-10 min of ischemia results in life-threatening arrhythmia in anaesthetized rats (Kajiwara et al., 2005[19]).
Assessment of ventricular arrhythmias
Ischemia-induced ventricular arrhythmias were determined in accordance with the Lambeth conventions (Walker et al., 1988[35]): premature ventricular beat (VEB) as a discrete and identifiable premature QRS complex (premature with respect to the P wave), ventricular tachycardia (VT) as a run of four or more VEB at a rate faster than the resting sinus rate and ventricular fibrillation (VF) as a signal for which individual QRS deflection can no longer be distinguishable from one another. Complex forms (bigeminy and salvos) were added to VEB count and not analyzed separately.
Biochemical studies
Immediately following to reperfusion period, the heart was excised and cleared of blood by rinsing in cold isotonic saline. In the IR and “Cr + IR” groups, the ischemic area was cut out whilst in the sham-operated animals the corresponding left ventricular region was taken. Only these tissue samples were snap frozen in liquid nitrogen, stored at -80 °C and used for glutathione (GSH), malondialdehyde (MDA), superoxide dismutase (SOD) and catalase assays. On the day of use, frozen tissue samples were quickly weighed and homogenized 1:10 in ice-cold phosphate-buffer saline (PBS). The homogenates were then centrifuged at 14000×g for 15 min at 4 °C. The supernatants were separated and used for enzyme activities assays and protein determination.
GSH level was measured using the method of Tietze (1969[33]).
Catalase activity was measured in tissues homogenates by the method of Aebi (1984[2]). The activity of SOD was determined according to Winterbourn et al. (1975[36]) and is based on the ability of superoxide dismutase to inhibit the reduction of nitro-blue tetrazolium (NBT) by superoxide. The end product of lipid peroxidation was estimated by measuring the level of MDA according to the method of Satoh (1978[29]).
Protein assay
Total protein concentration was measured by Bradford's method (Bradford, 1976[8]) using bovine serum albumin as a standard.
Statistical analysis
Data are shown as Mean ± SEM or percentage of incidence. The mean values were compared by independent sample T- test or one-way ANOVA (Tukey's post-hoc test).
Fisher's exact test was used to compare the differences in the incidence of VF between groups.
P-value < 0.05 was considered significant statistically.
Results
Hemodynamic parameters
Table 1(Tab. 1) summarizes the hemodynamic data. There were no significant differences at baseline values for heart rate and mean arterial blood pressure (MBP) among the experimental groups. Ischemia caused a marked reduction in blood pressure and the pressure remained at this reduced level during reperfusion period.
Table 1. Hemodynamics parameters in experimental groups.
Effects of crocin on reperfusion-induced arrhythmias
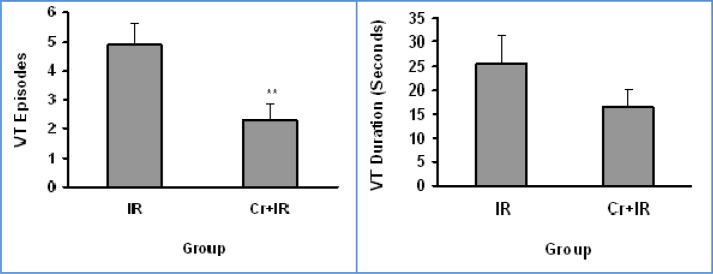
Crocin significantly reduced the number of VEBs compared with the IR group (p < 0.05).The number of VEBs were 31.1 ± 6.8 in the IR group and 12.6 ± 2.2 in the Cr+IR group (Figure 1(Fig. 1)). Crocin also reduced the VT episodes significantly compared with the IR group (p < 0.01). The number of VT episodes and VT duration were 4.3 ± 0.6 and 25.6 ± 6 s in the IR group, 2.2 ± 0.5 and 16.5 ± 4 s in the Cr+IR group (Figure 2(Fig. 2)), respectively. VF incidence is decreased significantly by crocin as compared to the IR group (p < 0.05). It was 67 % (12 of 18) in the IR group and 34 % (6 of 18) in the Cr+IR group (Figure 3(Fig. 3)).
Figure 1. VEB numbers (as Mean ± SEM) during 30 min reperfusion following 10 min ischemia in anaesthetized rats. IR, Ischemic-Reperfusion group; Cr+IR, group in which administration of crocin for 21 days was done before ischemia-reperfusion. * P < 0.05 versus IR group.

Figure 2. Ventricular tachycardia (VT) episodes and duration (seconds) during 30 min reperfusion following 10 min ischemia in rats. IR, Ischemic-Reperfusion group; Cr+IR, group in which administration of crocin for 21 days was done before ischemia-reperfusion. Data are shown as mean ± SEM. ** P < 0.01 versus IR group.
Figure 3. The incidence percentage of ventricular fibrillation (VF) during 30 min reperfusion following 10 min ischemia in anaesthetized rats. IR, Ischemic-Reperfusion group; Cr+IR, group in which administration of crocin for 21 days was done before ischemia-reperfusion. Data are shown as Mean ± SEM.

* P < 0.05 versus IR group
Antioxidant enzymes activities
Figure 4A(Fig. 4) shows mean heart tissue SOD activity. It was significantly lower in IR than control group (21.9 ± 5 vs. 29.7 ± 3.1 unit/mg protein, P < 0.01). The level of SOD activity in “Cr + IR” group did not have any significant difference compared to Crocin group (24.9 ± 4.7 vs 28.1 ± 3.5 unit/mg protein, P > 0.05). Figure 4B(Fig. 4) shows mean heart tissue catalase activity. Catalase activity decreased insignificantly in IR compared to the control group (23.6 ± 4.1 vs. 26.7 ± 3.5 units/mg protein, P = 0.054). Administration of crocin for 21 days significantly increased catalase activity compared to IR (28.9 ± 6.5 vs 23.6 ± 4.1 units/mg protein, P < 0.05).
Figure 4. Heart superoxide dismutase (SOD) (A) and catalase (CAT) activities (B) and heart glutathione (GSH) (C) and malondialdehyde (MDA) (D) levels following 10 min of ischemia and 30 min reperfusion. Data was shown as Mean ± SD.
* P < 0.05 compared to control group
** P < 0.01 compared to control group
# P < 0.05 compared to IR group
GSH and MDA levels
Figure 4C(Fig. 4) shows that ischemia-reperfusion caused a significant decrease in heart tissue GSH level (9.3 ± 1.4 vs. 11.6 ± 1.6 nmol/mg protein, P < 0.01). Figure 4D(Fig. 4) shows that MDA level was significantly higher in IR group compared with Control group (11.7 ± 1.5 vs. 9.5 ± 1.47 nmol/mg protein, P < 0.5). However, as shown Figures 4C and 4D(Fig. 4), GSH and MDA levels in Cr+IR and IR groups were not significantly different (9.8 ± 0.9) and 12.5 ± 1.7 nmol/mg protein, respectively.
Discussion
The present results show that in vivo reperfusion-induced arrhythmias are prevented by 21 days pretreatment of rats with crocin at a dose of 20 mg/kg with no significant alteration in hemodynamic parameters. This antiarrhythmic effect may have been partially exerted by stabilization of SOD levels and by amplification of catalase activity during IR injury.
The clinical importance of reperfusion arrhythmias is related to their documented role in sudden cardiac death. VT and VF during reperfusion period remain the most important causes of sudden death. In fact, reperfusion-induced arrhythmias are unresponsive to antiarrhythmic drugs with potential benefit in ischemia (Manning and Hearse, 1984[23]). Meanwhile, the standard antiarrhythmic drugs have limitations arising from their proarrhythmic and potentially toxic effects (Zhao et al., 2010[38]). Thus, research to finding new agents and treatment modalities for reperfusion arrhythmias are among the most important basic and clinical studies.
Generally, reperfusion-induced arrhythmias are believed to occur due to overproduction of oxygen-derived free radicals and calcium overload during the first minutes of reflow (Zhao et al., 2010[38]). Numerous antioxidants have been shown to prevent reperfusion induced arrhythmias in various animal species (Bernier et al., 1986[6]; Takusagawa et al., 2000[32]). Also treatment with diltiazem (a known calcium channel blocker), could be effective in prevention of reperfusion-induced arrhythmias (Kajiwara et al., 2005[19]).
Previous investigations suggested that some constituents of C. sativus (saffron) might have antioxidant properties (Abdullaev and Espinosa-Aguirre, 2004[1]). Crocin is the most important and abundant antioxidant among the pharmacologically active constituents of C. sativus. Also aqueous-ethanol extract of C. Sativus, via its calcium channel-blocking properties, has potent inhibitory effect on contractility and rate of guinea pig heart (Boskabady et al., 2008[7]). Furthermore, saffron and crocin have hypotensive (Imenshahidi et al., 2010[17]) and hypolipidemic properties via augmentation of antioxidant activities (Asdaq and Inamdar, 2010[4]). In the present study, prevention of arrhythmias was observed with intraperitoneal administration of 20 mg/kg/day of crocin in rat. The mechanism of action of crocin might be related to antioxidant or calcium channel-blocking effect and further electrophysiological studies are required to elucidate exactly how this agent prevents ventricular arrhythmias.
Most of previous studies in various organs emphasize on suppression of nearly all antioxidant defence systems, including lower SOD and catalase enzymes activities and lower GSH level following IR (Li and Jackson, 2002[21]; Rasoulian et al., 2008[27]). Superoxide dismutase (SOD) and catalase (CAT) are the most important antioxidant enzymes of tissues. Glutathione (GSH), a free radical scavenger, plays an important role in maintenance of cellular redox environment (Rasoulian et al., 2008[27]). A decrease in the activity of SOD and CAT can result in the decreased removal of superoxide ion (O2-) and hydrogen peroxide (H2O2) radicals (Rao and Vismanath, 2007[26]). Superoxide radicals are among the most important reactive oxygen species responsible for IR injury and could be converted to H2O2 by SOD activity. H2O2 itself is inactivated by glutathione peroxidase (GPX) and catalase (Paller et al., 1984[25]). In this study, there was a decrease in heart SOD activity and GSH level following myocardial IR in accordance with published data (Li and Jackson, 2002[21]; Abdullaev and Espinosa-Aguirre, 2004[1]). The decreased GSH level could be explained by its consumption for scavenging free radicals and maintaining the redox state of the cell during IR period (Schafer and Buettner, 2001[30]).
Catalase and SOD activities were recovered in the crocin treated group. The increase of Catalase activity by crocin in the IR-groups suggests that crocin has an important role in modulating antioxidative effects. Asdag and Inamdar (2010[4]) reported that crocin could return SOD and catalase activity to normal values in liver tissues of rats subjected to hyperlipidemic stress. Ochiai et al. (2007[24]) and Saleem et al. (2006[28]) in two independent studies showed protective roles for saffron and its carotenoid components on neuronal cell injuries in vivo and in vitro. In another experiment, Hosseinzadeh et al. (2005[16]) demonstrated that saffron extract and its active constituent, crocin, have protective effects on IR-induced oxidative stress in rat kidney. It has been documented that saffron consumption prevents cardiac injury and leads to decreased serum levels of cardiac troponin I in rats subjected to isoproterenol induced myocardial injury (Joukar et al., 2010[18]). Based on antioxidant properties of crocin, it could be suggested that cardioprotective effects of saffron may at least partially be related to antioxidant activity of its natural constituent, crocin.
We also assessed the effect of crocin on lipid peroxidation by measuring MDA, a stable metabolite resulting from free radical mediated lipid peroxidation. MDA level was increased significantly following myocardial IR injury, as have been reported in previous studies (Dhalla et al., 1999[11], 2000[10]). Cardiac preconditioning decreases MDA formation after prolonged ischemia and reperfusion (Turrens et al., 1992[34]). However, crocin did not decrease MDA level in present study.
We used the i.p.-route for crocin administration because it was previously reported that orally administered crocin was hardly absorbed into the blood circulation in rodents (Asai et al., 2005[3]). Crocin is easily transformed to crocetin by the intestinal bacteria of human or mouse (Lee et al., 2005[20]). So, crocins are hydrolyzed to crocetin before or during intestinal absorption, and absorbed crocetin is partly metabolized to mono- and diglucuronide conjugates (Asai et al., 2005[3]). Li et al. (2002[21]) reported that large amounts of crocin were eliminated via feces after oral administration. Crocin remained undetectable in plasma, further indicating that the intact form of crocin was not absorbed from the intestinal tract. Meanwhile, the plasma concentrations of crocetin after oral dose(s) of crocin were found to be low (Xi et al., 2007[37]). This indicates that crocetin was rapidly removed without accumulation in the body, which is consistent with the short plasma half-life of crocetin (Liu and Qian, 2002[22]). These are important aspects for therapeutic crocin applications.
In conclusion, the present experiment showed that crocin protects against severe reperfusion-induced arrhythmias in rat hearts in vivo and this could be partly related to stability or amplification of antioxidant system. Supplementary studies are required for clarifying the entire aspects of crocin effects on heart.
Notes
This paper is part of a MSc thesis by Zahra Jahanbakhsh.
Acknowledgements
Authors would like to thank Dr. Hedayat Sahraei, Professor Alireza Asgari and Mrs. Vafadar for their kind comments and helps. Financial support by Neuroscience Research Center of Baqiyatallah University of Medical Sciences and Razi Herbal Medicines Research Center of Lorestan University of Medical Sciences is gratefully acknowledged.
Conflict of interests
The authors declare that they have no competing interests.
References
- 1.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28:426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 3.Asai A, Nakano T, Takahashi M, Nagao A. Orally administered crocetin and crocins are absorbed into blood plasma as crocetin and its glucuronide conjugates in mice. J Agric Food Chem. 2005;53:7302–7306. doi: 10.1021/jf0509355. [DOI] [PubMed] [Google Scholar]
- 4.Asdaq SMB, Inamdar MN. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl Biochem Biotechnol. 2010;163:358–372. doi: 10.1007/s12010-009-8740-7. [DOI] [PubMed] [Google Scholar]
- 5.Baharvand B, Dehaj ME, Foadaddini M, Rasoulian B, Poorkhalili K, Aghai HW, et al. Delayed cardioprotective effects of hyperoxia preconditioning prolonged by intermittent exposure. J Surg Res. 2010;160:53–59. doi: 10.1016/j.jss.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 6.Bernier M, Hearse DJ, Manning AS. Reperfusion-induced arrhythmias and oxygen-derived free radicals. Studies with “anti-free radical” interventions and a free radical generating system in the isolated perfused rat heart. Circ Res. 1986;58:331–340. doi: 10.1161/01.res.58.3.331. [DOI] [PubMed] [Google Scholar]
- 7.Boskabady MH, Shafei MN, Shakiba A, Sefidi HS. Effect of aqueous-ethanol extract from Crocus sativus (saffron) on Guinea-pig isolated heart. Phytother Res. 2008;22:330–334. doi: 10.1002/ptr.2317. [DOI] [PubMed] [Google Scholar]
- 8.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970;34:30–38. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 10.Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia–reperfusion injury. Cardiovasc Res. 2000;47:446–456. doi: 10.1016/s0008-6363(00)00078-x. [DOI] [PubMed] [Google Scholar]
- 11.Dhalla NS, Golfman L, Takeda S, Takeda N, Nagano M. Evidence for the role of oxidative stress in acute ischemic heart disease: a brief review. Can J Cardiol. 1999;15:587–593. [PubMed] [Google Scholar]
- 12.Foadoddini M, Esmailidehaj M, Mehrani H, Sadraei SH, Golmanesh L, Wahhabaghai H, et al. Pretreatment with hyperoxia reduces in vivo infarct size and cell death by apoptosis with an early and delayed phase of protection. Eur J Cardiothorac Surg. 2010;39:233–240. doi: 10.1016/j.ejcts.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 13.Goyal SN, Arora S, Sharma AK, Joshi S, Ray R, Bhatia J, et al. Preventive effect of crocin of Crocus sativus on hemodynamic, biochemical, histopathological and ultrastuctural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine. 2010;17:227–232. doi: 10.1016/j.phymed.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Hicks JJ, Montes-Cortes DH, Cruz-Dominquez MP, Medina-Santillan R, Olivares-Corichi IM. Antioxidants decrease reperfusion induced arrhythmias in myocardial infarction with ST-elevation. Front Biosci. 2007;12:2029–2037. doi: 10.2741/2208. [DOI] [PubMed] [Google Scholar]
- 15.Hosseinzadeh H, Modaghegh MH, Saffari Z. Crocus sativus L. (saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evid Based Complement Alternat Med. 2009;6:343–350. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion induced oxidative damage in rats. J Pharm Pharm Sci. 2005;22:387–393. [PubMed] [Google Scholar]
- 17.Imenshahidi M, Hosseinzadeh H, Javadpour Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother Res. 2010;24:990–994. doi: 10.1002/ptr.3044. [DOI] [PubMed] [Google Scholar]
- 18.Joukar S, Najafipour H, Khaksari M, Sepehri G, Shahrokhi N, Dabiri S, et al. The effect of saffron consumption on biochemical and histopathological heart indices of rats with myocardial infarction. Cardiovasc Toxicol. 2010;10:66–71. doi: 10.1007/s12012-010-9063-1. [DOI] [PubMed] [Google Scholar]
- 19.Kajiwara H, Ohoi I, Tanonaka K, Takeo S. Effects of a novel cyclohexane dicarboximide derivative, ST-6, on reperfusion-induced arrhythmia in rats. J Pharmacol Sci. 2005;98:8–15. doi: 10.1254/jphs.fp0040815. [DOI] [PubMed] [Google Scholar]
- 20.Lee I, Lee J, Baek N, Kim D. Antihyperlipidemic effect of crocin isolated from the fructus of Gardenia jasminoides and its metabolite crocetin. Biol Pharm Bull. 2005;26:2106–2110. doi: 10.1248/bpb.28.2106. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol. 2002;282:C227–C241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- 22.Liu T, Qian Z. Pharmacokinetics of crocetin in rats. Yao Xue Xue Bao. 2002;37:367–369. [PubMed] [Google Scholar]
- 23.Manning AS, Hearse DJ. Reperfusion-induced arrhythmias: mechanisms and prevention. J Mol Cell Cardiol. 1984;16:497–518. doi: 10.1016/s0022-2828(84)80638-0. [DOI] [PubMed] [Google Scholar]
- 24.Ochiai T, Shimeno H, Mishima K, Iwasaki K, Fujiwara M, Tanaka H, et al. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim Biophys Acta. 2007;1770:578–584. doi: 10.1016/j.bbagen.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao PR, Vismanath RK. Cardioprotective activity of silymarin in ischemia-reperfusion-induced myocardial infarction in albino rats. Exp Clin Cardiol. 2007;12:179–187. [PMC free article] [PubMed] [Google Scholar]
- 27.Rasoulian B, Jafari M, Noroozzadeh A, Mehrani H, Wahhab-Aghar H, Hashemi-Madani MH, et al. Effects of ischemia-reperfusin on rat renal tissue antioxidant systems and lipid peroxidation. Acta Med Iranica. 2008;46:353–360. [Google Scholar]
- 28.Saleem S, Ahmad M, Shmad AS, Yousuf S, Ansari MA, Khan B, et al. Effect of saffron (Crocus sativus) on neurobehavioral and neurochemical changes in cerebral ischemia in rats. J Medicinal Food. 2006;9:246–253. doi: 10.1089/jmf.2006.9.246. [DOI] [PubMed] [Google Scholar]
- 29.Satoh K. Serum lipid peroxidation in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 30.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava R, Ahmed H, Dixit RK, Dharameveer, Saraf SA. Crocus sativus L. A comprehensive review. Pharmacogn Rev. 2010;4:200–208. doi: 10.4103/0973-7847.70919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takusagawa M, Komori S, Matsumura K, Osada M, Kohno I, Umetani K, et al. The inhibitory effects of carvedilol against arrhythmias induced by coronary reperfusion in anesthetized rats. J Cardiovasc Pharmacol Ther. 2000;5:105–112. doi: 10.1053/XV.2000.5494. [DOI] [PubMed] [Google Scholar]
- 33.Tietze F. Enzymic method for quantitative determination of nanogram amount of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 34.Turrens JF, Thornton J, Barnard ML, Snyder S, Liu G, Downey JM. Protection from reperfusion injury by preconditioning hearts does not involve increased antioxidant defenses. Am J Physiol. 1992;262:H585–H589. doi: 10.1152/ajpheart.1992.262.2.H585. [DOI] [PubMed] [Google Scholar]
- 35.Walker MJA, Curtis MJ, Hearse DJ, Campbell RWF, Janse MJ, Yellon DM, et al. The Lambeth conventions: Guidelines for the study of arrhythmias in ischemia infarction and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- 36.Winterbourn C, Hawkins R, Brian M, Carrell RW. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975;85:337–341. [PubMed] [Google Scholar]
- 37.Xi L, Qian Z, Du P, Fu J. Pharmacokinetic properties of crocin (crocetin digentiobiose ester) following oral administration in rats. Phytomedicine. 2007;14:633–636. doi: 10.1016/j.phymed.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 38.Zhao G, Gao H, Qiu J, Lu W, Wei X. The molecular mechanism of protective effects of grape seed Proanthocyanidin extract on reperfusion arrhythmias in rats in vivo. Biol Pharm Bull. 2010;33:759–762. doi: 10.1248/bpb.33.759. [DOI] [PubMed] [Google Scholar]
- 39.Zheng YQ, Liu JX, Wang JN, Xu L. Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007;23:86–94. doi: 10.1016/j.brainres.2006.12.064. [DOI] [PubMed] [Google Scholar]