Abstract
Previous research has shown the beneficial effects of aqueous extract of Piper sarmentosum (P.s) on atherosclerosis. The first stage in atherosclerosis is the formation of foam cell. The aim of this study was to investigate the effect of the methanol extract of P.s on fatty streaks by calculating neointimal foam cell infiltration in rabbits fed with high cholesterol diet. Thirty six male New Zealand white rabbits were divided equally into six groups: (i) C: control group fed normal rabbit chow; (ii) CH: cholesterol diet (1 % cholesterol); (iii) PM1: 1 % cholesterol with methanol extract of P.s (62.5 mg/kg); (iv) PM2: 1 % cholesterol with methanol extract of P.s (125 mg/kg); (v) PM3: 1 % cholesterol with methanol extract of P.s (250 mg/kg); (vi) SMV group fed 1 % cholesterol supplemented with Simvistatin drug (1.2 mg/kg). All animals were treated for 10 weeks. At the end of the treatment, the rabbits were fasted and sacrificed and the aortic tissues were collected for histological studies to measure the area of the neointimal foam cell infiltration using software. The thickening of intima ratio of atherosclerosis and morphological changes by scanning electron microscope were measured. The results showed that the atherosclerotic group had significantly bigger area of fatty streak compared to the control group. The area of fatty streak in the abdominal aorta was significantly reduced in the treatment groups which were similar with the SMV group. Similarly, there was a reduction in the number of foam cell in the treatment groups compared to the atherosclerotic group as seen under scanning microscope. In conclusion, histological study demonstrated that the methanol extract of the P.s could reduce the neointimal foam cell infiltration in the lumen of the aorta and the atherosclerotic lesion.
Keywords: Piper sarmentosum, extract, atherosclerosis, Simvistatin, histology, aorta
Abbreviations
Piper sarmentosum - P.s; control - C, atherogenic rabbits group - CH, PM1 - dose 62.5; PM2 - dose 125mg/kg; PM3 - dose 250 mg/kg; Simvastatin -SMV
Introduction
Hypercholesterolaemia is associated with an increased risk of various disorders, such as coronary heart disease and stroke (Hornstra, 1988[14]). These disorders are caused due to the narrowing of the blood vessels and then filled with fatty deposits, thereby leading to reduction in the blood flow to the organs. Coronary artery disease (CAD) is highly prevalent and one of the most important causes of morbidity and mortality in various parts of the world. Atherosclerosis is considered to be a chronic inflammatory disease of the arterial wall (Van der Wal et al., 1994[43]; Moreno and Fallon, 1997[23]; Ross, 1999[31]). Artherosclerosis is caused by hardening and narrowing of the arteries (Sundram et al., 1995[38]). There are many factors that facilitate development of the disorders of hypercholesterolaemia such as smoking, stress, diabetes and heredity (Osmund, 2001[26]). The mechanisms that may be involved in the development of atherosclerosis are oxidation of low-density lipoprotein-cholesterol (LDL-C), abnormal platelet aggregation, and inflammation (Olszanecki et al., 2005[25]).
Histological study of the atherosclerotic plaques reveals the presence of progressive infiltration and accumulation of lipids, inflammatory cells, smooth muscle cells, and an extracellular matrix in the arterial wall (Breslow, 1997[5]; Braunwald, 1997[4]). The earliest detectable change in the early phases of atherosclerosis is the accumulation of LDL particles in the subintimal space (Sánchez-Recalde and Kaski, 2001[33]). The LDL undergoes an oxidation process that activates the endothelium, this will lead to development of atherosclerotic plaque (Klatt and Esterbauer, 1996[19]).
Piper sarmentosum (P.s) is an herb which belongs to the family Piperaceae. It contains flavonoid compounds which have been reported to possess high antioxidative, free radical scavenging abilities and anti-inflammatory effect (Kannel et al., 1961[18]). The active extract P.s contains natural antioxidants like rutin, vitexin, naringenin, hesperitin, taxifolin/dihydroquercitin and quercetin which have high superoxide scavenging action (Vimala et al., 2003[44]; Ugusman et al., 2011[42]). P.s has been used traditionally used to cure many diseases (Vimala et al., 2003[44]). Phytochemically, the plant contains constituents like alkaloids (amide, flavonoids, pyrones) (Tutiwachwuttikul et al., 2006[40]) and it has also been reported to possess pharmacological properties such as antituberculosis (Hussain et al., 2008[15]) anticancer (Shahrul Hisham et al., 2009[36]), anti-angiogenic (Hussain et al., 2008[16]), anti-diabetic (Penchom et al., 1998[27]), neuromuscular blocker (Ridtitid et al., 1998[29]) antimalarial and antioxidant (Najib Nik et al., 1999[24]), and antiamoebic (Sawangjaroen et al., 2004[34]). Previous research studies showed that the aqueous extract of P.s improved endothelial function by promoting nitric oxide (NO) production in Human Umbilical Vein Endothelial Cells (HUVECs) (Ugusman et al., 2010[41]). P.s also reduced atherosclerosis lesion and inflammatory marker's like ICAM-1,VCAM-1 and C-RP (Amran et al., 2011[1]). The present study was conducted to determine the effect of methanol extract of P.s leaves on neointimal foam cell infiltration in rabbits fed with high cholesterol diet, and the morphological changes in the abdominal aorta were observed under scanning electron microscopy.
Materials and Methods
Animals and experimental protocol
Prior ethical approval was obtained from the Animal Ethics Committee, Universiti Kebangsaan Malaysia. Thirty six New Zealand white male rabbits with body weight of 1.8±2 kg were obtained from East Asia Rabbit Corporation Sdn. Bhd. Malaysia. The animals were housed separately in cages in an air-conditioned room with a 12 h light/dark cycle. All animals were housed individually with free access to water. The rabbits were divided randomly into six groups; control group (C; n=6) rabbits was fed the standard diet, atherogenic rabbits group (CH; n=6) was fed the standard diet enriched with 1 % cholesterol, treatment groups (PM1; n=6, PM2; n=6 and PM3; n=6), were fed with standard diet enriched with 1 % cholesterol plus different doses of methanol extract of P.s (62.5, 125 and 250 mg/kg/day) respectively. The Simvistatin group (Smv; n=6) was fed with the standard diet enriched with 1 % cholesterol plus Simvistatin drug (1.2 mg/kg/day, Merck, NJ) (Tsung-Ming et al., 2005[39]). The experiment was continued for 10 weeks. At the end of 10 weeks, the animals were fasted overnight and sacrificed by intravenous injection of pentobarbital (Nembutal, Abbott Laboratories, North Chicago, IL, 50 mg/kg body weight) and the abdominal aortic tissues were harvested for histological analysis.
High cholesterol diet
Analytical pure cholesterol powder (Sigma Chemical Co., St. Louis, USA) was mixed with the rabbit chow pellet (1 % cholesterol, w/w, in food pellet). For each 200 g of grounded rabbit chow pellet, 2 g of cholesterol was added and mixed with a 34 ml of chloroform where cholesterol was dissolved in 99.9 % chloroform and then mixed with grounded rabbit chow pellet. Chloroform was evaporated by exposing the diets as a thin layer at 50 °C in oven (Campbell et al., 2001[6]).
Plant material and extraction
The leaves of P.s were purchased from Ethano Resources Company and the leaves were identified by Forest Research Institute of Malaysia (FRIM). The leaves were cut to small sizes and then dried. We proceeded with the grinding the 300 g of leaves and powder was extracted with 2.5 L methanol (MeoH) using Soxhlet extractor. The extract was filtered and evaporated by using a rotator evaporator to give 80 g of methanolic crude extract. The crude extract was sticky and black which was kept in dark bottle in 4 ºC, until used. The crude extract was used in different doses of 62.5, 125 and 250 mg/kg and was then mixed with 5 ml of distilled water, and administered orally to the different groups (n=6/ group/extract) of rabbits.
Analysis of fatty streaks
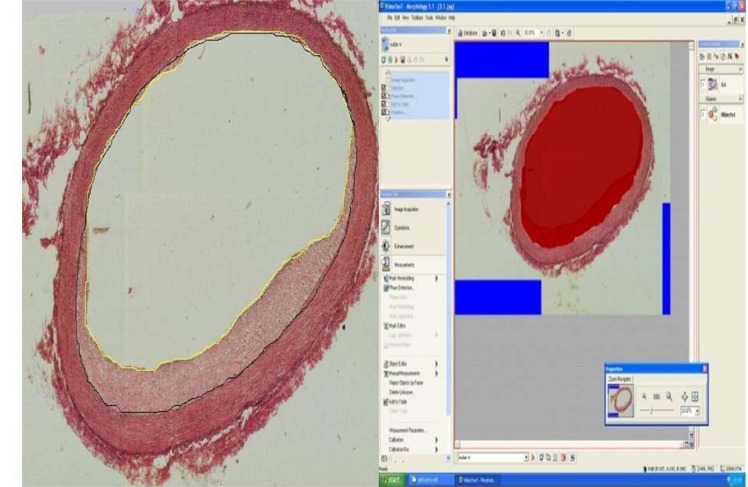
Following the sacrifice of the rabbits, the abdominal aorta was removed and the aorta was cut and stained with Haemotoxylin and Eosin (H & E). It was fixed in 10 % phosphate buffered formalin and then embedded in paraffin. From each sample, serial sections were made (3-5 sections/aorta) by using microtome (Leica RM2135). The procedure was based on the method by Seong et al. (2001[35]), the area of neointimal foam-cell infiltration was calculated where 20-fold magnification optical microscopic images were obtained using Pixelink color camera (USA). The lumen aortic surface was traced from the images using a computerized image analysis system Video Test T-Morphology 5.1 software with light microscope (Leica DM RXA2; German), and the area of the lumen was calculated. The internal elastic lamina was traced and its area was calculated. The neointimal foam-cell infiltration area was calculated by subtracting the area of the lumen from that of the internal elastic lamina (Figure 1(Fig. 1)).
Figure 1. Calculation of the neointimal foam-cell infiltration area using Video Test T-Morphology 5.1 software with camera; black circle indicates the internal elastic lamina was traced from the images and its area calculated. Yellow circle indicates the area of the aortic luminal surface and lumen area calculated. The area of the neointimal foam-cell was calculated by subtracting the area of the lumen from the internal elastic lamina. x25.
Determination of the thickening of intima and media
The thickening of intima and media were measured and the ratio taken between intima tunica and media tunica were calculated as follows: 100-fold magnification optical microscopic images were obtained using Pixelink color camera (USA) using a computerized image analysis system Video Test T-Morphology 5.1 software with light microscope (leica DM RXA2; German).
Scanning electron microscopy
The descending aorta was removed intact from each animal and re-immersed in fixative solution for a further 6 hours at 4 ºC. One centimeter long, from the aorta was prepared for examination by scanning electron microscopy (SEM). Samples for SEM was post fixed in 1 % aqueous Osmium tetroxide (OsO4) for 1 hour at 4 °C and dehydrated through a graded series of ethanol. Samples for SEM were critical-point dried from CO2, sputter-coated with gold, and examined under electron microscope (Tecnai G2, FEI Company) at an accelerating voltage of 100 kV.
Statistical analysis
Statistical analysis was carried out using the SPSS statistical package version 18 (SPSS Inc. USA). Distribution of data was examined by Kolmogrov-Smirnov test and found to be normal. ANOVA test was used to compare between the groups.
Results
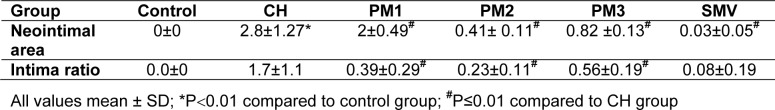
Table 1(Tab. 1) shows the effect of P.s on fatty streak in rabbits fed with 1 % cholesterol diet. The area of the fatty streak in the abdominal aorta was significantly reduced by both Simvistatin and treatment with P.s. The area of fatty streak was significantly increased in CH group compared to the control group. Among the treatment groups PM1, PM2 and PM3 there was a significant reduction in the area of the streak.
Table 1. Effect of Piper sarmentosum on fatty streak in groups fed with 1 % cholesterol diet.
Histological examination showed thickening of tunica intima: tunica media and the foam cell layer of the abdominal aorta in the groups of rabbits that received 1 % cholesterol daily compared to the control group. In the treatment group, there was significant reduction in the thickening of the intima and foam cell compared to the CH group P0.01 (Table 1(Tab. 1)).
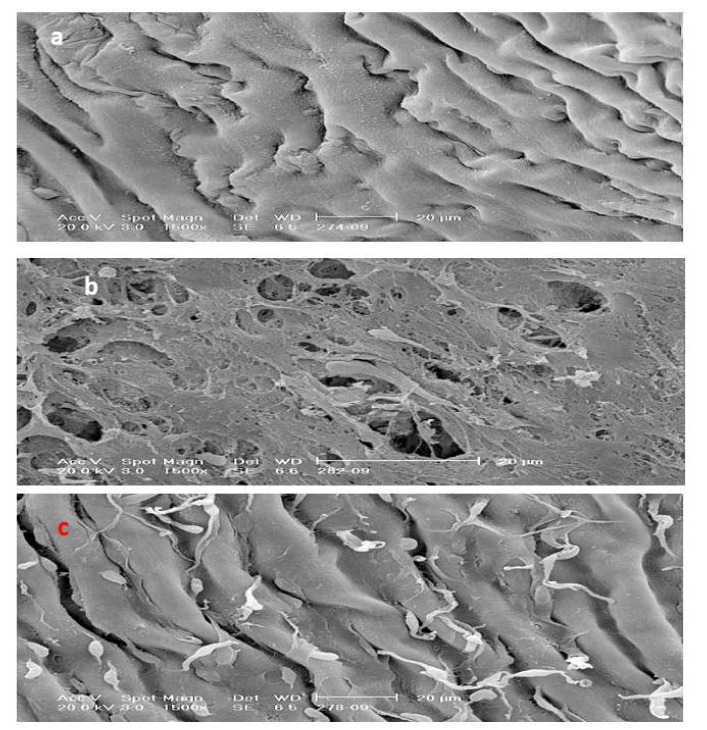
Under the scanning electron microscopy study, no foam cell could be observed in the control group. In the CH group, many foam cells were observed covering all the intima layer. However, in PM3 there was significant reduction in the number of foam cells compared to the CH group similarly SMV group (Figure 2(Fig. 2)).
Figure 2. Scanning electron micrograph from a lesion in the luminal abdominal aorta (a) control group showed no lipid or foam cell appearance, (b) hypercholestrolaemic rabbit, there is marked irregularity of the surface caused by accumulation of numerous foam cells covering all the intimal endothelium surface. Adhering leukocytes have tail-like protrusions and appear to be spreading over or migrating to the aortic intima and (c) treatment with P.s, there is less accumulation of adherent leukocytes on the surface (Bar = 20 µm).
Discussion
Hypercholesterolemia is a major risk factor for coronary heart disease, and the Framingham study had reported that a 1 % increase of plasma cholesterol level is equivalent to a 2 % increase in the incidence of coronary heart disease (Kannel et al., 1961[18]). The present study showed that dietary treatment of rabbits with high-cholesterol diets caused atherosclerotic lesions in rabbit model and these findings were in accordance with earlier studies (Yi et al., 2000[48]; Yamakoshi at al., 2000[47]; Amran et al., 2010[2]). In the present study, it was observed that the severity of the atherosclerosis in aorta was associated with hypercholesterolemia which was in accordance with past investigations.
It has been shown that high cholesterol diet produced tunica intimal thickening that contained foam cell (Prasad et al., 1994[28]). Hypercholesterolemia is also one of the important factors that cause endothelial dysfunction in human arteries (Minor et al., 1990[22]). Atheromatous lesions develop in the subendothelial space due to the accumulation of cholesterol ester which form the foam cells. The mechanism of foam cell formation still remains unclear because macrophages have few LDL receptors but there is much evidence to show that oxidized LDL is responsible for cholesterol loading of macrophages foam cell formation and atherosclerosis.
Histological analysis in the present study demonstrated that methanol extract of P.s could reduce the neointimal foam-cell infiltration in the lumen aorta. Inflammation plays an important role in the development of atherosclerosis. Hence, significant reduction in the inflammatory lesion may be due to anti-inflammatory action of P.s. This study demonstrated that P.s possessed antiatherogenic activity since the supplementation of P.s reduces the foam cell number and the thickening of the tunica intima layer. The reduction in the tunica intima thickening due to supplementation with P.s was not clearly understood. The protective activity of P.s on atherosclerotic lesion may be attributed to its antioxidant action because it reduces the production of free radicals, decreasing the oxidized LDL and alleviating the subsequent damage to the heart tissue (Zhang et al., 2001[49]). The effect is probably due to its active compound such as naringenin which has antiatherogenic effect. Naringenin inhibition of VACM-1 and MCP-1 expressions, followed by the prevention of monocyte adhesion and macrophage infiltration in aortic endothelium. It was reported that naringenin significantly inhibited aortic fatty streak formation in rabbits on high cholesterol diets (Chul-Ho et al., 2001[7]; Seong et al., 2001[35]).
Atherosclerosis, which is a chronic inflammatory disease of arteries, is characterized by a thickening of the vascular wall and an infiltration of macrophages and lymphocytes. In animals with diet-induced or genetically determined hyperlipidemia, the earliest morphological changes in arteries include focal adherence of mononuclear leukocytes to the endothelium and accumulation of monocyte-derived foam cells in the intima (Klurfeld, 1985[20]; Rogers et al., 1986[30]). Our previous results have shown that the supplementation of water extract of P.s markedly reduced atherosclerosis lesion (Amran et al., 2010[2]). The present study showed that methanol extract of P.s also reduced atherosclerotic lesion by the reducing the fatty streaks.
The oxidative modification of LDL plays an important role in the development of atherosclerosis (Steinberg et al., 1989[37]; Jialal and Devaraj, 1996[17]). There are many antioxidant components in P.s such as rutin, vitexin, naringenin and quercetin which have potent action in protecting LDL from oxidation. The antioxidant activity of P.s extract was found to possess high scavenger free radical activity 98 % for the aqueous extract and 78 % for the methanol extract. Other studies have also shown that consumption of flavonoid antioxidant is inversely related to the risk of developing coronary heart disease (Hertog et al., 1993[13]). Past research reports showed a link between flavonoid and atherosclerosis due to the antioxidant activities of flavonoid which inhibits the aggregation and adhesion of platelets in the blood (Seong et al., 2001[35]). It has also been shown that flavonoids reduce LDL oxidation, which is an important step in atherogenesis (De Whalley et al., 1990[8]). The present results in experiments with rabbits, although not directly applicable to human, suggest that P.s is effective as an anti-atherosclerotic agent. Foam cells observed under both light and transmission electron microscope in cholesterol group (CH) caused reduction of the lumen surface similar to the results of past studies (Salazar et al., 2007[32]). The protective effect of flavonoids against chronic diseases have been attributed to their free radical-scavenging property. Interestingly, in the case of CVD, flavonoids have been shown to reduce low density lipoprotein (LDL) oxidation which is an important step in the pathogenesis of atherogenesis (De Whalley et al., 1990[8]). The accumulation of foam cells in the vascular wall is one of the characteristic features of atherosclerotic lesions. Studies in experimental animals and humans have indicated that most of the foam cells, especially in early atherosclerotic lesions, are monocyte-derived macrophages (Watanabe et al., 1985[46]). Under scanning electron micrograph in CH group the adhering leukocytes have tail-like protrusions and appear to be spreading over or migrating to the aortic intima which was shown in another report (Haraoka et al., 1995[12]). Increased fatty streak lesion formation with increasing duration of cholesterol diet was reported by Walker et al. (1986[45]). Increased accumulation of leukocytes has been described as early response to hyperlipidemia (Gerrity, 1981[10]; Faggiotto et al., 1984[9]). However, in treatment groups with P.s there was less accumulation of adherent leukocytes on the surface compared to the hypercholesterolemic rabbits. The appearance of adherent leukocytes may play a role in initiating the development of the fatty intimal lesion and this has been shown in previous studies (Walker et al., 1986[45]). Several pathways could be suggested where by these cells participate. Study by Bevilacqua (1985[3]), shows that interleukin-1 strongly promotes leukocyte adherence to endothelium. The accumulation of leukocyte may be due to expression of endothelial leukocyte adhesion molecules (Gimbrone and Bevilacqua, 1988[11]).
Conclusion
In conclusion histological study demonstrated that the methanol extract of P.s could reduce the neointimal foam cell infiltration in the lumen aorta and atherosclerotic lesion in rabbits high cholesterol diet.
Acknowledgement
The research was supported by grant received from the Ministry of Science, Technology and Innovation, Malaysia. The authors wish to thank Universiti Kebangsaan Malaysia, Furley Marketing Sdn. Bhd. Malaysia for the plant extract and Mrs. Zanariyah for the technical help received.
References
- 1.Amran AA, Zakaria Z, Othman F, Das S, Al-Mekhlafi H, Nordin NMM. Changes in the vascular cell adhesion molecule-1, intercellular adhesion molecule-1 and c-reactive protein following administration of aqueous extract of Piper sarmentosum on experimental rabbits fed with cholesterol diet. Lipids Health Dis. 2011;10:2. doi: 10.1186/1476-511X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amran AA, Zakaria Z, Othman F, Das S, Raj S, Nordin NA. Aqueous extract of Piper sarmentosum decreases atherosclerotic lesions in high cholesterolemic experimental rabbits. Lipids Health Dis. 2010;9:44. doi: 10.1186/1476-511X-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA. Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes and related leukocyte cell lines. J Clin Invest. 1985;76:2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braunwald E. Shattuck Lecture –cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 5.Breslow JL. Cardiovascular disease burden increases, NIH funding decreases. Nature Medicine. 1997;3:600–601. doi: 10.1038/nm0697-600. [DOI] [PubMed] [Google Scholar]
- 6.Campbell H, Efendy L, Smith S, Campbell R. Molecular basis by which garlic suppresses atherosclerosis. J Nutr. 2001;131:1006–9. doi: 10.1093/jn/131.3.1006S. [DOI] [PubMed] [Google Scholar]
- 7.Chul-Ho L, Tae-Sook J, Yang-Kyu C, Byung-Hwa H, Goo-Taeg O, Eun-Hee K, et al. Antiatherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem Biophys Res Commun. 2001;284:681–8. doi: 10.1006/bbrc.2001.5001. [DOI] [PubMed] [Google Scholar]
- 8.De Whalley CV, Rankin SM, Hoult JR, Jessup WDS. Flavonoids inhibit the antioxidative modification of low density lipoproteins. Biochem Pharmacol. 1990;39:1743–1749. doi: 10.1016/0006-2952(90)90120-a. [DOI] [PubMed] [Google Scholar]
- 9.Faggiotto A, Ross R, Harker L. Studies of hypercholesterolemia in the non-human primate I. Changes that lead to fatty streak formation. Atheriosclerosis. 1984;4:323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- 10.Gerrity RG. The role of the monocyte in atherogenesis. I: Transition of blood borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981;103:181–190. [PMC free article] [PubMed] [Google Scholar]
- 11.Gimbrone MA, Bevilacque MP. Vascular endothelium functional modulation at the blood interface. In: Simionescu N, Simionescu M, editors. Endothelial cell biology in health and disease. New York: Plenum Press; 1988. pp. 255–269. [Google Scholar]
- 12.Haraoka S, Shimokama T, Watanabe T. Participation of T lymphocytes in atherogenesis: sequential and quantitative observation of aortic lesions of rats with diet-induced hypercholesterolaemia using en face double immunostaining. Virchows Archiv. 1995;426:307–315. doi: 10.1007/BF00191369. [DOI] [PubMed] [Google Scholar]
- 13.Hertog MGL, Feskens EJM, Hollman PCH, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 14.Hornstra G. Dietary lipids and cardiovascular diseases. Oleagineux. 1988;43:75–81. [Google Scholar]
- 15.Hussain K, Ismail Z, Sadikun A, Ibrahim P. Analysis of proteins, polysaccharides, glycosaponins contants of Piper sarmentosum Roxb. and anti-TB evaluation for bio enhancing/interation effects of leaf extracts with isoniazid (INH) Natural Product Radiance. 2008;7:204–208. [Google Scholar]
- 16.Hussain K, Ismail Z, Sadikun A, Ibrahim P, Malik A. In vitro antiangiogenesis activity of standardized extract of Piper sarmentosum Roxb. Journal Riset Kimia. 2008;1:146–50. [Google Scholar]
- 17.Jialal I, Devaraj S. Low-density lipoprotein oxidation, antioxidants, and atheroscelerosis: a clinical biochemistry perspective. Clin Chem. 1996;4:498–506. [PubMed] [Google Scholar]
- 18.Kannel WB, Dawber TR, Kagan A, Revostski N, Strokes J. Factors of risk in the development of coronary heart disease-six year follow-up experience: the Framingham Study. Ann Intern Med. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 19.Klatt P, Esterbauer H. Oxidative hypothesis of atherogenesis. J Cardiovasc Risk. 1996;3:346–351. doi: 10.1177/174182679600300402. [DOI] [PubMed] [Google Scholar]
- 20.Klurfeld DM. Identification of foam cells in human atherosclerotic lesions as macrophages using monoclonal antibodies. Arch Pathol Lab Med. 1985;109:445–449. [PubMed] [Google Scholar]
- 21.Lee A, Dyer JR, Craig DD. Isolation, synthesis, and evolutionary ecology of Piper amides. In: Dyer LA, Palmer AND, editors. A model genus for studies of phytochemistry, ecology, and evolution. New York: Kluwer/Plenum; 2004. pp. 117–139. [Google Scholar]
- 22.Minor RL, Myers PR, Guerra RJ, Bates JN, Harrison DG. Diet-induced atherosclerosis increases the release of nitrogen oxides from rabbit aorta. J Clin Invest. 1990;86:2109–2116. doi: 10.1172/JCI114949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno PR, Fallon JT. Inflammation in acute coronary syndromes. In: Schultheiss H, Schwimmbeck P, editors. The role of immune mechanism in cardiovascular disease. Berlin: Springer; 1997. pp. 213–229. [Google Scholar]
- 24.Najib Nik A, Rahman N, Furuta T, Kojima S, Takane K, Ali Mohd M. Antimalarial activity of extracts of Malaysian medicinal plants. J Ethnopharmacol. 1999;64:249–54. doi: 10.1016/s0378-8741(98)00135-4. [DOI] [PubMed] [Google Scholar]
- 25.Olszanecki R, Jawien J, Gajda M, Mateuszuk L, Gebska A, Korabiowska M, et al. Effect of curcumin on atherosclerosis in apoE/LDLR-double knockout mice. J Physiol Pharmacol. 2005;56:627–35. [PubMed] [Google Scholar]
- 26.Osmund CE. Basic biochemistry of food nutrients. 1st. Enugu, Nigeria: Immaculate Publ. Ltd.; 2001. [Google Scholar]
- 27.Penchom P, Suwan ST, Rungravi T, Hirosh W, Jeevan KB, Shigetoshi K. Hypoglycemic effect of the water extract of Piper sarmentosum in rats. J Ethnopharmacol. 1998;60:27–32. doi: 10.1016/s0378-8741(97)00127-x. [DOI] [PubMed] [Google Scholar]
- 28.Prasad K, Kalra J, Lee P. Oxygen free radicals as a mechanism of hypercholesterolemic atherosclerosis: effects of probucol. Int J Angiol. 1994;3:100–112. [Google Scholar]
- 29.Ridtitid W, Rattanaprom W, Thaina P, Chittrakarn S, Sunbhanich M. Neuromuscular blocking activity of methanolic extract of Piper sarmentosum leaves in rat phrenic nerve-hemidiaphragm preparation. J Ethnopharmacol. 1998;61:135–142. doi: 10.1016/s0378-8741(98)00025-7. [DOI] [PubMed] [Google Scholar]
- 30.Rogers KA, Hoover RL, Castellott JJJ, Robinson JM, Karnovsky MJ. Dietary cholesterol-induced changed in macrophage characteristics relationship to atherosclerosis. Am J Pathol. 1986;125:284–291. [PMC free article] [PubMed] [Google Scholar]
- 31.Ross R. Atherosclerosis: an inflammatory disease. New Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 32.Salazar J, Ramirez A, de Hoz R, Rojas B, Ruiz E, Teierina T, et al. Alterations in the choroid in hypercholesterolemic rabbits: reversibility after normalization of cholesterol levels. Exp Eye Res. 2007;84:412–422. doi: 10.1016/j.exer.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Sánchez-Recalde A, Kaski JC. Diabetes mellitus, inflamación y aterosclerosis coronaria: perspectiva actualy future. Rev Esp Cardiol. 2001;54:751–763. doi: 10.1016/s0300-8932(01)76390-7. [DOI] [PubMed] [Google Scholar]
- 34.Sawangjaroen AN, Sawangjaroen AK, Poonpanang P. Effects of Piper longum fruit, Piper sarmentosum root and Quercus infectoria nut gall on caecal amoebiasis in mice. J Ethnopharmacol. 2004;91:357–360. doi: 10.1016/j.jep.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Seong CC, Hyo SK, Tae SJ, Song HB, Young BP. Naringin has an antiatherogenic effect with the inhibition of intercellular adhesion molecule-1 in hypercholesterolemic rabbits. J Cardiovasc Pharmacol. 2001;38:947–955. doi: 10.1097/00005344-200112000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Shahrul Hisham ZA, Wan Haifa Haryani WO, Zaidah ZA, Muhd Fauzi S, Sahidan S, Rohaya Megat AW. Intrinsic anticarcinogenic effects of Piper sarmentosum ethanolic extract on a human hepatoma cell line. Cancer Cell Int. 2009;9:6. doi: 10.1186/1475-2867-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinberg D, Parthasarathy S, Carew TW, Knoo JC, Witztum JL. Beyond cholesterol: modification of low-density lipoprotein that increase its atherogenicity. New Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 38.Sundram K, Hayes KC, Siru OH. Dietary 18:2 and 16: 0 may be required to improve the serum LDL/HDL cholesterol ratio in noncholesterolemic men. J Nutr Biochem. 1995;6:179–187. [Google Scholar]
- 39.Tsung-Ming L, Mei-Shu L, Tsai-Fwu C, Nen-Chung C. Effect of simvastatin on left ventricular mass in hypercholesterolemic rabbit. Am J Physiol. 2005;288:H1352–8. doi: 10.1152/ajpheart.00527.2003. [DOI] [PubMed] [Google Scholar]
- 40.Tutiwachwuttikul P, Phansa P, Pootaeng-on Y, Tylor WC. Chemical constituents of the roots Piper sarmentosum. Chem Pharmaceut Bull. 2006;54:149–51. doi: 10.1248/cpb.54.149. [DOI] [PubMed] [Google Scholar]
- 41.Ugusman A, Zakaria Z, Hui Ck, Nordin NA. Piper sarmentosum inhibits ICAM-1 and Nox4 gene expression in oxidative stress-induced human umbilical vein endothelial cells. Clinics. 2010;65:709–14. doi: 10.1186/1472-6882-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ugusman A, Zakaria Z, Hui Ck, Nordin NA, Zaleha M. Rutin enhanced nitric oxide synthesis in human umbilical vein endothelial cells. Nat Pro, Natural Products for Health & Beauty. 3rd international conference on NPH, 2011; 16-18 March, Bangkok, Thailand.2011. [Google Scholar]
- 43.Van der Wal AC, Becker AE, van der Loss CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]
- 44.Vimala S, Mohd Ilham A, Abdul Rashid A, Rohana S. Natural antioxidants: Piper sarmentosum and Morinda elliptica. Malaysian J Nutr. 2003;9:41–51. [PubMed] [Google Scholar]
- 45.Walker LN, Reidy MA, Bowyer DE. Morphology and cell kinetics of fatty streak lesion formation in hypercholesterolemic rabbit. Am J Pathol. 1986;125:450–459. [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe T, Hirata M, Yoshigawa Y, Nagafuchi Y, Toyoshima H, Watanabe T. Role of macrophages in atherosclerosis. Sequential observations of cholesterol-induced rabbit aortic lesion by the immunoperoxidase technique using monoclonal anti-macrophage antibody. Lab Invest. 1985;53:80–90. [PubMed] [Google Scholar]
- 47.Yamakoshi J, Piskula K, Izumi T, Tobe K, Saito M, Kataoka S, et al. Isoflavone aglycone-rich extract without soy protein attenuates atherosclerosis development in cholesterol-fed rabbits. J Nutr. 2000;130:1887–1893. doi: 10.1093/jn/130.8.1887. [DOI] [PubMed] [Google Scholar]
- 48.Yi S, Nancy L, William P, Clarie H. Effect of cholesterol diet on vascular function and atherogenesis in rabbits. Exp Biol Med. 2000;224:166–171. doi: 10.1046/j.1525-1373.2000.22416.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z, Chang Q, Zhu M, Huang Y, Ho WKK, Chen ZY. Characterization of antioxidants present in hawthorn fruits. J Nutr Biochem. 2001;12:144–152. doi: 10.1016/s0955-2863(00)00137-6. [DOI] [PubMed] [Google Scholar]