Abstract
Previous studies have reported that oleuropein, the major constituent of olive leaves, has cardioprotective effects. There is no report related to oleuropein and ischemic-reperfusion injuries (cardiac dysfunction and myocardial infarction) as well as preconditioning in rat hearts. 56 male Wistar rats were divided into 7 groups (n=8). Group 1 as the control group and groups 2 to 7 as the treatment groups that received a single dose of oleuropein (100 mg/kg, i.p.) 1, 3, 6, 12, 24 and 48 hours before the excision of the heart, respectively. After these times, their hearts were excised and subjected to 30 min regional ischemia and 120 min reperfusion under Langendorff apparatus. Electrocardiogram and intraventricular pressures were monitored and recorded throughout the procedure. Finally, infarct size was measured by triphenyltetrazolium chloride staining. Compared to the control group, oleuropein significantly reduced infarct size and reperfusion-induced cardiac dysfunction in groups 2 and 3. Oleuropein markedly attenuated both ischemic and reperfusion arrhythmias in groups 2 and 3. There was no significant difference between other groups (4 to 7) than the control group. Heart rate had no significant difference among all of the groups. These results indicate that pretreatment of rats with a single dose of intraperitoneal oleuropein could protect their heart against ischemic-reperfusion injury for at least 3 hours. However, it has no preconditioning effect, since oleuropein had not cardioprotective effects 24 hour later.
Keywords: oleuropein, ischemia, reperfusion, preconditioning, arrhythmia
Introduction
Oleuropein, the main constituent of olive leaves, is a phenolic complex that has many biological roles, including hypolipidemic (Jemai et al., 2008[12]), hypoglycemic (Al-Azzawie and Alhamdani, 2006[1]), anti-inflammatory (Puel et al., 2006[25]), anti-ischemic (Andreadou et al., 2006[2]), anti-atherosclerotic (Coni et al., 2000[6]), antimicrobial (Zhao et al., 2009[38]; Jiang et al., 2008[14]; Zanichelli et al., 2005[37]) and antidiabetic (Al-Azzawie and Alhamdani, 2006[1]; Jemai et al., 2009[13]) properties. Most studies have attributed these activities to its antioxidant activity. However, the results of some studies have shown that oleuropein can also affect intracellular signaling pathways (Sato et al., 2007[28]; Santiago-Mora et al., 2011[27]). Oleuropein and its metabolite, hydroxytyrosol, have a number of pharmacological properties against cardiovascular disorders (Singh et al., 2008[29]). In 1978, Petkov and Manolov reported that intravenous administration of a single dose of oleuropein (10-40 mg/kg) has antiarrhythmic effect against barium chloride induced-arrhythmia in rabbits. Also, it had antiarrhythmic effects opposed to calcium chloride and aconitine induced-arrhythmia in rats (Petkov and Manolov, 1978[23]). In 2004, Manna and his colleagues (Manna et al., 2004[19]) for the first time, using isolated rat heart model, showed the experimental evidence of cardioprotective effect of oleuropein (20 µg/g wet weight through perfusion fluid for 15 min before global ischemia) against acute ischemic-reperfusion injury. In 2006, Andreadou and his co-workers (2006[2]) demonstrated anti-ischemic effect of oleuropein (10 or 20 mg/kg orally for 3 weeks) in rabbits that was evident with reduced infarct size and oxidative stress. In 2007, Andreadou et al. reported that intraperitoneal administration of 100 and 200 mg/kg oleuropein two days before injection of a toxic dose of doxorubicin (20 mg/kg, i.p, once time), attenuated doxorubicin-induced cardiotoxicity in rats. This protective effect was evident with reduced oxidative stress and inhibition of lipid peroxidation. There was not any significant difference between doses of 100 and 200 mg of oleuropein (Andreadou et al., 2007[3]).
In this study, we wanted to know whether the intraperitoneal administration of a single dose of oleuropein (100 mg/kg) has cardioprotective and preconditioning effects against ischemic and reperfusion arrhythmia and dysfunction in isolated rat hearts. Preconditioning is a phenomenon thereby short period(s) of a sublethal stimulus like ischemia, or pharmacological agents like nitroglycerin, before a prolonged lethal ischemia renders that organ more resistance to ischemic-reperfusion injury (Murry et al., 1986[22]; Baharvand et al., 2010[4]). Preconditioning is a biphasic mode with an early phase that initiates immediately after the stimulus and lasts for 1-3 h followed by a late phase that starts 12-24 h later and continues about 24- 48 h (Baharvand et al., 2010[4]).
Materials and Methods
Animals
Male Wistar rats, weighing 250-300 g (8-12 weeks of age) obtained from Pasture Institute (Tehran, Iran), were housed under standard condition with 22±2 oC, 55 % humidity and 12 light/dark cycle. All animals were supplied with a standard laboratory diet and tap water ad libitum. This study was confirmed by the Animal Ethic Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Heart isolation
Rats were anaesthetized with sodium thiopental (60 mg/kg, i.p.) and heparinized by injection of 1000 IU intraperitoneally. Then, the hearts were excised and aorta cannulated to perfuse retrogradly under Langendorff apparatus with filtered Krebs solution. It contains NaCl 118 mM, KCl 4.7 mM, CaCl2 2.5 mM, MgSo4 1.2 mM, Glucose 10 mM, NaHco3 25 mM and KH2Po4 1.2 Mm gassed with 95 % O2 and 5 % Co2. The perfusion pressure was maintained at 70-80 mmHg. Next, a water filled balloon was inserted into the left ventricle to monitor intraventricular pressure through Narko pressure transducer connected to the powerlab data acquisition system (Lab chart 6, ADIstrument, Australia). The hearts were warmed by a water filled jacket. Afterwards, coronary flow was collected and measured at certain time points. Any heart with systolic pressure less than 80 mmHg or severe disrrhythmia was excluded.
Ischemic-reperfusion injury
A 5-0 silk suture was passed around the left anterior descending coronary artery (LAD). After 20 min stabilization to induce ischemia, both ends of the thread were passed through a polyethylene tube and held tightly in place with a small clip for 30 min. Then, the LAD was reoccluded to reperfuse the ischemic area for 120 min.
Measurement of infarct size
At the end of reperfusion, the thread was tightened again and the hearts were perfused by 1 % Evans Blue to separate ischemic and non-ischemic areas. Then, the heart was freezed and cut into 2 mm slices. Next, the slices were stained with triphenyltetrazolium chloride (TTC) that shows the viable cells as red and the infarcted cells as white color. The slices were photographed and the infarct size area was determined by Image Tool software. Finally, infarct size was expressed as percentage of ischemic area.
Assessment of ischemic and reperfusion arrhythmia
Electrocardiogram (ECG) was monitored by placing two electrodes on the right atrium and the apex of the hearts. Arrhythmia were monitored during ischemia and reperfusion and analyzed separately according to the Lambeth conventions (Walker et al., 1988[33]): ventricular ectopic beats (VEBs) as a wide premature QRS complex without P wave, ventricular tachycardia (VT) as a run of four or more VEBs and ventricular fibrillation (VF) was considered as a signal in which the individual QRS complex can no longer be distinguishable. Complex forms (bigenimy and salvos) were added to VEB counts as well as their episodes. In addition, severity of arrhythmia was quantified by a scoring system (Pourkhalili et al., 2009[24]) in which VEBs alone were given score of 1, bigenimy/salvos score of 2, ventricular tachycardia score of 3, ventricular fibrillation score of 4, SVF (ventricular fibrillation lasting more than 120 seconds) score of 5. The allocated number was the most severe type of arrhythmia observed in that heart.
Experimental groups
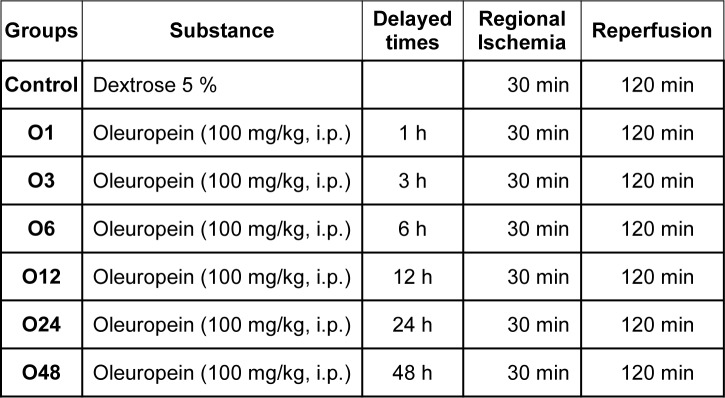
56 rats were divided into 7 groups as follows (Table 1(Tab. 1)): Group 1, rats were given 1 ml 5 % dextrose (vehicle) and their hearts were only subjected to 30 min ischemia and 120 min reperfusion Group 2 to 7, rats were given oleuropein (100 mg/kg, i.p.) 1, 3, 6, 12, 24 and 48 h before their hearts subjected to 30 min ischemia and 120 min reperfusion under Langendorff apparatus. This dose was chosen according to the previous studies on heart (Andreadou et al., 2007[3]) which did not have any significant effect on systolic and diastolic pressure in anesthetized rats in our pilot study (data not shown). Andreadou et al. have shown that intraperitoneal administration of 100 and 200 mg/kg oleuropein 2 days before intraperitoneal administration of doxorubicin could attenuate doxorubicin-induced cardiotoxicity and there was not any difference between the cardioprotective effects of these doses. As previously noted, we wanted to know whether this dose of oleuropein (100 mg/kg, i.p) has cardioprotective and preconditioning like effects independent of the changes in hemodynamic parameters. So, we chose only a single dose of oleuropein before ischemia to induce preconditioning.
Table 1. Table1: The experimental groups.
Statistics
Data were shown as Mean ± SEM or the percentage of incidence. Heart rate (HR), left ventricular end diastolic pressure (LVEDP), left ventricular developed pressure (LVDP) and coronary flow (CF) were analyzed by two-way ANOVA repeated measure test with time as one variable and treatment as another variable. Infarct size, VEBs episodes, VT and VF numbers and durations were analyzed by one-way ANOVA with Tukey test as post hoc test. The incidence of VT and VF were analyzed by Fisher Exact test. The scores of arrhythmia were analyzed by non-parametric Kraskul-Wallis test. Finally, P values less than 0.05 were considered to be statistically significant.
Results
Hemodynamic parameters
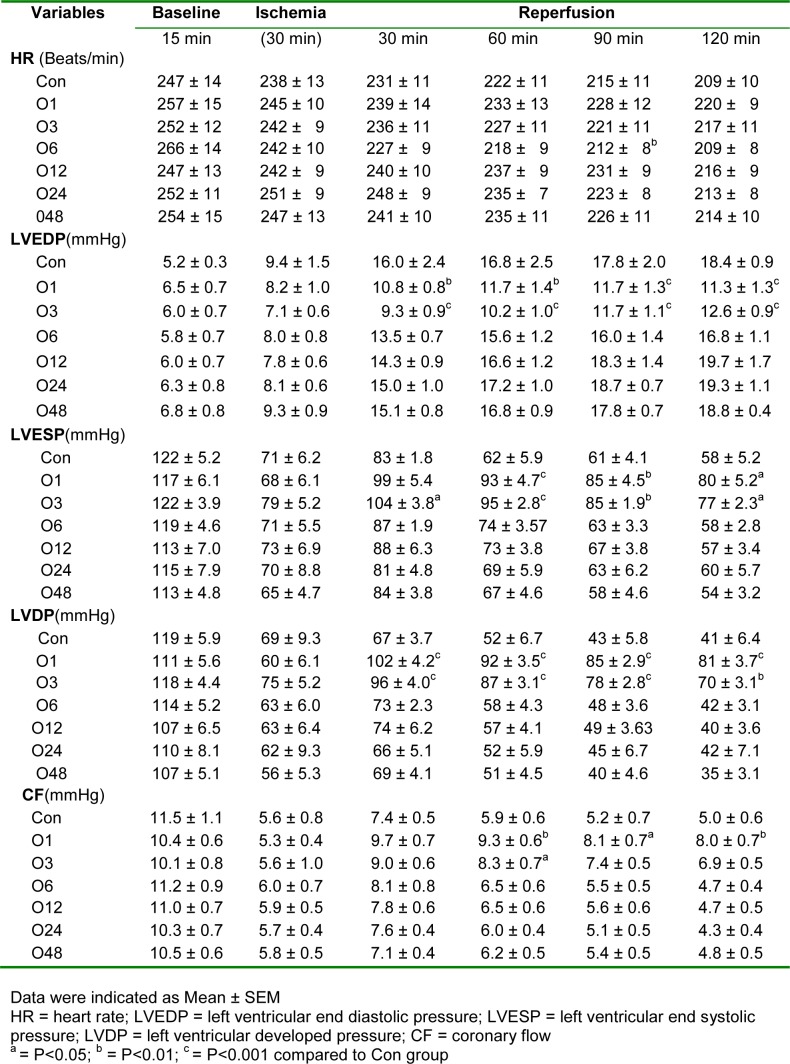
These parameters, including heart rate, LVEDP, left ventricular end systolic pressure (LVESP), LVDP and CF, were monitored during baseline, at the end of 30 min ischemia and every 30 min during reperfusion. As Table 2(Tab. 2) shows, although the heart rate reduced significantly during the ischemic and reperfusion procedure, there was not any significant difference among all groups. LVEDP had not any marked difference between groups at baseline time, however, it was increased slightly during ischemia with no significancy in all groups. During reperfusion, LVEDP was increased severely in all groups. In comparison to the control (Con) group, LVEDP was increased with less severity in groups O1 and O3. LVESP had not any considerable difference at baseline and ischemic times among groups. But it was significantly increased during reperfusion which was significantly higher in O1 and O3 groups than the Con group. Similarly, LVDP was decreased markedly during ischemia and increased again during reperfusion. But LVDP was significantly higher in O1 and O3 groups than the Con group during reperfusion. CF has been considerably decreased during ischemia and increased again during reperfusion. Although, these data show that CF is higher in O1 and O3 groups, there was only a significant difference between O1 and Con groups.
Table 2. Hemodynamic parameters.
Ischemic-induced arrhythmia
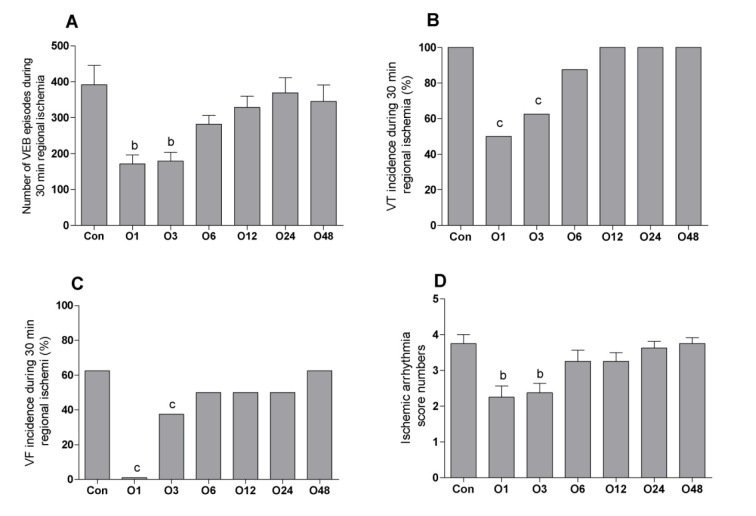
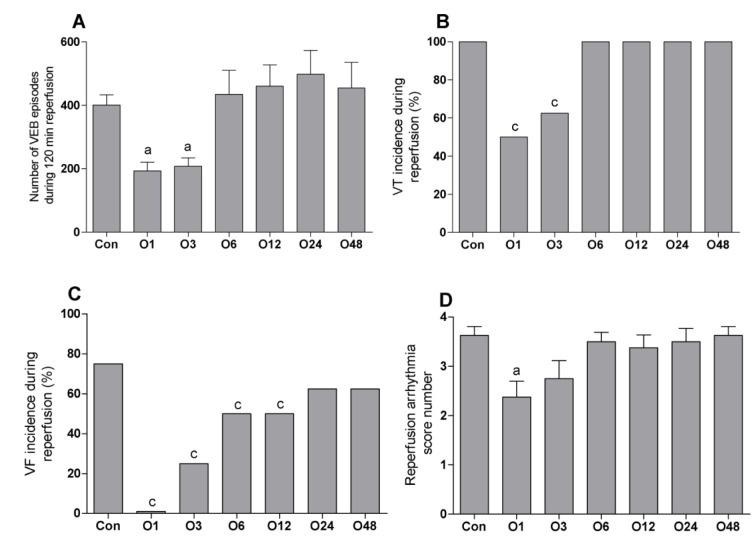
Figure 1(Fig. 1) shows the episodes of VEBs, the incidence percentages of VT and VF and the scores of arrhythmia during 30 min regional ischemia. The episodes of VEBs were decreased significantly from 391 ± 57 in the Con to 171 ± 25 and 179 ± 24 in O1 and O3 groups, respectively. There was not any marked difference between O6 (281 ± 24), O12 (329 ± 30), O24 (369 ± 42) and O48 (345 ± 45) groups than the Con group (Figure 1A(Fig. 1)). The incidence of VT% was decreased significantly from 100 % in the Con group to 50 and 62.5 % in O1 and O3 groups, respectively. The incidence of VT% was 87.5, 100, 100 and 100 % in O6, O12, O24 and O48 groups, respectively (Figure 1B(Fig. 1)). VF% was 62.5 % in Con group and decreased significantly to 0 and 37.5 % in O1 and O3 groups, respectively. There was not any considerable different between the incidence of VF in O6 (50 %), O12 (50 %), O24 (50 %) and O48 (62.5 %) groups (Figure 1C(Fig. 1)). Similarly, the score number of ischemic arrhythmia was only significantly decreased in O1 (2.25 ±0.25) and O3 (2.37 ± 0.26) than Con group (3.75 ± 0.25). It was 3.2 ± 0.31, 3.2 ± 0.25, 3.6 ± 0.18 and 3.7 ± 0.16 in O6, O12, O24 and O48 groups, respectively (Figure 1D(Fig. 1)).
Figure 1. Effect of intraperitoneal administration of a single dose of oleuropein on the magnitude of ischemic arrhythmia in isolated rat hearts at different times following the administration of oleuropein. VEB, ventricular ectopic beat; VT, ventricular tachycardia; VF, ventricular fibrillation; Con, control group and O1, O3, O6, O12, O24 and O48 means rats were pretreated with a single dose of intraperitoneal oleuropein (100 mg/kg, i.p.) 1, 3, 6, 12, 24 and 48 before excision of the heart. b = P<0.01 and c = P<0.001 compared to con group.
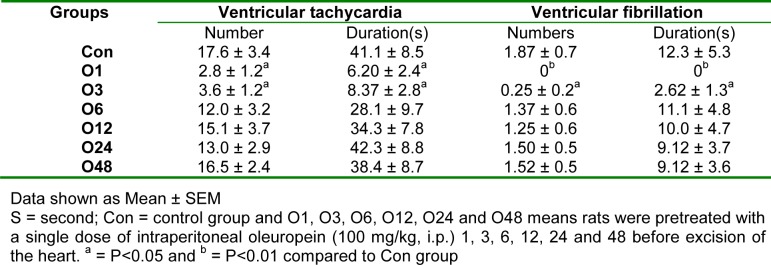
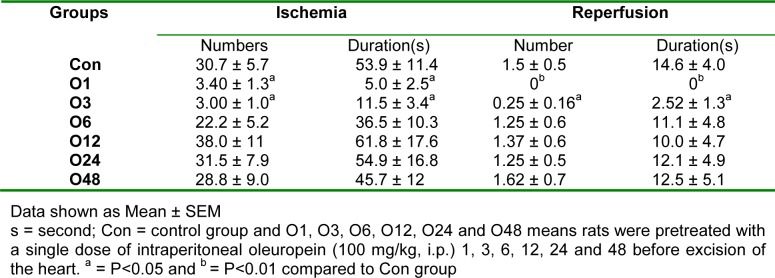
Table 3(Tab. 3) indicates that there was only a significant difference in VT and VF numbers and duration between O1 and O3 groups compared to the Con group.
Table 3. Effect of intraperitoneal administration of a single dose of oleuropein on the ventricular tachycardia and ventricular fibrillation numbers and durations during 30 min regional ischemia in isolated rat hearts.
Reperfusion-induced arrhythmias
Figure 2(Fig. 2) presents the episodes of VEBs, the incidence percentage of VT and VF and the arrhythmia scores during 120 min reperfusion following 30 min regional ischemia. VEBs episodes were markedly decreased in O1 (193 ± 27) and O3 (207 ± 26) compared to the Con group (400 ± 32). There was not any significant difference between other groups and the Con group. It was 434 ± 76, 460 ± 67, 497 ± 75, and 454 ± 81 in O6, O12, O24 and O48 groups, respectively (Figure 2A(Fig. 2)). The incidence percentage of VT also significantly decreased in O1 (50 %) and O3 (62.5 %) groups compared to the Con group (100%) and it was occurred in all hearts of O6, O12, O24 and O48 groups (Figure 2B(Fig. 2)). The incidence of VF was considerably deceased in O1 (0 %), O3 (25 %), O6 (50 %) and O12 (50 %) groups compared to the Con group. The incidence of VF was 62.5 % in O24 group and 62.5 % in O48 group (Figure 2C(Fig. 2)). Figure 2D(Fig. 2) shows that the score of arrhythmia was only decreased significantly in O1 group (2.37 ± 0.32) than the Con group (3.62 ± 0.18). It was 2.75 ± 0.32 in O3, 3.50 ± 0.19 in O6, 3.37 ± 0.26 in O12, 3.50 ± 0.26 in O24 and 3.62 ± 0.18 in O48 groups, respectively.
Figure 2. Effect of intraperitoneal administration of a single dose of oleuropein on the magnitude of reperfusion arrhythmia in isolated rat hearts at different times following the administration of oleuropein. VEB, ventricular ectopic beat; VT, ventricular tachycardia; VF, ventricular fibrillation; Con, control group and O1, O3, O6, O12, O24 and O48 means rats were pretreated with a single dose of intraperitoneal oleuropein (100 mg/kg, i.p.) 1, 3, 6, 12, 24 and 48 before excision of the heart. a = P<0.05 and c = P<0.001 compared to con group.
Table 4(Tab. 4) shows the VT and VF numbers and durations during 120 min reperfusion. Compared to the Con group, they were only significantly reduced in O1 and O3 groups.
Table 4. Effect of intraperitoneal administration of a single dose of oleuropein on the ventricular tachycardia and ventricular fibrillation numbers and durations during 120 min reperfusion following 30 min regional ischemia in isolated rat hearts.
Infarct size assessment
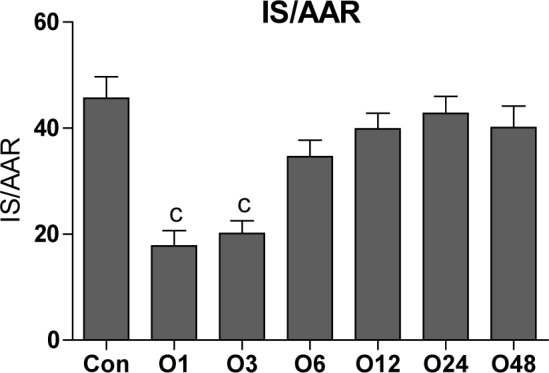
The same as VF and VT incidence percentages, the mean of the infarct size was significantly decreased in O1 and O3 groups compared to the Con group (45.6 ± 4). The infarct size was 17.8 ± 2.9, 20.1 ± 2.4, 34.6 ± 3.1, 39.9 ± 3, 42.7 ± 3.2 and 40.1 ± 4.1 in O1, O3, O6, O12, O24 and O48 groups, respectively (Figure 3(Fig. 3)).
Figure 3. Effect of intraperitoneal administration of a single dose of oleuropein on the myocardial infarct size induced by 30 min regional ischemia and 120 min reperfusion in isolated rat hearts at different times following the administration of oleuropein. Con, control group and O1, O3, O6, O12, O24 and O48 means rats were pretreated with a single dose of intraperitoneal oleuropein (100 mg/kg, i.p.) 1, 3, 6, 12, 24 and 48 before excision of the heart. c = P<0.001 compared to con group.
Discussion
The main findings of this study show that the intraperitoneal administration of a single dose of oleuropein (100 mg/kg) has a cardioprotective, but not preconditioning effect against ischemic-reperfusion injury in isolated rat heart for about three hours. This protective effect was evident with reduced infarct size, diminished the magnitude of ischemic and reperfusion arrhythmia and improved cardiac dysfunction. Acute coronary syndromes as a consequence of ischemic injuries to the myocardium are still responsible for a large proportion of all hospital administrations and all causes of death around the world (Williams and Benjamin, 2000[35]; Hausenloy and Yellon, 2007[10]; Huikuri et al., 2001[11]; Misra et al., 2009[20]). The malignant arrhythmia, including VT and VF, are still the major complications during acute phase of myocardial infarction (Kaneko et al., 2009[15]). Although the relief of related ischemia by coronary artery bypass graft (CABG) could reduce the incidence of arrhythmia, it seems that CABG itself exerts effects on arrhythmiogenic substrates (Wu et al., 2003[36]). It appears many factors involve in the initiation of arrhythmia, but the main factor is the deficiency of the myocardial defense system (Wu et al., 2003[36]). It has been suggested that the generation of a large quantity of free radicals plays an important role in the establishment of ischemic and reperfusion arrhythmia. Hence, administration of free radical scavengers has antiarrhythmic effects (Somova et al., 2004[30]). Since the heart has low regenerative ability (Molojavyi et al., 2001[21]), increasing the antioxidant defense system of the myocardium has a great relevance. In this study, we wanted to know whether the intraperitoneal administration of a single dose of oleuropein would have cardioprotective and preconditioning-like effects in isolated rat hearts. If so, how long does it take? Preconditioning is a phenomenon thereby short period(s) of sublethal ischemia or administration of a single dose of some drugs make the heart more tolerant against the subsequent prolonged ischemia (Murry et al., 1986[22]; Baharvand et al., 2010[4]). Preconditioning has two phases: early and delayed phases. The early phase, initiates immediately after the insertion of the stimulus and ends 3 hours later, is caused by post-translation of proteins. The delayed phase starts 12-24 hours following the insertion of the stimulus and continues up to about 72 hours later, is related to the gene expression (Baharvand et al., 2010[4]). Epidemiological data have indicated that the lower incidence of cardiovascular diseases and cancers in Mediterranean area is associated with high consumption of natural phenolic antioxidants, especially oleuropein, through their traditional diet (Waterman and Lockwood, 2007[34]). Oleuropein is the main constituent of olive leaf extracts that has anti-inflammatory (Puel et al., 2004[26]), antidiabetic (Al-Azzawie and Alhamdani, 2006[1]; Jemai et al., 2009[13]), anti-atherosclerosis (Coni et al., 2000[6]; Visioli and Galli, 1994[32]), antimicrobial (Tranter et al., 1993[31]; Fleming et al., 1973[7]) and anti-tumoral (Hamdi and Castellon, 2005[8]) effects. Although most studies have attributed these effects to the antioxidant property of oleuropein (Coni et al., 2000[6]; Visioli and Galli, 1994[32]; Kruk et al., 2005[17]), some studies also reported that oleuropein could change intracellular signaling pathway (Sato et al., 2007[28]; Santiago-Mora et al., 2011[27]). Our data showed that intraperitoneal administration of a single dose of oleuropein had anti-ischemic effects that were evident with reduced malignant VT and VF arrhythmia during ischemia and reperfusion, reduced infarct size and improved cardiac dysfunctions during reperfusion. This study showed that a single dose of oleuropein also has a cardioprotective effect that is consistent with Petkov and Manolov's work. They reported that the intravenous administration of a single dose of oleuropein (40 mg/kg) reduced the magnitude of calcium chloride and aconitine-induced arrhythmia in anaesthetized rats. They also addressed that this dose of oleuropein had a hypotensive effect in cats and dogs, but not in rabbits, as a dose dependent manner (Petkov & Manolov, 1978[23]). But in our study, oleuropein (100 mg/kg) had not any effect on blood pressure before isolation of the heart, and on the baseline coronary flow under Langendorff apparatus, which may be due to the manner of oleuropein injection. On the other hand, we injected 40 mg/kg oleuropein into the tail vein of three anaesthetized rats for 1 minute. We observed that blood pressure reduced immediately following oleuropein injection and rapidly return to baseline values (data not shown). We do not have any explanation for this transient hypotensive effect. There are a few studies about oleuropein and heart. Andreadou et al. (2007[3]) reported that pretreatment of rats with 100 and 200 mg/kg intraperitoneal oleuropein doses for two days attenuated the doxorubicin-induced cardiotoxicity through its antioxidant activity. The difference between these doses was not statistically significant. In our study, only one dose of 100 mg/kg oleuropein was administered that its cardioprotective effects last for 3 hours. It seems these effects are related to its antioxidant activity and it does not seem oleuropein altered the intracellular signaling pathways. During ischemia and reperfusion, the generation of oxygen free radicals increase in very high quantities that the antioxidant defense mechanism of the heart could not scavenge them (Krematinos, 2008[16]). Hence, it seems that oleuropein in our study has increased the antioxidant activity of the myocardium. There are two other studies about cardioprotective effect of oleuropein, which are different from our study. Manna et al. (2004[19]) using isolated rat heart model reported that perfusing the isolated rat hearts, with 50 µg/g wet weight of oleuropein for 15 min before global ischemia, had anti-ischemic and antioxidant effects. They have not reported any effect related to arrhythmia and cardiac dysfunctions. Andreadou et al. (2006[2]) indicated the anti-infarct and anti-ischemic effects of oleuropein in anesthetized rabbit heart model. In their study, rabbits were pretreated orally with 10-20 mg/kg oleuropein for 3 weeks which was different with the design of our study. These two studies concluded that oleuropein exerted its effects via its antioxidant property (Andreadou et al., 2006[2]; Manna et al., 2004[19]). On the other hand, it appears that oleuropein could not precondition the heart against lethal ischemia. Because it was not observed two phases of cardioprotection which is seen in preconditioning phenomenon: early and delayed phases (Andreadou et al., 2006[2]; Hausenloy and Yellon, 2008[9]; Luh and Yang, 2006[18]; Murry et al., 1986[22]). Also, some data show that oleuropein could change the intracellular pathways, especially the expression of some genes (Sato et al., 2007[28]; Santiago-Mora et al., 2011[27]). Then, further studies need to determine the effect of oleuropein in another animal species with different models. Another important point of our study is that all of the cardioprotective effects of a single dose of intraperitoneal oleuropein stopped 6 hours later. It appears that the plasma concentration of oleuropein decreased below the level of its cardioprotective dose. Previous studies reported that following its absorption from the gastrointestinal tract, oleuropein rapidly distributed throughout the body and metabolized mainly in the conjugated forms and then excreted in the urine (Jemai et al., 2008[12]; Luh and Yang, 2006[18]; Bazoti et al., 2010[5]). In this study, we did not measure the plasma concentration of oleuropein, hence further in vivo studies need to be determined; 1) How does the plasma concentration of oleuropein change in various times following its administration? 2) How long do the cardioprotective effects of oleuropein last for? And 3) Whether oleuropein has cardiac preconditioning in in vivo models?
In summary, our study emphasizes that a single dose of intraperitoneal administration of oleuropein (100 mg/kg) has anti-infarct, antistunning and antiarrhythmic, but no preconditioning effects in rat hearts. Hence, it is likely that future studies could prove the beneficial effects of oleuropein in managing of coronary heart disease, ischemic-reperfusion injuries and cardiac surgery in human beings.
Acknowledgement
We are great to thank the Vice-chancellors of Shahid Sadoughi University of Medical Sciences of Yazd and Khorramabad Medical University that provided us a grant.
Conflict of interests
The authors declare that they have no competing interests.
References
- 1.Al-Azzawie HF, Alhamdani MS. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci. 2006;78:1371–1377. doi: 10.1016/j.lfs.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 2.Andreadou I, Iliodromitis EK, Mikros E, Constantinou M, Agalias A, Magiatis P, et al. The olive constituent oleuropein exhibits anti-ischemic, antioxidative, and hypolipidemic effects in anesthetized rabbits. J Nutr. 2006;136:2213–2219. doi: 10.1093/jn/136.8.2213. [DOI] [PubMed] [Google Scholar]
- 3.Andreadou I, Sigala F, Iliodromitis EK, Papaefthimiou M, Sigalas C, Aligiannis N, et al. Acute doxorubicin cardiotoxicity is successfully treated with the phytochemical oleuropein through suppression of oxidative and nitrosative stress. J Mol Cell Cardiol. 2007;42:549–558. doi: 10.1016/j.yjmcc.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Baharvand B, Dehaj ME, Foadaddini M, Rasoulian B, Poorkhalili K, Aghai HW, et al. Delayed cardioprotective effects of hyperoxia preconditioning prolonged by intermittent exposure. J Surg Res. 2010;160:53–59. doi: 10.1016/j.jss.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 5.Bazoti FN, Gikas E, Tsarbopoulos A. Simultaneous quantification of oleuropein and its metabolites in rat plasma by liquid chromatography electrospray ionization tandem mass spectrometry. Biomed Chromatogr. 2010;24:506–515. doi: 10.1002/bmc.1319. [DOI] [PubMed] [Google Scholar]
- 6.Coni E, Di BR, Di PM, Masella R, Modesti D, Mattei R, et al. Protective effect of oleuropein, an olive oil biophenol, on low density lipoprotein oxidizability in rabbits. Lipids. 2000;35:45–54. doi: 10.1007/s11745-000-0493-2. [DOI] [PubMed] [Google Scholar]
- 7.Fleming HP, Walter WM, Jr , Etchells JL. Antimicrobial properties of oleuropein and products of its hydrolysis from green olives. Appl Microbiol. 1973;26:777–782. doi: 10.1128/am.26.5.777-782.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamdi HK, Castellon R. Oleuropein, a non-toxic olive iridoid, is an anti-tumor agent and cytoskeleton disruptor. Biochem Biophys Res Commun. 2005;334:769–778. doi: 10.1016/j.bbrc.2005.06.161. [DOI] [PubMed] [Google Scholar]
- 9.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: new strategies for cardioprotection. Diabetes Obes Metab. 2008;10:451–459. doi: 10.1111/j.1463-1326.2007.00762.x. [DOI] [PubMed] [Google Scholar]
- 10.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: united at reperfusion. Pharmacol Ther. 2007;116:173–191. doi: 10.1016/j.pharmthera.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 12.Jemai H, Bouaziz M, Fki I, El FA, Sayadi S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem Biol Interact. 2008;176:88–98. doi: 10.1016/j.cbi.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Jemai H, El FA, Sayadi S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J Agric Food Chem. 2009;57:8798–8804. doi: 10.1021/jf901280r. [DOI] [PubMed] [Google Scholar]
- 14.Jiang JH, Jin CM, Kim YC, Kim HS, Park WC, Park H. Anti-toxoplasmosis effects of oleuropein isolated from Fraxinus rhychophylla. Biol Pharm Bull. 2008;31:2273–2276. doi: 10.1248/bpb.31.2273. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko H, Anzai T, Naito K, Kohno T, Maekawa Y, Takahashi T, et al. Role of ischemic preconditioning and inflammatory response in the development of malignant ventricular arrhythmias after reperfused ST-elevation myocardial infarction. J Cardiac Failure. 2009;15:775–781. doi: 10.1016/j.cardfail.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Kremastinos DTH. Olive and oleuropein. Hellenic J Cardiol. 2008;49:295–296. [PubMed] [Google Scholar]
- 17.Kruk I, boul-Enein HY, Michalska T, Lichszteld K, Kladna A. Scavenging of reactive oxygen species by the plant phenols genistein and oleuropein. Luminescence. 2005;20:81–89. doi: 10.1002/bio.808. [DOI] [PubMed] [Google Scholar]
- 18.Luh SP, Yang PC. Organ preconditioning: the past, current status, and related lung studies. J Zhejiang Univ Sci B. 2006;7:331–341. doi: 10.1631/jzus.2006.B0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manna C, Migliardi V, Golino P, Scognamiglio A, Galletti P, Chiariello M, et al. Oleuropein prevents oxidative myocardial injury induced by ischemia and reperfusion. J Nutr Biochem. 2004;15:461–466. doi: 10.1016/j.jnutbio.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Misra MK, Sarwat M, Bhakuni P, Tuteja R, Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15:RA209–RA219. [PubMed] [Google Scholar]
- 21.Molojavyi A, Preckel B, Comfere T, Mullenheim J, Thamer V, Schlack W. Effects of ketamine and its isomers on ischemic preconditioning in the isolated rat heart. Anesthesiology. 2001;94:623–629. doi: 10.1097/00000542-200104000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 23.Petkov V, Manolov P. Pharmacological studies on substances of plant origin with coronary dilatating and antiarrhythmic action. Comp Med East West. 1978;6:123–130. doi: 10.1142/s0147291778000198. [DOI] [PubMed] [Google Scholar]
- 24.Pourkhalili K, Hajizadeh S, Tiraihi T, Akbari Z, Esmailidehaj M, Bigdeli MR, et al. Ischemia and reperfusion-induced arrhythmias: role of hyperoxic preconditioning. J Cardiovasc Med. 2009;10:635–642. doi: 10.2459/JCM.0b013e32832997f3. [DOI] [PubMed] [Google Scholar]
- 25.Puel C, Mathey J, Agalias A, Kati-coulibaly S, Mardon J, Obled C, et al. Dose-response study of effect of oleuropein, an olive oil polyphenol, in an ovariectomy/inflammation experimental model of bone loss in the rat. Clin Nutr. 2006;25:859–868. doi: 10.1016/j.clnu.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Puel C, Quintin A, Agalias A, Mathey J, Obled C, Mazur A, et al. Olive oil and its main phenolic micronutrient (oleuropein) prevent inflammation-induced bone loss in the ovariectomised rat. Br J Nutr. 2004;92:119–127. doi: 10.1079/BJN20041181. [DOI] [PubMed] [Google Scholar]
- 27.Santiago-Mora R, Casado-Díaz A, De Castro MD, Quesada-Gómez JM. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: the effect on differentiation in stem cells derived from bone marrow. Osteoporos Int. 2011;22:675–684. doi: 10.1007/s00198-010-1270-x. [DOI] [PubMed] [Google Scholar]
- 28.Sato H, Genet C, Strehle A, Thomas C, Lobstein A, Wagner A, et al. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun. 2007;362:793–798. doi: 10.1016/j.bbrc.2007.06.130. [DOI] [PubMed] [Google Scholar]
- 29.Singh I, Mok M, Christensen AM, Turner AH, Hawley JA. The effects of polyphenols in olive leaves on platelet function. Nutr Metab Cardiovasc Dis. 2008;18:127–132. doi: 10.1016/j.numecd.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Somova LI, Shode FO, Mipando M. Cardiotonic and antidysrhythmic effects of oleanolic and ursolic acids, methyl maslinate and uvaol. Phytomedicine. 2004;11:121–129. doi: 10.1078/0944-7113-00329. [DOI] [PubMed] [Google Scholar]
- 31.Tranter HS, Tassou SC, Nychas GJ. The effect of the olive phenolic compound, oleuropein, on growth and enterotoxin B production by Staphylococcus aureus. J Appl Bacteriol. 1993;74:253–259. doi: 10.1111/j.1365-2672.1993.tb03023.x. [DOI] [PubMed] [Google Scholar]
- 32.Visioli F, Galli C. Oleuropein protects low density lipoprotein from oxidation. Life Sci. 1994;55:1965–1971. doi: 10.1016/0024-3205(94)00529-x. [DOI] [PubMed] [Google Scholar]
- 33.Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, et al. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- 34.Waterman E, Lockwood B. Active components and clinical applications of olive oil. Altern Med Rev. 2007;12:331–342. [PubMed] [Google Scholar]
- 35.Williams RS, Benjamin IJ. Protective responses in the ischemic myocardium. J Clin Invest. 2000;106:813–818. doi: 10.1172/JCI11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu ZK, Iivainen T, Pehkonen E, Laurikka J, Tarkka MR. Perioperative and postoperative arrhythmia in three-vessel coronary artery disease patients and antiarrhythmic effects of ischemic preconditioning. Eur J Cardiothoracic Surg. 2003;23:578–584. doi: 10.1016/s1010-7940(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 37.Zanichelli D, Baker TA, Clifford MN, Adams MR. Inhibition of Staphylococcus aureus by oleuropein is mediated by hydrogen peroxide. J Food Prot. 2005;68:1492–1496. doi: 10.4315/0362-028x-68.7.1492. [DOI] [PubMed] [Google Scholar]
- 38.Zhao G, Yin Z, Dong J. Antiviral efficacy against hepatitis B virus replication of oleuropein isolated from Jasminum officinale L. var. grandiflorum. J Ethnopharmacol. 2009;125:265–268. doi: 10.1016/j.jep.2009.06.030. [DOI] [PubMed] [Google Scholar]