Abstract
Responsible development of any technology, including nanotechnology, requires protecting workers, the first people to be exposed to the products of the technology. In the case of nanotechnology, this is difficult to achieve because in spite of early evidence raising health and safety concerns, there are uncertainties about hazards and risks. The global response to these concerns has been the issuance by authoritative agencies of precautionary guidance to strictly control exposures to engineered nanomaterials (ENMs). This commentary summarizes discussions at the “Symposium on the Health Protection of Nanomaterial Workers” held in Rome (25 and 26 February 2015). There scientists and practitioners from 11 countries took stock of what is known about hazards and risks resulting from exposure to ENMs, confirmed that uncertainties still exist, and deliberated on what it would take to conduct a global assessment of how well workers are being protected from potentially harmful exposures.
Keywords: Control procedures, occupational exposure limits, precautionary guidance, toxicity
For almost two decades that the current generation of ENMs has been in commerce, there have been increasing numbers of workers involved in the research, manufacture, production, and use of them. There are more than 1600 “nanoenabled” products in commerce, all required workers for that to happen (http://www.nanotechproject.org/cpi/). This creation and utilization of ENMs occurred ahead of government, employer, and worker knowledge about potential hazards. However, based on the concerns about small particles (ultrafines) in air pollution epidemiology, known health effects of incidental nanoparticles such as diesel and welding fumes, and results of early range-finding animal studies, there was a wave of precautionary guidance issued in the mid-2000s by governments, organizations, and scientists (Table 1) (Aitken et al., 2004; Ambroise et al., 2006; Antonini et al., 2007; BauA, 2007; Borm et al., 2006; Castranova 2000; Chalupa et al., 2004; Dockery et al., 1993; Donaldson et al., 2005; Environmental Defense Fund, 2007; Garshick et al., 2004; Gwinn & Vallyathan, 2006; Hett, 2004; HSE, 2004; Ibald-Mulli et al., 2002; Maynard & Kuempel, 2005; NIOSH, 2005; Oberdörster & Yu, 1990; Oberdörster et al., 2005; Ostiguy et al., 2006; Pietropaoli et al., 2004; Pope et al., 2002, 2004; Royal Society and Royal Academy of Engineering, 2004; Safe Work Australia, 2010; Shvedova et al., 2005; Tran et al., 2005). This guidance essentially called for strict control of exposure to ENMs until more was learned about any actual hazards and adverse health effects. The issue was complicated due to the vast heterogeneity of ENMs that can result from the combination of numerous physico-chemical properties (e.g. size, shape, composition, crystal structure, surface characteristics, and impurities) and the fact that there could be a range of toxic potentials associated with this universe of materials. Nonetheless, since airborne ENMs follow the laws of aerosol physics, control technologies used for fine dusts and powders and pharmaceuticals were believed to be, and ultimately shown to be, effective in controlling exposure (NIOSH, 2005; Plitzko et al., 2013). In the ensuing years since the early guidance was issued, more information on hazards of ENMs was developed but still there are many uncertainties and the need for precautionary approaches to exposure control and worker protection is still warranted (Schulte et al., 2014).
Table 1.
Precautionary guidance on ENMs by authoritative organizations.
| Source | Precautionary guidance on ENMs by authoritative organizations |
|---|---|
| Royal Society and The Royal Academy of Engineering (2004) | “We believe that chemicals in the form of nanoparticles and nanotubes should be treated separately to those in a larger form … while HSE performs a wider review of the adequacy of current regulation to assess and control workplace exposure … we have recommended that it consider setting lower occupational exposure levels …” p. 82 |
| NIOSH (2005) | “Given the limited amount of information about health risks that may be associated with nanomaterials, taking measures to minimize worker exposure is prudent.” p. vii |
| IRRST (Ostiguy, 2010) | “Production and use of NM can signify different types of risks… It is essential that the senior management of every establishment makes OSH an action priority…” p. 63 “Given the current absence of standards and many uncertainties related to toxicity… it is recommended that a precautionary approach be adopted to NM production handling, storage, transportation and use control plans.” p. 69 |
| BAuA (2007) | “… it cannot be ruled out that exposure to nanomaterials might have specific effects different to the effects in the micrometer range … Until specific limit values are laid down for nanoparticles … it should therefore be striven to minimize exposure.” p. 5 |
| Netherlands (SER, 2009) | “The basic principles are that substances attended by uncertain or unknown risks which include nanoparticles – should be treated as hazardous (or extremely hazardous) substances.” p. 7 http://www.ser.nl/en/publications/publications/2009/2009_01.aspx |
Regarding the protection of the nanomaterial workforce, it is one thing to know what to do, it is another to know whether it is being done. A core element of public health in general, and occupational health in particular, is the evaluation of the use of recommended control procedures (Hanlon, 1974; Levy & Wegman, 1983). The evaluation of the nanomaterial workforce protection involves surveillance of hazards and controls to answer the question “Are employers worldwide following the precautionary guidance of authoritative organizations and strictly controlling exposure to engineered nanomaterials?”
The answer to the question is complicated by the fact that nanotechnology is a decentralized enabling technology. There is no nanotechnology industry, but there are many applications that occur in companies throughout the world. The challenge is how to obtain information on which companies are producing, manufacturing, and using nanomaterials in a manner that could lead the generation of airborne nanomaterials in the breathing zone of workers. Some preliminary information from the U.S. based on a National Institute for Occupational Health (NIOSH) survey showed that in a subset of companies producing carbon nanotubes (CNTs) and other nanomaterials, there was 80% (95% CI: 63–92%) using respirators (Schubauer-Berigan et al., 2015). While this is not the most preferred control approach, it showed some indication of these companies following precautionary guidance. However, the extent to which the participants in the investigations were representative of all companies is unknown. Also, the extent to which respirators were used indicated a lack of following the hierarchy of controls which is the widely recommended approach for controlling occupational hazards (NIOSH, 2005; Plog & Quinlan, 2002). The hierarchy of controls includes elimination, substitution, engineering controls, administrative controls, and personal protection equipment (Schulte et al., 2008). In contrast, published exploratory studies from various countries showed relatively high levels of exposure to engineered nanomaterials measured in a variety of workplaces (Pietroiusti & Magrini, 2014). Therefore, it is still of great importance to identify globally the adherence to precautionary guidance to protect workers. To this end, a multistep process was initiated. The first step was the development by NIOSH of a survey instrument to assess adherence to precautionary controls and guidance. This instrument was developed and received public review (Federal Register, 2013). However, to apply it to the full complement of workplaces in the U.S. or globally is a difficult and expensive task because of the number and diversity of workplaces and potential reticence of employers to participate. The number and the location of workers exposed to ENMs in these workplaces are difficult to identify. Many companies that use nanomaterials do not identify themselves as such. Rather, nanotechnology is a part of their range of capabilities, or they identify themselves as producing “advanced materials”. Figure 1 shows the dilemma in trying to estimate the number of workers and their locations. Using the number of “nanoenabled” products (as derived by Project on Emerging Technologies at the Woodrow Wilson International Center for Scholars (http://www.nanotechproject.org)) as a surrogate for workers or locations, Figure 1 shows an increase in the slope of the trend (through 2020 and a straight line projection beyond). However, the number of workers or locations associated with each product is not known, could it be 10, 100, 1000, or 10 000? There is also a vast number of ENMs in or that potentially could be in commerce. Finding the locations where nanomaterials are manufactured and used is difficult not only for assessing adherence to precautionary guidance but also for identifying cohorts of similarly exposed workers for epidemiological investigations (Schmid et al., 2009; Schulte et al., 2009; Riediker et al., 2012).
Figure 1.
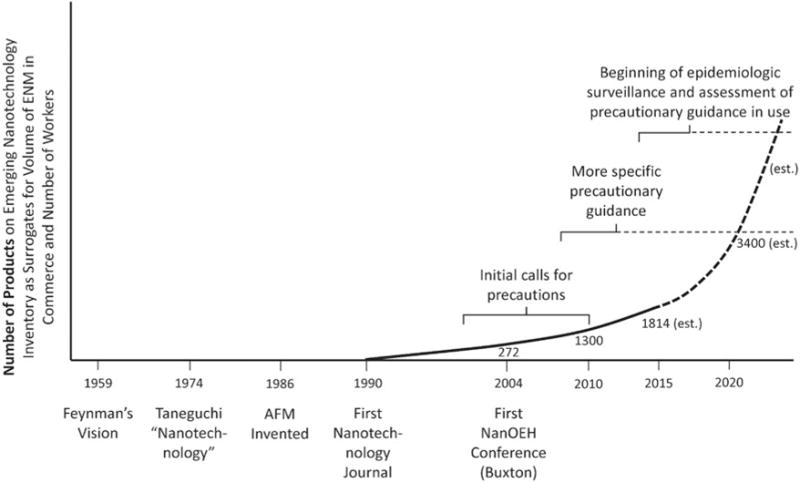
Number of nano-enabled products in commerce.
To further attempt to characterize the use of precautions globally, the International Commission on Occupational Health (ICOH), Scientific Committee on Nanomaterial Workers Health, proposed a global assessment as part of a work plan developed in 2012 and then held an international symposium in Rome in February 2015 on the health protection of nanomaterial workers. The symposium “Symposium on the Health Protection of Nanomaterial Workers” identified the state of knowledge about nanomaterial hazards, risks, and risk management as a prelude to developing a study to assess global adherence to precautionary guidance to control exposures to ENMs. This commentary is a summary of the discussions at the symposium.
Overview of the state of knowledge to protect workers
Figure 2 illustrates the cycle of steps to protect workers. It shows the worker protection is a multi-level and multifactorial effort. While research is a driver of the cycle in that it provides new information on hazards, another is risk management. Employers need to know what to do to protect workers even in the face of uncertainty. Two general approaches have been utilized to develop guidance (Figure 3) (Schulte et al., 2010). One involves the case where there are adequate physico-chemical and toxicity data. This can lead to specific guidance and occupational exposure limits such as those developed for CNTs (NIOSH, 2013). The other approach is where there is limited information on one substance or categories of substances and various pragmatic approaches are used. This is illustrated by the British Standards Institute (BSI) and NanoReference Values developed by the Dutch and Germans efforts and various risk-based grouping approaches (BSI, 2007; CEN, 2012; IFA, 2009; SER, 2009).
Figure 2.
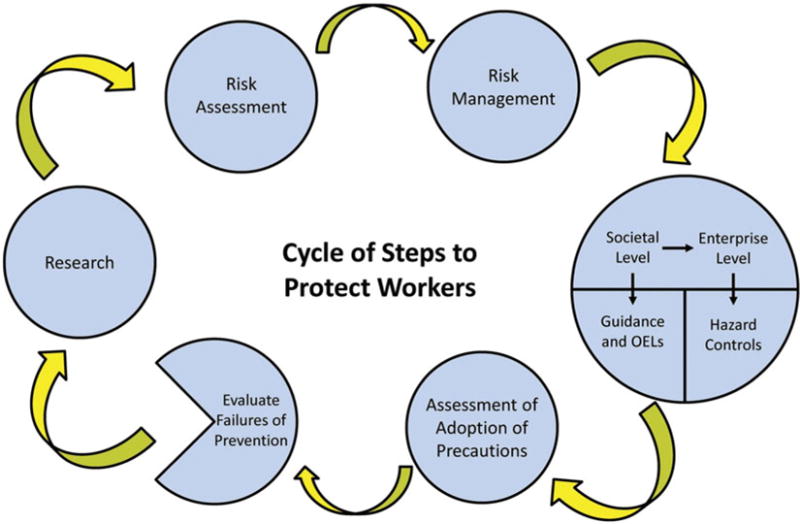
Cycle of steps to protect workers.
Figure 3.
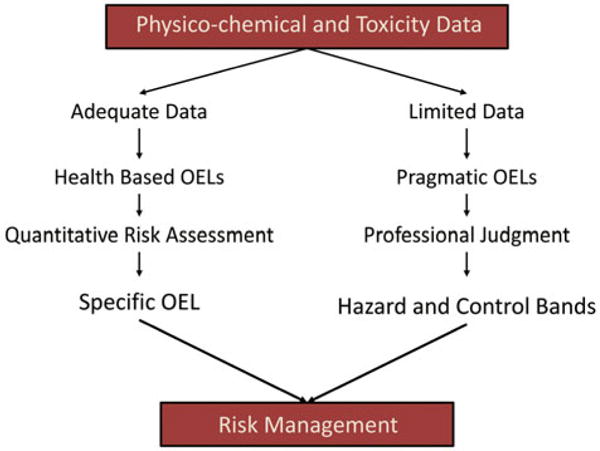
Pathways to risk management based on adequacy of data.
Underpinning the development of occupational exposure limits (OELs) and enterprise-level risk management is the need for appropriate exposure assessment. Exposure assessment is central for risk assessment since risk is a product of hazard and exposure. Exposure assessment is also necessary to inform the employer if the protective risk management activities are sufficient and if any relevant threshold limits are complied with. In threshold limit compliance, control data must be of sufficient quality as described, for example, in BS-EN-482 (2012) for Europe (CEN, 2012). This means that an appropriate time-base comparable with the threshold limit (i.e., normally 8 h shift or short-term 15 min) is necessary. An example of a standard protocol for exposure assessment is the NanoGem (a German-funded project on nanosafety, exposure, and risk (Asbach et al., 2015). A critical issue in measuring engineered nanomaterial exposure in the air is to determine if the measured exposure is significantly above background. Background emissions from equipment and sources in the workplace as well as from external environmental sources not related to ENMs can confound measurements. Many of these background emissions come from diesel engines, electric motors, and air pollution. Generally, they are carbonaceous (Dahm et al., 2015; Schauer, 2003). Background nanoparticles may be health hazards and should be considered in risk assessments of workplaces where ENM exposures are the focus. Figure 4 illustrates the various generic components of nanomaterial (nanoparticles) exposure in the workplace.
Figure 4.
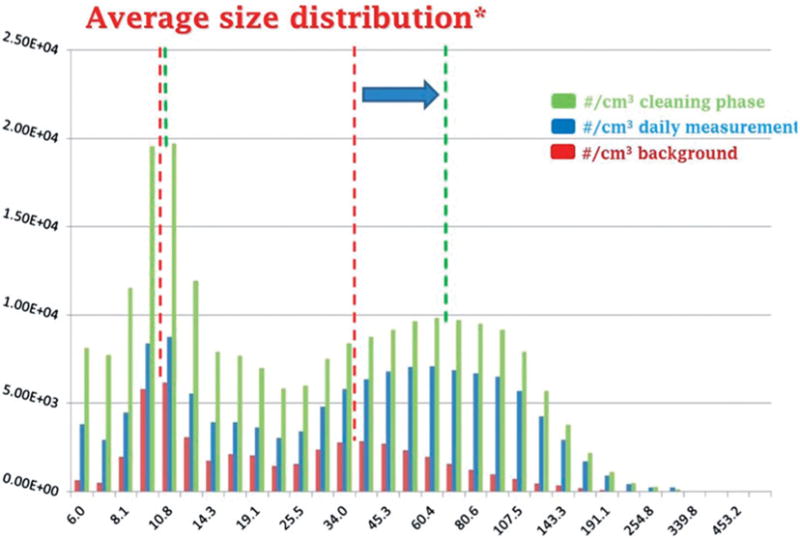
Relative counts of nanoparticles by size in a workplace exposure assessment.
One approach that may be useful to supplement exposure assessment is biological monitoring of nanomaterial workers (Bergamaschi et al., 2015; Erdely et al., 2011; Iavicoli et al., 2014). Biological monitoring such as assessing lead in blood in exposed workers is a tool with a long history in occupational health. There are various reasons for the expansion of biological monitoring in recent years, mainly due to its extensive use in occupational health risk assessment (Manno et al., 2010). One reason is represented by the development and the validation of new biomarkers of exposure. There is growing literature on the identification and measurement of ENMs in biological matrices, although methods are still rather complex and expensive (Iavicoli et al., 2014). Another reason in favor of biomonitoring is the discovery of increasingly sensitive and/or specific biomarkers of effect, which may be quite useful in assessing workers’ early responses to exposure. Available data obtained from laboratory animals and exposed workers suggest that biomarkers of effect particularly for oxidative stress, inflammation but also genotoxicity, are useful in biomonitoring workers exposed to nanomaterials (Bergamaschi et al., 2015; Nel et al., 2006). This foundational research may also be informed by the use of “omics” technologies to screen ENMs in in vitro studies before they enter commerce (Iavicoli et al., 2014). Also important in assessing biomarkers of exposure and effect is the need to consider susceptibility factors which may modulate toxicokinetic and toxicodynamic processes (Iavicoli et al., 2016). Despite these advances, there is still a need to be able to distinguish ENMs from background and to fully validate biomarkers targeted in biological monitoring. This validation effort may take many years (Schulte & Hauser, 2012)
An adequate exposure assessment should be complemented by an efficient hazard surveillance. To this aim, employers or governments should identify where nanomaterials are handled in an enterprise or in groups of enterprises. One pioneering example of hazard surveillance on a national level is the EpiNano Program in France (Guseva Canu et al., 2013). The focus is carbon nanotubes and titanium dioxide nano-objects. Regulation in France requires declaration by enterprises where these materials occur. Then if workers in the enterprise are identified and registered into the EpiNano Program, they become part of an ongoing epidemiologic surveillance research effort.
The efforts of Bundesanstalt für Arbeitsschutz und Arbeitsmedizin (BAuA) surveys in Germany, presented at the symposium, illustrate the issues that will probably be seen globally in assessing adherence to precautions. In Germany, 1750 companies and research institutes were invited to participate in a survey (Plitzko et al., 2013); only 450 responded so the low (26%) response rate show a limited representativeness; 76% of respondents were from industry and 23% from research. The survey also showed that 63% of the responders were in companies with less than 10 employees working with ENMs. Also, the potential exposure is rather recent; 46% of responders had been handling nanomaterials for 5 years or less. This is a clear indication of rapid development in this field. The main activities involving nanomaterials were mixing, dispersing, filling, bagging, feeding, and decanting; 81% of responders said they used “safety” measures (otherwise undefined), 16% said they needed no such measures; 75% reported no need for additional regulation but 24% wanted more specific guidance.
From research to practice
Ultimately, the protection of nanomaterial workers will depend on the utility and application of guidance (which could include control or risk assessment information) for employers. Because of the global nature of the use of nanotechnology, the World Health Organization (WHO) is developing guidelines to assess appropriate health protection activities for nanomaterial workers. The approach WHO is taking is to follow the evidence assessment involving systematic review and utilizing the Grading of Recommendations Assessment, Development and Evaluation (GRADE) System (Kortum, 2015). The aim of WHO guidelines is to facilitate improvements in occupational health and safety of nanotechnologies in a broad range of manufacturing and social environments. The target group in the first phase are policy-makers in low- and medium-income countries whereas in the second phase, there will be an implementation guide for employers and workers.
Beyond guidelines, regulation plays a part in worker protection. The NANoREG project is a European approach for safety testing of manufactured nanomaterials. It includes 59 partners from 14 European countries and involves collaboration between authorities, industry, and scientists (Figure 5) to obtain the knowledge required for appropriate risk management and to create the basis for common approaches, mutually acceptable datasets, and harmonized risk management practices.
Figure 5.
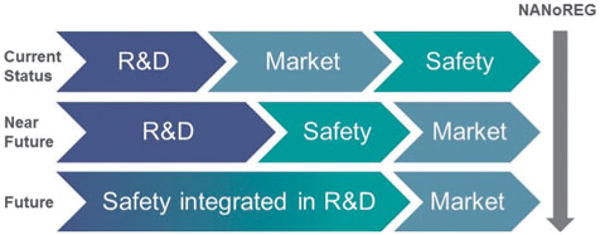
Inclusion of safety earlier in the innovation process.
A major area of concern in assessing adherence to precautionary guidance is evaluating the experiences in transitional and developing countries. These countries are often dependent on information and guidance developed elsewhere. They have, however, a rich diversity of workplaces ranging from early industrial to “high tech”. The experience in Argentina illustrates approaches that are useful. Generally, the focus has been on small companies, with an emphasis on containment, ventilation, good practices, and personal protective equipment (PPE). The control banding Nanotool is one instrument that has been used as was the DuPont-EDF NanoRisk Framework, and NIOSH “Approaches to Safe Nanotechnology” (Environmental Defense Fund, 2007; NIOSH, 2005).
Another issue when considering nanotechnology and workers’ health is that nanotechnology crosses other key enabling technologies such as microelectronics, photonics, biotechnologies, advanced materials, and advanced manufacturing systems. Nanotechnologies indicate new ways of manufacturing encompassing all industrial sectors and the entire value chain. Cross-cutting nanotechnology is the Responsible Research and Innovation concept that globally has been promoted in response to the rising demand for sustainable growth. It includes corporate social responsibility (CSR) and environmental safety and health (ESH). Critical for including occupational safety and health (OSH) in new technologies is an active “research to practice” effort. Such transfer will be better accomplished by harmonized methods and collaboration among organizations. Choosing the right way to the future is becoming more demanding. Scientific knowledge and practical experience are the best tools for navigation (Rantanen, 2008). Focusing on safety earlier in the innovation process is critical for responsible development of a technology. This new focus is illustrated in Figure 5. A technology is not being developed responsibly if workers are not protected (Schulte et al., 2014).
Global assessment of the adherence to precautionary guidance
The objectives of a global assessment are to generate an up-to-date picture of the global nanowork situation, to identify the exposure panorama qualitatively if not quantitatively, to identify hotspots in need of information and interventions, and to generate knowledge for subsequent action plans. The development and administration of such an assessment is a major effort. The value of ICOH directing the assessment is that it has a global occupational safety and health focus.
The development and administration of such a survey is a major effort. The ICOH scientific committee is in the process of soliciting parties to assist in the survey by planning, funding, and administering it. Potential collaborators could be WHO, the WHO Collaborating Center Network, International Labor Organization (ILO), and other international associations and corporations. A three tier approach was envisioned at the Rome Symposium. It included (1) collection and analysis of relevant studies, e.g., NIOSH (Schubauer-Berigan et al., 2015), (BAuA, EpiNano) (BauA, 2007; Guseva Canu et al., 2013; Guseva Canu et al., 2015), (2) development and early testing of a questionnaire in a pilot study, and (3) the global assessment. Various operational questions still need resolution. These include identifying a sampling frame, determining how to maintain confidentiality of data, maximizing the motivation for participation, and funding.
A key rationale for participating in the survey is that the responsible development of a new technology, such as nanotechnology, cannot be achieved if workers are harmed by it. During the early utilization of the technology when uncertainty about hazards and risks is high, it is part of responsible development to assure that precautions are being (Schulte et al., 2014). Business, government, labor, and other organizations must invest in developing and coordinating such evaluations.
In summary, there are still major uncertainties about the toxic potential of engineered nanomaterials. Consequently, the precautionary guidance issued by various authoritative organizations globally still is pertinent. The participants at the symposium concluded that part of whether nanotechnology is being developed responsibly is determining the extent to which precautionary guidance is being followed. The ICOH assessment of adherence to this guidance is a missing piece in occupational safety and efforts to protect the nanomaterial workforce.
Acknowledgments
The authors wish to acknowledge Dr. Evelyn Kortum (WHO) who presented at the symposium. The authors would also like to thank Candace Tsai, Maurizio Manno, and Michael Riediker for their comments on early drafts of this manuscript.
Footnotes
This work was authored as part of the Contributor’s official duties as an Employee of the United States Government and is therefore a work of the United States Government. In accordance with 17 U.S.C. 105, no copyright protection is available for such works under U.S. Law.
Declaration of interest
The authors report that they have no conflicts of interest. This work has not been published elsewhere. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the organizations to which they are affiliated.
References
- Aitken R, Creely K, Tran C. Nanoparticles: An Occupational Hygiene Review. Norwich, UK: HSE Books; 2004. [Google Scholar]
- Ambroise D, Wild P, Moulin JJ. Update of a meta-analysis on lung cancer and welding. Scand J Work Environ Health. 2006;32:22–31. doi: 10.5271/sjweh.973. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Stone S, Roberts JR, Chen B, Schwegler-Berry D, Afshari AA, Frazer DG. Effect of short-term stainless steel welding fume inhalation exposure on lung inflammation, injury, and defense responses in rats. Toxicol Appl Pharmacol. 2007;223:234–45. doi: 10.1016/j.taap.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Asbach C, Kuhlbusch T, Stahlmecke B, Kaminski H, Kiesling M, Voetz M, et al. Measurement and monitoring strategy for assessing workplace exposure to airborne nanomaterials. In: Wohlleben W, Kuhlbusch T, Lehr C, Schnekenburger J, editors. Safety of Nanomaterials along their Lifecycle: Release, Exposure and Human Hazard. New York: Taylor and Francis; 2015. pp. 233–46. [Google Scholar]
- BAuA (BundesanstaltfürArbeitsschutzundArbeitsmedizin) Guidance for handling and use of nanomaterials at the workplace. 2007 Available at: http://www.baua.de/en/Topics-from-A-to-Z/Hazardous-Substances/Nanotechnology/pdf/guidance.pdf?__blob=publicationFile&v=2.
- Bergamaschi E, Poland C, Guseva Canu I, Prina-Mello A. The role of biological monitoring in nano-safety. Nano Today. 2015;10:274–77. [Google Scholar]
- Borm PJ, Robbins D, Haubold S, Kuhlbusch T, Fissan H, Donaldson K, et al. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol. 2006;3:11. doi: 10.1186/1746-8977-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BSI. Guide to Safe Handling and Disposal of Manufactured Nanomaterials. London: British Standard Institution PD 6699-2; 2007. p. 32. [Google Scholar]
- Castranova V. From coal mine dust to quartz: mechanisms of pulmonary pathogenicity. Inhal Toxicol. 2000;12:7–14. doi: 10.1080/08958378.2000.11463226. [DOI] [PubMed] [Google Scholar]
- CEN (de Comité Normalisation Européen) EN482 Workplace Exposure – General Requirements for the Performance of Procedures for the Measurement of Chemical Agents. Brussels, Belgium: Comité Européende Normalisation (CEN); 2012. [Google Scholar]
- Chalupa DC, Morrow PE, Oberdörster G, Utell MJ, Frampton MW. Ultrafine particle deposition in subjects with asthma. Environ Health Perspect. 2004;112:879. doi: 10.1289/ehp.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm MM, Schubauer-Berigan MK, Evans DE, Birch ME, Fernback JE, Deddens JA. Carbon nanotube and nanofiber exposure assessments: an analysis of 14 site visits. Ann Occup Hyg. 2015;6:705–23. doi: 10.1093/annhyg/mev020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six US cities. N Engl J Med. 1993;329:1753–9. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Tran L, Jimenez LA, Duffin R, Newby DE, Mills N, et al. Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol. 2005;2:10–4. doi: 10.1186/1743-8977-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Defense Fund. Nanorisk framework. DuPont. 2007 Available at: http://qsinano.com/wp-content/uploads/2014/05/nano_risk_framework_dupont.pdf. Accessed on 5 January 2016.
- Erdely A, Liston A, Salmen-Muniz R, Hulderman T, Young SH, Zeidler-Erdely PC, et al. Identification of systemic markers from a pulmonary carbon nanotube exposure. J Occup Environ Med. 2011;53:S80–6. doi: 10.1097/JOM.0b013e31821ad724. [DOI] [PubMed] [Google Scholar]
- Federal Register. Survey of Nanomaterial Risk Management Practices. Vol. 78. Washington, DC: Federal Register; 2013. [Google Scholar]
- Garshick E, Laden F, Hart JE, Rosner B, Smith TJ, Dockery DW, Speizer FE. Lung cancer in railroad workers exposed to diesel exhaust. Environ Health Perspect. 2004;112:1539–43. doi: 10.1289/ehp.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseva Canu I, Boutou-Kempf O, Delabre L, Ducamp S, Iwatsubo Y, Marchand JL, Imbernon E. French registry of workers handling engineered nanomaterials as an instrument of integrated system for surveillance and research. J Phys: Conf Series. 2013;429(1) doi: 10:1088/1742-6596/429/1/012066. [Google Scholar]
- Guseva Canu I, Ducros C, Ducamp S, Delabre L, Audignon-Durand S, Durand C, et al. Standardized non-instrumental tool for characterizing workstations concerned with exposure to engineered nanomaterials. J Phys: Conf Serv. 2015;617 doi: 10.1088/1742-6596/617/1/012036. [DOI] [Google Scholar]
- Gwinn MR, Vallyathan V. Nanoparticles: health effects-pros and cons. Environ Health Perspect. 2006;114:1818–25. doi: 10.1289/ehp.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon JJ. Public Health Administration and Practice. St. Louis, MO: CV Mosby Company; 1974. [Google Scholar]
- Hett A. Nanotechnology: Small Matter, Many Unknowns. Zurich: Swiss Reinsurance Company; 2004. [Google Scholar]
- HSE. Nanotechnology. Horizons Scanning Information Note No HSIN1. London: Health and Safety Executive; 2004. [Google Scholar]
- Iavicoli I, Leso V, Manno M, Schulte PA. Biomarkers of nanomaterial exposure and effect: current status. J Nanopart Res. 2014;16:1–33. [Google Scholar]
- Iavicoli I, Leso V, Schulte PA. Biomarkers of susceptibility: state of the art and implications for occupational exposure to engineered nanomaterials. Toxicol Appl Pharmacol. 2016 doi: 10.1016/j.taap.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibald-Mulli A, Wichmann HE, Kreyling W, Peters A. Epidemiological evidence on health effects of ultrafine particles. J Aerosol Med. 2002;15:189–201. doi: 10.1089/089426802320282310. [DOI] [PubMed] [Google Scholar]
- Institut for Arbeitsschutzder Deutschen Gesetzlichen Unfallversicherung (IFA) Criteria for Assessment of the Effectiveness of Protective Measures. Germany: IFA; 2009. [Google Scholar]
- Kortum E. Developing WHO guidelines for protecting workers from potential risks of manufactured nanomaterials. 2015 Available at: http://www.nanotec.or.th/en/wp-content/uploads/2015/09/WHO-V-Murashov_NANO-HEALT.pdf. Accessed on 5 January 2016.
- Levy BS, Wegman PH, editors. Occupational Health: Recognizing and Preventing Work-related Disease. Boston: Little Brown and Company; 1983. [Google Scholar]
- Manno M, Viau C, Cocker J, Colosio C, Lowry L, Mutti A, et al. Biomonitoring for occupational health risk assessment (BOHRA) Toxicol Lett. 2010;192:3–16. doi: 10.1016/j.toxlet.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Kuempel ED. Airborne nanostructured particles and occupational health. J Nanopart Res. 2005;7:587–614. [Google Scholar]
- Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–7. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- NIOSH. Approaches to Safe Nanotechnology: An Information Exchange with NIOSH. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 2005. Available at: http://www.cdc.gov/niosh/topics/nanotech/pdfs/Approaches_to_Safe_Nanotechnology.pdf. Accessed on 13 August 2013. [Google Scholar]
- NIOSH. Current Intelligence Bulletin 65: Occupational Exposure to Carbon Nanotubes and Nanofibers. Cincinnati, OH: Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 2013. (DHHS (NIOSH) Publication 2013-145). [Google Scholar]
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Yu CP. The carcinogenic potential of inhaled diesel exhaust: a particle effect? J Aerosol Sci. 1990;21:S397–401. [Google Scholar]
- Ostiguy C, Lapointe G, Ménard L, Cloutier Y, Trottier M, Boutin M, et al. Nanoparticles: current knowledge about occupational health and safety risks and prevention measures. Montreal: Disponibleen; 2006. (Studies and research, IRSST, Report R-470). Available at: http://www.irsst.qc.ca/media/documents/pubirsst/r-470.pdf. Accessed on 5 January 2016. [Google Scholar]
- Ostiguy C, Roberge B, Woods C, Soucy B. Nanoparticles: current knowledge about OSH risks and prevention measures. 2nd. Montreal: Disponibleen; 2010. (IRSST Report R-656). Available at: http://www.irsst.qc.ca/medica/documents/pubirsst/r-456.pdf.[Links]. [Google Scholar]
- Pietroiusti A, Magrini A. Engineered nanoparticles at the workplace: current knowledge about workers risk. Occup Med. 2014;64:319–30. doi: 10.1093/occmed/kqu051. [DOI] [PubMed] [Google Scholar]
- Pietropaoli A, Frampton M, Oberdörster G, Cox C, Huang L, Marder V, Utell M. Blood markers of coagulation and inflammation in healthy human subjects exposed to carbon ultra fine particles. In: Heinrich U, editor. Effects of Air Contaminants on the Respiratory Tract-Interpretations from Molecular to Meta Ananlysis. Stuttgart, Germany: Fraunhofer IRB Verlag; 2004. pp. 181–94. (INIS Monographs). [Google Scholar]
- Plitzko S, Thim C, Bachmann V. Zweite Fragebogenaktion zu Aspekten des Arbeitsschutzes bei der Herstellung und bei Tätigkeiten mit Nanomaterialien in Deutschland. Bundesanstalt Für Arbeitsschutz Und Arbeitsmedizin. Gefahrstoffe-Reinhaltlung Luft. 2013;73:7–13. [Google Scholar]
- Plog BA, Quinlan P. Fundamentals of Industrial Hygiene. 5th. Itasa, IL: National Safety Council; 2002. [Google Scholar]
- Pope CA, III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–41. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–7. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Rantanen J. Risks in Modern Society. Netherlands: Springer; 2008. Challenges to global governance in the changing world of work; pp. 17–59. [Google Scholar]
- Riediker M, Schubauer-Berigan MK, Brouwer DH, Nelissen I, Koppen G, Frijns E, et al. A road map toward a globally harmonized approach for occupational health surveillance and epidemiology in nanomaterial workers. J Occup Environ Med. 2012;54:1214–23. doi: 10.1097/JOM.0b013e31826e27f1. [DOI] [PubMed] [Google Scholar]
- Royal Society and Royal Academy of Engineering. Nanoscience and Nanotechnologies: Opportunities and Uncertainties. London: Royal Society and Royal Academy of Engineering; 2004. [Google Scholar]
- Safe Work Australia. Work health and safety assessment tool for handling engineered nanomaterials. 2010 Available at: http://www.safeworkaustralia.gov.au/sites/swa/about/publications/pages/at201008workhealthandsafetyassessmenttool. Accessed on 5 January 2016.
- Schauer JJ. Evaluation of elemental carbon as a marker for diesel particulate matter. J Expo Anal Environ Epidemiol. 2003;13:443–53. doi: 10.1038/sj.jea.7500298. [DOI] [PubMed] [Google Scholar]
- Schmid A, Goel S, Wang W, Beiu V, Carrara S, Riediker M. Chances and risk of nanomaterials for health and environment. In: Akan O, Bellavista P, Cao J, Dressler F, Ferrari D, Gerla M, et al., editors. Nature-Net. Vol. 20. Berlin, Heidelberg: Springer; 2009. pp. 128–33. [Google Scholar]
- Schubauer-Berigan MK, Dahm MM, Schulte PA, Hodson L, Geraci CL. Characterizing adoption of precautionary risk management guidance for nanomaterials, an emerging occupational hazard. J Occup Environ Hyg. 2015;12:69–75. doi: 10.1080/15459624.2014.946515. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Geraci CL, Murashov V, Kuempel ED, Zumwalde RD, Castranova V, et al. Occupational safety and health criteria for responsible development of nanotechnology. J Nanopart Res. 2014;16:2153. doi: 10.1007/5/1051-013-2153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte P, Geraci C, Zumwalde R, Hoover M, Kuempel E. Occupational risk management of engineered nanoparticles. J Occup Environ Hyg. 2008;5:239–49. doi: 10.1080/15459620801907840. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Hauser JE. The use of biomarkers in occupational health research, practice and policy. Toxicol Lett. 2012;213:91–9. doi: 10.1016/j.toxlet.2011.03.027. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Schubauer-Berigan MK, Mayweather C, Geraci CL, Zumwalde R, Mc Kernan JL. Issues in the development of epidemiologic studies of workers exposed to engineered nanoparticles. J Occup Environ Med. 2009;51:323–35. doi: 10.1097/JOM.0b013e3181990c2c. [DOI] [PubMed] [Google Scholar]
- Schulte P, Murashov V, Zumwalde R, Kuempel E, Geraci C. Occupational exposure limits for nanomaterials: state of the art. J Nanopart Res. 2010;12:1971–87. [Google Scholar]
- SER. Advisory Report 0901. Nanoparticles in the Workplace: Health and Safety Precautions. The Hague: Social and Economic Council of the Netherlands; 2009. [Google Scholar]
- Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol-Lung Cell Mol Physiol. 2005;289:L698–708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- Tran C, Donaldson K, Stones V, Fernandez T, Ford A, Christofi N, et al. As Coping Study to Identify Hazard Data Needs for Addressing the Risks Presented by Nanoparticles and Nanotubes. Edinburgh, UK, London: Institute of Occupational Medicine; 2005. [Google Scholar]


