Abstract
Aim: The objective of this study was to develop a reference range for urine calcium excretion (both 24-hour and fasting) for African American women compared to White women. In addition, the variables that determine urine calcium excretion were identified. Material: Data were analyzed for baseline studies of healthy postmenopausal volunteers who participated in seven separate studies conducted at one site. Methods: Some studies included fasting urine Ca/Cr and others 24-hour urine calcium excretion. 24-hour urine calcium was considered with and without correction for urinary creatinine excretion. Calcium was measured initially by atomic absorption spectrophotometry and more recently by an automated method (ADVIA 2400 Chemistry System). Results: Participants were considered healthy based on history and physical and routine laboratory studies. Those screened who had a history of nephrolithiasis were excluded. A reference range for 24-hour urine calcium and fasting urine calcium/creatinine was developed. Reference intervals of 11 – 197 mg/24-hour urine calcium excretion and of 0.007 – 0.222 of fasting Ca/Cr were found for African American women compared to 21 – 221 mg/24 hours and 0.019 – 0.264 in White women, respectively. Urine creatinine excretion was higher in African Americans consistent with their higher muscle mass. Conclusion: Urine calcium excretion is lower in postmenopausal African American than White women. The reference range developed should be considered in the diagnosis of hypocalciuric states and may also be useful in the diagnosis of hypercalciuria.
Keywords: African American, urine calcium, fasting urine calcium, ethnicity, creatinine
Introduction
African Americans have a superior calcium economy when compared to Whites. Calcium conservation through renal reabsorption is enhanced, resulting in higher bone density and lower risk for fracture [1]. The incidence of nephrolithiasis is also lower in African Americans, presumably at least in part because of their lower calcium excretion [2].
Measurement of urinary calcium excretion for clinical purposes may be useful in evaluating nephrolithiasis, primary hyperparathyroidism, and hypocalciuric disorders such as Familial Hypocalciuric Hypercalcemia as well as malabsorption. Reference values for urine calcium excretion have been suggested both for 24-hour urine excretion and fasting urine/creatinine excretion (a study that is convenient) [3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22]. Published values for a hypercalciuric cut-off vary. For example, the urine calcium to creatinine ratio upper limit has been reported to be as low as 0.21 but as high as 0.37 [23]. Similarly, the upper limit of the reference range for 24-hour urine excretion has been variously suggested to be 200 mg or 250 mg/day [6, 19]. Lower reference values are reported on a restricted dietary calcium intake. Importantly, these values have been primarily determined in White populations; African Americans have been less extensively studied.
We have measured urine calcium excretion at baseline prior to any intervention in a large number of healthy African American women in several clinical trials, as well as in White women [24, 25, 26, 27]. Here, we examine these combined data to answer the following questions: 1). What is the reference range for 24-hour urine calcium and fasting urine calcium/creatinine in postmenopausal African American women? 2). How does this reference range compare with White women?
Methods
These data include baseline values from six previous clinical trials and a current clinical trial between 1990 and 2015 [24, 26, 28, 29, 30]. Only postmenopausal Black and White ethnic groups were selected for this analysis. Purposes of this study were to establish reference intervals (RI) for fasting urine calcium/creatinine and 24-hour urinary calcium for combined ethnic groups as well as Black and White women separately and to find the predictors of 24-hour urinary calcium. 24-hour urine calcium was considered uncorrected and corrected for creatinine excretion to reduce collection bias. Both the corrected and uncorrected 24-hour urine calcium variables were analyzed.
Serum PTH was measured by the Allegro intact PTH immunoassay, purchased from Nichols Institute (San Juan Capistrano, CA, USA). Serum 25-hydroxyvitamin D was measured by a radioimmunoassay purchased from DiaSorin (previously named INCSTAR when we first used the kits; Stillwater, MN, USA). The intra-assay CV was 4.1%, and the interassay CV was 7.0%. Calcium was measured by atomic absorption spectrophotometry (PerkinElmer 560, Norwalk, CT, USA) initially and more recently by an automated method (ADVIA 2400 Chemistry System). Serum creatinine was measured by the method of Heinegard and Tiderstrom [31]. eGFR was calculated using the CKD-EPI formula, which is corrected for race, sex, age, and levels of serum creatinine.
Descriptive statistics (i.e., mean, median, standard deviation, first quartile, and third quartile) were generated and presented as mean (sd) and median (q1-q3). Normality of distributions of clinical variables and laboratory markers was evaluated using visual observation of histograms and the Kolmogorov-Smirnov test. Differences of each variable between groups were examined using the Wilcoxon rank-sum test. When a test of normality revealed that the variables for calcium excretion were not normally distributed, a nonparametric approach was taken to calculate 95% reference intervals (RI) [32, 33] and 90% confidence limits [34] for fasting urine calcium, 24-hour urine calcium, and 24-hour urine calcium corrected for creatinine. The RI was calculated for the combined groups as well as separately for Black and White ethnic groups.
Multiple linear regression models were developed using log-transformed 24-hour urinary calcium variables (both the corrected for creatinine and the uncorrected) as the dependent variables to perform adjusted analyses. Fasting calcium/creatinine, race, study, parathyroid hormone, serum calcium, eGFR, dietary calcium intake, age, body weight, 25(OH)D, and 1,25(OH)D were used as the explanatory variables in all models. A study variable was used to adjust models for data coming from multiple studies. Model coefficients, p-values, and R-square values were examined for each model in order to determine the predictors of 24-urinary calcium and how much variability is explained by each model. Different models were developed to examine predictors for Black and White ethnic groups separately. Least square means were calculated using Tukey-Kramer adjustment. Model assumptions were checked via residual analysis and graphic summaries. All calculations were performed using SAS 9.3 and 9.4 (SAS Institute, Cary, NC, USA). Results were considered statistically significant when p < 0.05.
Results
Participants
The Bone Mineral Research Center at Winthrop-University has conducted studies in healthy Black and White women. Each of these studies has been concluded and published, except for one that is ongoing [24, 25, 26, 27]. Participants in these studies have been determined to be eligible for inclusion by history, physical, and routine blood chemistries. None of the subjects had a history of nephrolithiasis. Osteoporosis was excluded by history or bone density measurement. The values here represent baseline studies, prior to any intervention. Fasting blood was obtained in the morning in each of these studies.
Demographic and laboratory values
Only postmenopausal women were included in this analysis. Race was self-declared. There were 1,042 participants, 561 Black and 481 White. Of these, a total of 318 participants had measurement of both fasting and 24-hour urine values. There were significant differences in the two populations as would be expected in these combined studies (Table 1). The Black women were older and heavier. Fasting urine calcium/creatinine and 24-hour calcium were lower in African Americans. 24-hour urine calcium (uncorrected) was lower in African Americans, and this was also the case with correction for creatinine or body weight. Serum PTH, calcium, 1,25(OH)2D, and creatinine were higher in African Americans, and serum 25(OH)D was lower. Dietary calcium intake was similar.
Table 1. Baseline comparisons between Blacks and Whites.
| Variable | Black | White | p-value | ||||
|---|---|---|---|---|---|---|---|
| N | Mean (sd) | Median (q1-q3) | N | Mean (sd) | Median (q1-q3) | ||
| Age (years) | 561 | 64 (8) | 64 (58 – 69) | 481 | 59 (8) | 57 (53 – 64) | < 0.0001 |
| BMI (kg/M2) | 552 | 30 (5) | 29 (26 – 33) | 481 | 26 (4) | 25 (23 – 28) | < 0.0001 |
| Weight (kg) | 560 | 79 (14) | 77 (68 – 86) | 481 | 68 (10) | 66 (60 – 74) | < 0.0001 |
| Dietary Ca intake (mg) | 543 | 790 (483) | 686 (458 – 1,008) | 466 | 779 (379) | 730 (519 – 984) | 0.370 |
| 25(OH)D (nmol/L) | 554 | 46 (17) | 46 (33 – 59) | 456 | 69 (26) | 67 (51 – 83) | < 0.0001 |
| 1, 25(OH)2D (pmol/L) | 290 | 109 (38) | 102 (79 – 135) | 426 | 90 (33) | 85 (67 – 108) | < 0.0001 |
| Fasting urine Ca/Cr | 352 | 0.07 (0.05) | 0.05 (0.03 – 0.09) | 463 | 0.11 (0.06) | 0.09 (0.06 – 0.14) | < 0.0001 |
| 24-hour urine Ca/Cr×1,000 | 285 | 80 (46) | 72 (44 – 108) | 235 | 138 (70) | 126 (89 – 179) | < 0.0001 |
| 24-hour urine Ca (mg) | 287 | 80 (50) | 71 (43 – 111) | 240 | 98 (56) | 91 (54 – 132) | < 0.001 |
| 24-hour urine Cr (mg) | 286 | 1,033 (376) | 997 (790 – 1,235) | 237 | 754 (271) | 769 (561 – 939) | < 0.0001 |
| PTH (pg/mL) | 557 | 50 (24) | 46 (34 – 62) | 470 | 36 (15.3) | 33 (25 – 44) | < 0.0001 |
| Serum Ca (mg/dL) | 553 | 9.3 (0.53) | 9.3 (9 – 9.6) | 475 | 9.6 (0.4) | 9.6 (9.3 – 9.8) | < 0.0001 |
| Serum Cr (mg/dL) | 557 | 0.84 (0.19) | 0.80 (0.7 – 1.0) | 477 | 0.77 (0.2) | 0.8 (0.6 – 0.9) | < 0.0001 |
| eGFR(mL/min/1.73m2) | 557 | 87 (18) | 87 (73 – 103) | 477 | 84 (18) | 84 (69 – 101) | 0.006 |
*p-values are from Wilcoxon rank-sum test.
Distribution and reference range for urine calcium excretion
The distribution of fasting urine calcium/creatinine and 24-hour urine calcium excretion is given in Figure 1, Figure 2, Figure 3, and Figure 4. The figures show data on Black and White participants combined and separately, with the mean, minimum, and maximal values. The excretion data were not normally distributed. In Table 2, values are presented with the 95% nonparametric reference intervals with confidence limits, along with the 24-hour urine calcium corrected for creatinine.
Figure 1. Distribution of fasting calcium/creatinine for postmenopausal women – Black and White combined.
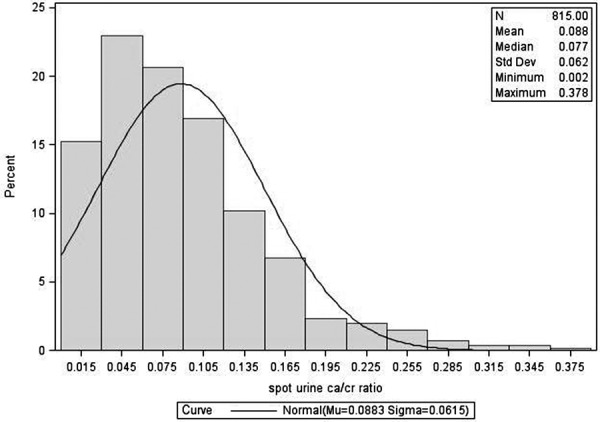
Figure 2. Distribution of fasting calcium/creatinine for postmenopausal women – Black and White separately.
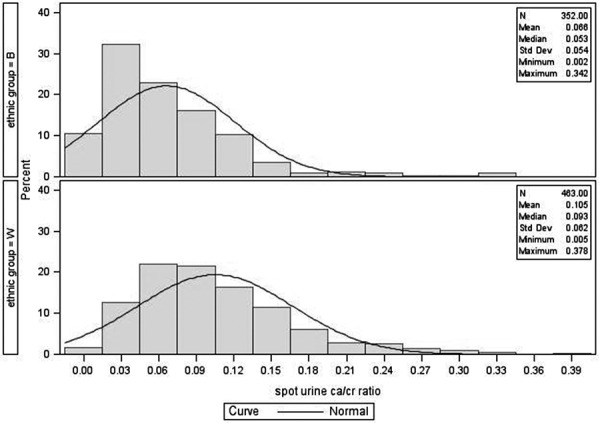
Figure 3. Distribution of 24-hour urine calcium for postmenopausal women – Black and White combined.
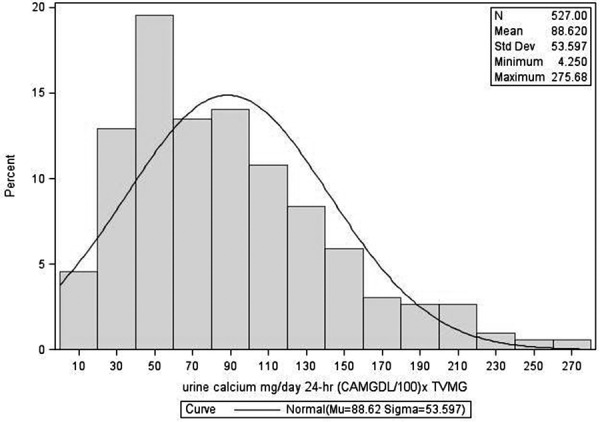
Figure 4. Distribution of 24-hour urine calcium for postmenopausal women – Black and White separately.
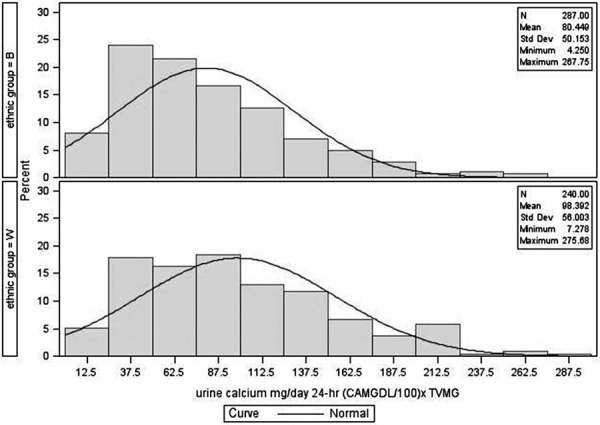
Table 2. Nonparametric 95% reference intervals and their 90% confidence limits.
| Variable | Race | N | Median (q1-q3) | Reference Interval (90% confidence limit) | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Fasting urine Ca/Cr | Black and White | 815 | 0.08 (0.04 – 0.12) | 0.008 (0.007 – 0.011) | 0.249 (0.232 – 0.274) |
| Black | 352 | 0.05 (0.03 – 0.09) | 0.007 (0.005 – 0.008) | 0.222 (0.165 – 0.275) | |
| White | 463 | 0.09 (0.06 – 0.14) | 0.019 (0.014 – 0.023) | 0.264 (0.238 – 0.293) | |
| 24-hour urine calcium (mg) |
Black and White | 527 | 79 (48 – 118) | 14 (10 – 16) | 214 (205 – 232) |
| Black | 287 | 70 (42 – 111) | 11 (8 – 15) | 197 (186 – 249) | |
| White | 240 | 91 (54 – 132) | 21 (9 – 24) | 221 (214 – 261) | |
| 24-hour urine Ca/Cr×1,000 |
Black and White | 520 | 94 (56 – 140) | 20 (17 – 23) | 268 (252 – 282) |
| Black | 285 | 72 (44 – 108) | 17 (8 – 20) | 189 (178 – 223) | |
| White | 235 | 127 (89 – 179) | 33 (20 – 40) | 291 (275 – 356) | |
The reference range in this population for fasting urine Ca/Cr is 0.007 – 0.222 for Black and 0.019 – 0.264 for White women. For 24-hour urine calcium excretion, the range in Black women was 11 – 197 mg, and in White women was 21 – 221 mg. 24-hour urine calcium corrected for creatinine excretion is also given in Table 2.
Correlations with urine calcium excretion
Spearman correlation coefficient between fasting and 24-hour urine calcium excretion was 0.55 (p < 0.0001) and the correlation was slightly higher when the 24-hour urine was corrected for creatinine. For the combined groups, significant inverse correlations were found between fasting urine Ca/Cr and age, weight, PTH and serum creatinine. A significant direct correlation was found with each of the vitamin D metabolites. Correlations tended to be less significant between these variables and 24-hour urine calcium excretion, with age (r = –0.13, p = 0.003) and PTH (r = –0.09, p = 0.04) being significantly correlated with this measurement.
Multiple linear regression models
A simultaneous multiple linear regression model (model r-square = 0.36, F = 12.02, p < 0.0001) including all variables was developed to determine the predictors of 24-hour urine calcium (Table 3). This adjusted model revealed fasting urinary calcium/creatinine ratio (β = 5.3, se = 0.75, p < 0.0001), race-Black (β = –0.42, se = 0.11, p < 0.0001), dietary calcium intake (β = 0.0003, se = 0.0001, p = 0.021), age (β = –0.015, se = 0.006, p = 0.014) and weight (β = 0.013, se = 0.004, p = 0.001) were significantly associated with 24-hour urine calcium. β-coefficients were based on logarithm values of 24-hour urine calcium. For example, the β-co-efficient for the Black race is –0.42. We can de-transform this as (exp(–0.42) = 0.66, 1 – 0.66 = 0.34), hence we can say that Black women have 34% lower urine calcium compared to White women, holding all other variables constant. Separate models were developed for Black and White groups. For Black women (model r-square = 0.39, F = 4.02, p < 0.001), only fasting urine calcium/creatinine ratio was associated with the 24-hour urine calcium whereas for White women (model r-square = 0.38, F = 9.97, p < 0.0001), dietary calcium intake, age and weight were associated in addition to the fasting urine calcium/creatinine ratio. The contribution of these other variables was minimal.
Table 3. Independent predictors of 24-hour urine calcium – from multivariable models.
| Variable | 1Overall (n = 265) | 2Black (n = 74) | 3White (n = 191) | |||
|---|---|---|---|---|---|---|
| 4Estimate (SE) | p-value | Estimate (SE) | p-value | Estimate (SE) | p-value | |
| Age | –0.015 (0.006) | 0.014 | –0.008 (0.012) | 0.509 | –0.018 (0.006) | 0.005 |
| Weight (kg) | 0.013 (0.004) | 0.001 | 0.003 (0.008) | 0.709 | 0.016 (0.004) | < 0.0001 |
| Race (B) | –0.42 (0.11) | < 0.0001 | – | – | – | – |
| Fasting urine ca/cr | 5.3 (0.75) | < 0.0001 | 14.6 (2.6) | < 0.0001 | 4.2 (0.73) | < 0.0001 |
| PTH (pg/mL) | 0.0002 (0.003) | 0.922 | 0.011 (0.006) | 0.096 | –0.003 (0.003) | 0.258 |
| Serum calcium (mg/dL) | 0.19 (0.11) | 0.079 | 0.22 (0.26) | 0.405 | 0.26 (0.11) | 0.021 |
| eGFR (mL/min/1.73 m2) | 0.004 (0.003) | 0.196 | 0.008 (0.007) | 0.257 | 0.003 (0.004) | 0.396 |
| Dietary calcium intake (mg) | 0.0003 (0.0001) | 0.02 | 0.0001 (0.0003) | 0.862 | 0.0003 (0.0001) | 0.02 |
| 25(OH)D (nmol/L) | 0.001 (0.001) | 0.508 | 0.005 (0.003) | 0.1 | –0.0004 (0.001) | 0.746 |
| 1,25(OH)2D (pmol/L) | 0.001 (0.002) | 0.428 | –0.004(0.004) | 0.325 | 0.003 (0.002) | 0.173 |
1Model for Black and White women combined with model r-square = 0.36, p < 0.0001; 2Model for Black ethnic group only with model r-square = 0.39, p < 0.001; 3Model for White ethnic group only with model r-square = 0.38, p < 0.0001. 4Estimates correspond to logarithm of 24-hour urine calcium.
A priori models
Urinary calcium
Two separate models were developed for 24-hour urine calcium. Race, 25(OH)D, and 1,25(OH)2D were used as covariates in one model (F = 8.25, p < 0.0001), only race and 25(OH)D were used for another (F = 6.62, p = 0.002). Adjusted mean 24-hour urine calcium was found to be 25 mg lower in Black compared to White women (p < 0.0001) from the former model and 16 mg lower in Black compared to White (p = 0.001) in the latter model. A separate model was developed using race and calcium intake as covariates (F = 7.3, p < 0.001); the adjusted mean for Black was again 16 mg lower than for White women (p < 0.001). Thus, urine calcium is consistently lower in Black compared to White women regardless of adjusting factors.
PTH
An unadjusted analysis showed that PTH is 16 pg/mL higher in Black compared to White women (p < 0.0001). To verify that this relationship still exists regardless of their 25(OH)D levels, a model was developed for PTH using race and 25(OH)D as covariates (F = 47.81, p < 0.0001). Adjusted mean PTH was still 11 pg/mL higher among Black compared to White women, p < 0.0001.
Fasting Ca/Cr
Unadjusted analysis showed a mean difference of 0.04 in fasting urine Ca/Cr between Black and White women (p < 0.001) (Table 1). An adjusted model was developed using race, BMI, and 25(OH)D as covariates (F = 40.86, p < 0.0001), to verify this finding. This model revealed that the adjusted mean difference stayed the same 0.04, p < 0.0001. A separate model was developed using race and calcium intake as covariates (F = 59.4, p < 0.0001) which also showed the similar difference in adjusted mean fasting ca/cr between Black and White women (p < 0.0001).
All models were examined using 24-urine calcium corrected for creatinine as the dependent variable to reduce urine collection bias. The results were comparable when uncorrected data were used.
Discussion
This study provides the reference intervals for urinary calcium excretion in postmenopausal African American women of 11 – 197 mg/24-hour and fasting calcium/creatinine of 0.007 – 0.222. These values may be compared to 21 – 221 mg and 0.019 – 0.264 in White women, respectively. Rather than simply observing that urine calcium excretion is lower in African Americans, our study provides a reference range based on a large number of healthy postmenopausal women [15, 35, 36, 37, 38].
Urinary calcium excretion has been reported as under 200 mg/day in normal individuals in contrast with kidney stone formers. Pak et al. [19] have studied urinary calcium excretion on a calcium-restricted diet to identify stone-formers with absorptive hypercalciuria. In an examination of 39 publications, they derived an upper limit of 219 mg/day, and suggest an optimal cut-off of 172 mg/day on a restricted diet. These values are consistent with our values in White women who were not on a restricted diet.
On an unrestricted diet, Coe et al. [16] suggested 250 mg/day as the upper limit, based on White women and using the 95th percentile. In our report, we provide percentiles, along with confidence intervals for women on an unrestricted diet. In the Nurses’ Health Study, a range of 73 – 302 mg/day was reported for 24-hour urine calcium excretion in White women as compared to 32 to 240 mg/day for Black women (n = 146), with mean values of 183 and 118 mg/day, respectively [36]. Calcium intake in the Black women in their study exceeded the current recommended dietary allowance. The calcium intake was lower in our population, which may partially explain the higher values in the Nurse’s Health Study.
The risk for kidney stones is presumably less in African American women (at least in part) because of their lower calcium excretion, and it may be appropriate to use the “White” reference range for hypercalciuria. However, it could be useful to consider the lower calcium excretion of African Americans in the diagnosis of hypercalciuria associated with primary hyperparathyroidism [35, 39]. Racial differences could lead to misdiagnosis in conditions associated with hypocalciuria such as idiopathic hypocalciuric hypercalcemia [39]. We suggest that the lower reference ranges for African Americans should be considered in these conditions.
An issue to be considered is the use of urine creatinine for comparison in Black and White populations. African Americans have a larger muscle mass and as a result a higher urine creatinine excretion. In this study, the higher urine creatinine excretion was also apparent. Thus, when using the fasting urine/calcium excretion, the ratio will be lower in African Americans for each quantity of calcium excreted. For this reason, we considered comparisons using 24-hour urine calcium excretion uncorrected for creatinine. Of course, for the fasting urine this is not possible and could be problematic in the case of the fasting Ca/Cr in evaluation of hypocalciuric status.
The explanation for the lower urinary calcium excretion in African Americans has not been clearly elucidated. However, it is present during childhood, and, along with greater calcium absorption, is responsible for the increased skeletal mass acquired during adolescence [15]. Dietary calcium intake was similar in Black and White women in the current study, but serum 1,25(OH)2D and PTH were higher in Black women, which may be speculated as the reason for the difference in calcium excretion. In our multivariable models, however, it was evident that ethnicity was the primary determinant of the difference in calcium excretion, and it is likely that there is a genetic influence. The issue of weight and age being responsible for the cross-racial differences previously addressed by us in an age and weight matched study in premenopausal women [40]. Moreover, our multivariable analysis adjusted for age and BMI.
The lower serum 25OHD in African Americans is wellknown and is believed to be due to decreased absorption of ultraviolet rays as the result of increased skin pigmentation [1]. Lower total serum 25OHD levels may also be genetically determined through influence on vitamin D binding protein concentration, which results in higher free 25OHD than suggested by total 25(OH)D [41]. In addition, as observed in this study, African Americans have a higher prevalence of obesity, which also lowers serum 25OHD levels. The lower serum 25OHD could be considered as a cause of the lower urine calcium excretion if serum 25OHD levels influenced intestinal calcium absorption. However, calcium absorption is not decreased in African Americans, and it is calcitriol (not 25OHD) that controls calcium absorption [1, 42]. The higher calcitriol in this study may result from the higher PTH values. Serum calcium has mainly been reported to be the same or higher in African Americans; we do not have an explanation for why it was slightly lower in this study population.
There are several limitations in this study. It is a post-hoc analysis of several studies that were not concurrent, and there are baseline differences due to age and weight. However, statistical correction was carried out for contribution of the individual studies. In one of our previous studies, Black and White subjects were matched for age and weight, and the racial difference in urinary calcium persisted [43]. Strengths are that this was a population shown to be healthy, and the population size was large.
In conclusion, we provide a reference range for postmenopausal African American women for 24-hour urine calcium excretion and fasting urine calcium/creatinine. This reference range is of significance because urine calcium excretion is shown to be lower in African American women. Clinicians should be aware of this finding in diagnosing hypercalciuria and also in considering hypocalciuric states such as idiopathic hypocalciuric hypercalcemia.
Acknowledgment
This study was supported by NIH grants: R01-AR037520, P01-DK042618, R01-AG015325, R01-AG032440, and Merck-Interaction between Calcium Intake and Vitamin D Status.
Dr. John Aloia had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
John F. Aloia, Albert Shieh, Mageda Mikhail, and Shahidul Islam have no conflict of interest.
References
- 1. Aloia JF African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr. 2008; 88: 545S–550S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maloney ME Springhart WP Ekeruo WO Young MD Enemchukwu CU Preminger GM Ethnic background has minimal impact on the etiology of nephrolithiasis. J Urol. 2005; 173: 2001–2004. [DOI] [PubMed] [Google Scholar]
- 3. Gökçe C Gökçe O Baydinç C Ilhan N Alaşehirli E Ozküçük F Taşçi M Atilkeler MK Celebi H Arslan N Use of random urine samples to estimate total urinary calcium and phosphate excretion. Arch Intern Med. 1991; 151: 1587–1588. [DOI] [PubMed] [Google Scholar]
- 4. Pak CY Sakhaee K Pearle MS Detection of absorptive hypercalciuria type I without the oral calcium load test. J Urol. 2011; 185: 915–919. [DOI] [PubMed] [Google Scholar]
- 5. Jones AN Shafer MM Keuler NS Crone EM Hansen KE Fasting and postprandial spot urine calcium-to-creatinine ratios do not detect hypercalciuria. Osteoporos Int. 2012; 23: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Worcester EM Coe FL New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol. 2008; 28: 120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nordin BE Assessment of calcium excretion from the urinary calcium/creatinine ratio. Lancet. 1959; 2: 368–371. [DOI] [PubMed] [Google Scholar]
- 8. Heaney RP Recker RR Ryan RA Urinary calcium in perimenopausal women: normative values. Osteoporos Int. 1999; 9: 13–18. [DOI] [PubMed] [Google Scholar]
- 9. Choi IS Jung ES Choi YE Cho YK Yang EM Kim CJ Random urinary calcium/creatinine ratio for screening hypercalciuria in children with hematuria. Ann Lab Med. 2013; 33: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogawa Y Yonou H Hokama S Oda M Morozumi M Sugaya K Urinary saturation and risk factors for calcium oxalate stone disease based on spot and 24-hour urine specimens. Front Biosci. 2003; 8: a167–a176. [DOI] [PubMed] [Google Scholar]
- 11. Lavocat MP Freycon MT Muchrif M [Comparative study of 24-hour calciuria and urinary calcium/creatinine ratio in children over 4 years of age]. Pediatrie. 1992; 47: 565–568. [PubMed] [Google Scholar]
- 12. Strohmaier WL Hoelz KJ Bichler KH Spot urine samples for the metabolic evaluation of urolithiasis patients. Eur Urol. 1997; 32: 294–300. [PubMed] [Google Scholar]
- 13. Koyun M Güven AG Filiz S Akman S Akbas H Baysal YE Dedeoglu N Screening for hypercalciuria in schoolchildren: what should be the criteria for diagnosis? Pediatr Nephrol. 2007; 22: 1297–1301. [DOI] [PubMed] [Google Scholar]
- 14. Knapp EL Factors influencing the urinary excretion of calcium; in normal persons. J Clin Invest. 1947; 26: 182–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. So NP Osorio AV Simon SD Alon US Normal urinary calcium/creatinine ratios in African-American and Caucasian children. Pediatr Nephrol. 2001; 16: 133–139. [DOI] [PubMed] [Google Scholar]
- 16. Coe FL Evan A Worcester E Kidney stone disease. J Clin Invest. 2005; 115: 2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryan LE Ing SW Idiopathic hypercalciuria and bone health. Curr Osteoporos Rep. 2012; 10: 286–295. [DOI] [PubMed] [Google Scholar]
- 18. Wasserstein A Epidemiology and Natural History of Nephrolithiasis. Clinic Rev Bone Miner Metab.. 2011; 9: 165–180. [Google Scholar]
- 19. Pak CY Sakhaee K Moe OW Poindexter J Adams-Huet B Pearle MS Zerwekh JE Preminger GM Wills MR Breslau NA Bartter FC Brater DC Heller HJ Odvina CV Wabner CL Fordtran JS Oh M Garg A Harvey JA Alpern RJ Defining hypercalciuria in nephrolithiasis. Kidney Int. 2011; 80: 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pak CY Oata M Lawrence EC Snyder W The hypercalciurias. Causes, parathyroid functions, and diagnostic criteria. J Clin Invest. 1974; 54: 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Assimos D Re: defining hypercalciuria in nephrolithiasis. J Urol. 2012; 187: 925. [DOI] [PubMed] [Google Scholar]
- 22. Emamghorashi F Davami MH Rohi R Hypercalciuria in Jahrom’s school-age children: what is normal calcium-creatinine ratio? Iran J Kidney Dis. 2010; 4: 112–115. [PubMed] [Google Scholar]
- 23. Vieth R Chan PC MacFarlane GD Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001; 73: 288–294. [DOI] [PubMed] [Google Scholar]
- 24. Aloia JF Vaswani A Yeh JK Ross PL Flaster E Dilmanian FA Calcium supplementation with and without hormone replacement therapy to prevent postmenopausal bone loss. Ann Intern Med. 1994; 120: 97–103. [DOI] [PubMed] [Google Scholar]
- 25. Aloia JF Vaswani A Yeh JK Flaster E Risk for osteoporosis in black women. Calcif Tissue Int. 1996; 59: 415–423. [DOI] [PubMed] [Google Scholar]
- 26. Aloia JF Talwar SA Pollack S Yeh J A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med. 2005; 165: 1618–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aloia JF Dhaliwal R Shieh A Mikhail M Islam S Yeh JK Calcium and vitamin d supplementation in postmenopausal women. J Clin Endocrinol Metab. 2013; 98: E1702–E1709. [DOI] [PubMed] [Google Scholar]
- 28. Aloia JF Vaswani A Ma R Flaster E Aging in women – the four-compartment model of body composition. Metabolism. 1996; 45: 43–48. [DOI] [PubMed] [Google Scholar]
- 29. Aloia J Bojadzievski T Yusupov E Shahzad G Pollack S Mikhail M Yeh J The relative influence of calcium intake and vitamin D status on serum parathyroid hormone and bone turnover biomarkers in a double-blind, placebo-controlled parallel group, longitudinal factorial design. J Clin Endocrinol Metab. 2010; 95: 3216–3224. [DOI] [PubMed] [Google Scholar]
- 30. Aloia JF Dhaliwal R Shieh A Mikhail M Fazzari M Ragolia L Abrams SA Vitamin D supplementation increases calcium absorption without a threshold effect. Am J Clin Nutr. 2014; 99: 624–631. [DOI] [PubMed] [Google Scholar]
- 31. Heinegård D Tiderström G Determination of serum creatinine by a direct colorimetric method. Clin Chim Acta. 1973; 43: 305–310. [DOI] [PubMed] [Google Scholar]
- 32. Altman D Practical statistics for medical research. London: Chapman and Hall; 1980. [Google Scholar]
- 33. Bland M An introduction to Medical Statistics. Osford Medical Publications. (3rd edition); 2000. [Google Scholar]
- 34. Hahn G Meeker W Statistical Intervals: A guide for Practitioners. John Wiley & Sons; 1991. [Google Scholar]
- 35. Taha W Singh N Flack JM Abou-Samra AB Low urine calcium excretion in African American patients with primary hyperparathyroidism. Endocr Pract. 2011; 17: 867–872. [DOI] [PubMed] [Google Scholar]
- 36. Taylor EN Curhan GC Differences in 24-hour urine composition between black and white women. J Am Soc Nephrol. 2007; 18: 654–659. [DOI] [PubMed] [Google Scholar]
- 37. Bikle DD Ettinger B Sidney S Tekawa IS Tolan K Differences in calcium metabolism between black and white men and women. Miner Electrolyte Metab. 1999; 25: 178–184. [DOI] [PubMed] [Google Scholar]
- 38. Meier DE Luckey MM Wallenstein S Clemens TL Orwoll ES Waslien CI Calcium, vitamin D, and parathyroid hormone status in young white and black women: association with racial differences in bone mass. J Clin Endocrinol Metab. 1991; 72: 703–710. [DOI] [PubMed] [Google Scholar]
- 39. Shinall MC Dahir KM Broome JT Differentiating familial hypocalciuric hypercalcemia from primary hyperparathyroidism. Endocr Pract. 2013; 19: 697–702. [DOI] [PubMed] [Google Scholar]
- 40. Aloia JF Vaswani A Ma R Flaster E Comparison of body composition in black and white premenopausal women. J Lab Clin Med. 1997; 129: 294–299. [DOI] [PubMed] [Google Scholar]
- 41. Powe CE Evans MK Wenger J Zonderman AB Berg AH Nalls M Tamez H Zhang D Bhan I Karumanchi SA Powe NR Thadhani R Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013; 369: 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aloia JF Chen DG Yeh JK Chen H Serum vitamin D metabolites and intestinal calcium absorption efficiency in women. Am J Clin Nutr. 2010; 92: 835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aloia JF Mikhail M Pagan CD Arunachalam A Yeh JK Flaster E Biochemical and hormonal variables in black and white women matched for age and weight. J Lab Clin Med. 1998; 132: 383–389. [DOI] [PubMed] [Google Scholar]


