Abstract
Introduction
Campylobacter jejuni is the leading bacterial food-borne pathogen within the European Union, and poultry meat is an important vehicle for its transmission to humans. However, there is limited knowledge about how this organism persists in broiler litter and faeces. The aim of this study was to assess the impact of a number of environmental parameters, such as temperature, humidity, and oxygen, on Campylobacter survival in both broiler litter and faeces.
Materials and methods
Used litter was collected from a Campylobacter-negative broiler house after final depopulation and fresh faeces were collected from transport crates. Samples were confirmed as Campylobacter negative according to modified ISO methods for veterinary samples. Both sample matrices were inoculated with 9 log10 CFU/ml C. jejuni and incubated under high (≥85%) and low (≤70%) relative humidity conditions at three different temperatures (20°C, 25°C, and 30°C) under both aerobic and microaerophilic atmospheres. Inoculated litter samples were then tested for Campylobacter concentrations at time zero and every 2 hours for 12 hours, while faecal samples were examined at time zero and every 24 hours for 120 hours. A two-tailed t-test assuming unequal variance was used to compare mean Campylobacter concentrations in samples under the various temperature, humidity, and atmospheric conditions.
Results and discussion
C. jejuni survived significantly longer (P≤0.01) in faeces, with a minimum survival time of 48 hours, compared with 4 hours in used broiler litter. C. jejuni survival was significantly enhanced at 20°C in all environmental conditions in both sample matrices tested compared with survival at 25°C and 30°C. In general, survival was greater in microaerophilic compared with aerobic conditions in both sample matrices. Humidity, at the levels examined, did not appear to significantly impact C. jejuni survival in any sample matrix. The persistence of Campylobacter in broiler litter and faeces under various environmental conditions has implications for farm litter management, hygiene, and disinfection practices.
Keywords: humidity, temperature, aerobic, microaerophilic, broilers, atmosphere, Campylobacter jejuni
There are an estimated 9.4 million campylobacteriosis cases per annum within the European Union (EU), resulting in the loss of 0.35 million disability-adjusted life years (DALYs) and costing approximately €2.4 billion each year (1). Campylobacter is the most commonly reported bacterial food-borne pathogen in humans, with broiler meat identified as a major source (2). In the Republic of Ireland, there is cause for concern as 83% of domestically produced broiler batches were positive for Campylobacter and corresponding processed carcasses had the second highest (98%) prevalence of Campylobacter contamination within the EU (3). This is of particular concern given that in 2014 the farm gate value of the Irish broiler industry was approximately €133 million (4).
One potential source of Campylobacter contamination may be transmission due to carry-over from previously infected flocks. Research has demonstrated that Campylobacter carry-over between flocks is not a significant risk factor when litter is removed, and houses are cleaned and disinfected before restocking with a new batch of chicks (5–8). In contrast, other studies have identified carry-over between flocks as a potential risk factor, particularly where litter is not replaced between crops (9, 10). It has also been reported that Campylobacter may be found in the wider farm environment prior to the introduction of new flocks (11). Evans and Sayers found no evidence of Campylobacter survival in broiler units in the United Kingdom after cleaning and disinfection was carried out (5). However, a number of studies have suggested that campylobacters can enter a ‘viable but non-culturable’ (VBNC) state, particularly when exposed to atmospheric concentrations of oxygen or heat stresses (12, 13), making their detection in houses prior to restocking difficult. It is possible therefore that Campylobacter could remain viable in the broiler environment and capable of colonising new flocks even though it cannot be detected using traditional culture-based methods.
Campylobacter survival in-vivo is impacted by a wide range of factors such as temperature, atmosphere, sample matrix, and moisture. A previous study on Campylobacter survival in sheep, goose, and hen faeces found survival ranged from 2 to 14 days, with greatest survival at temperatures of 10°C–20°C in association with high rainfall (14–16). Moriarty et al. (15) found that Campylobacter survived in goose faeces on pasture for up to 7 days in winter compared with less than 2 days in summer. Further studies have demonstrated that Campylobacter is highly sensitive to desiccation. Oosterom et al. (17) found that following inoculation, viable Campylobacter cells could only be isolated from the surface of tiles that were visibly wet. The study also found that the drying of pig skin lead to significant declines in Campylobacter populations. Similarly, Doyle and Roman (18) found drying Campylobacter jejuni in the presence of skimmed milk caused significant declines in these populations. However, rates of decline differed between strains of C. jejuni investigated. Campylobacter also prefers microaerophilic atmospheres for growth. Chynoweth et al. (19) found that Campylobacter survival was better in microaerophilic than aerobic atmospheres. The study noted that the difference in their survival was greatest at higher temperatures of approximately 37°C, but their survival in microaerophilic conditions was still higher at temperatures as low as 5°C. A further study found that Campylobacter concentrations grew to 3×108 at 17% oxygen compared with 8×108 at 3% oxygen, highlighting that the organism performs better in lower oxygen concentrations (20). These studies suggest that many factors can influence the environmental survival of C. jejuni.
At present there is a lack of knowledge surrounding how long Campylobacter can persist within environmental niches in broilers and how this may impact transmission.
The aim of this study was to assess the survival of C. jejuni in broiler litter and faeces in-vitro under various environmental conditions that could be found within a broiler house.
Materials and methods
Inoculation of litter and faeces
Used litter (wood shavings) was collected in a sterile bag from a commercial broiler growing unit, housing a 42-day-old Campylobacter-negative flock. Samples were confirmed as Campylobacter negative using the following ISO enrichment and direct plating methods. The litter and faecal samples (10 g) were serially diluted and plated on modified charcoal-cefoperazone-deoxycholate agar (mCCDA), then incubated for 48 hours at 37°C under microaerophilic conditions. A further 10 g from each sample was inoculated into Bolton broth and incubated at 37°C for 24 hours; then, a 10 µl loop of the enriched culture was spread onto an mCCDA plate and incubated for 48 hours (37°C; 5–10% O2) (21–23). Samples of litter (70 g) were placed in 500 ml sterile containers (Sarstedt, Co. Wexford, Ireland) and inoculated with 6 ml of a 9 log10 CFU/ml overnight culture of C. jejuni (strain number PA-346) previously isolated from a broiler flock. Samples were homogenised by shaking for 30 seconds and mixed manually. For each replicate, 5×70 g quantities of litter were inoculated and homogenised before being pooled into a single sample (350 g) and mixed again (24).
Fresh broiler faeces from the same Campylobacter-negative flock was collected from transport crates as birds arrived at the processing plant. Samples were confirmed as Campylobacter negative using enrichment in Bolton broth and plated on mCCDA as previously described. Faecal material (70 g) was inoculated by mixing 6 ml of the 9 log10 CFU/ml overnight C. jejuni (strain number PA-346) culture in 500 ml containers (Sarstedt), then homogenised for 30 seconds, and mixed manually as done previously with the litter (24). For each replicate of the experiment, 5×70 g (350 g) quantities of inoculated sample were prepared and pooled into a single sample and mixed again.
Environmental conditions
Sealable 2.5 litre plastic boxes (BioMérieux, Hampshire, United Kingdom) were used as relative humidity (RH) control chambers. An RH meter (“Traceable” Digital Hygrometer/Thermometer, Fisher Scientific, Dublin, Ireland) was attached to the inner surface of the lid of all boxes.
High RH conditions (≥85%) were obtained by placing 2×20 ml sterile plastic bottles containing 10 ml of sterile water into each box. Sealed boxes were then incubated aerobically or under microaerophilic conditions in order to mimic the atmospheric conditions of litter in broiler houses (25). Microaerophilic conditions (5–10% O2; 10% CO2) were induced by adding an atmospheric gas generation pack (CampyGen 2.5 L, Thermo Fisher Scientific, Dublin, Ireland) to each box. For aerobic conditions, sealed boxes were incubated without any gas packs.
Low RH conditions (≤70%) were obtained by the same method but by omitting the addition of water to the boxes.
In total, Campylobacter survival was assessed under four atmospheric conditions (aerobic and low RH; aerobic and high RH; microaerophilic and low RH; microaerophilic and high RH) at three temperatures (20°C, 25°C, and 30°C). All experiments were repeated in triplicate.
Campylobacter survival in litter and broiler faeces
Samples of inoculated litter (13 g) were placed in sterile petri dishes, spread evenly, and the petri dishes, in turn, were placed inside separate sealed boxes for each atmospheric condition and sampling time point. This was done to ensure the atmosphere was not breached between sampling points.
To quantify initial Campylobacter concentrations in the inoculated litter, three samples (3 g each) were collected at time zero, serially diluted in maximum recovery diluent (MRD; Oxoid, Hampshire, United Kingdom), 0.1 ml volume spread plated on mCCDA (Oxoid), and incubated (42°C; 5–10% O2; 48 hours). The remaining litter samples were then incubated under each atmospheric condition and temperature as described above and sampled every 2 hours over a 12-hour period. At each sampling point, 3×3 g samples of litter were collected and cultured as previously described. The remaining 4 g of litter was used to calculate% litter moisture at each time point under each condition/temperature. RH was recorded at each sampling point. Likewise, Campylobacter survival in broiler faeces was monitored every 24 hours over a 5-day period.
Statistical analysis
A two-tailed t-test assuming unequal variance was used to compare mean Campylobacter concentrations between different time points, temperatures, and atmospheric conditions. A t-test was also used to compare mean litter moisture (%) between sampling time points for each atmospheric condition at each temperature. A P value of ≤0.05 was considered statistically significant.
Results
Relative humidity and (%) moisture content
Relative humidity (%RH) varied depending on sample matrix, atmospheric conditions, temperature, and sampling time point. Lower RH values were generally observed under conditions where water had not been added to the boxes. However, the initial RH in all boxes containing faecal material was greater than 85%; this was due to the faeces being wet at time zero (data not shown). In these cases, the RH declined to less than 70% over 24–48 hours due to the boxes not having any additional water added. A statistically significant (P≤0.05) reduction in% moisture in the litter was observed after 6 and 4 hours when samples were stored under aerobic low RH conditions at 25°C and 30°C, respectively (data not shown).
Campylobacter survival in the litter
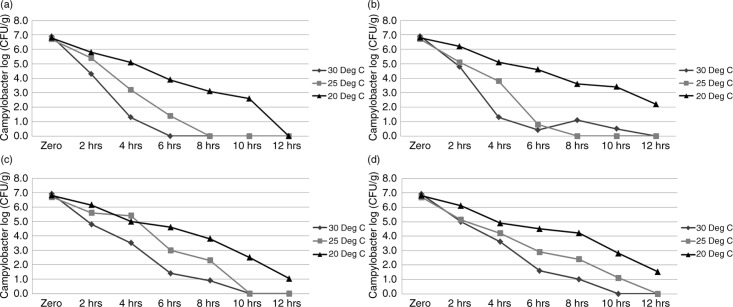
C. jejuni PA-436 survived for longer and in greater concentrations at 20°C under all atmospheric conditions tested (P≤0.05) when compared to the higher incubation temperatures (P≤0.05). Campylobacter concentrations decreased by 2–5 log10 CFU/g over a 12-hour period under all environmental conditions in the litter. Specifically, concentrations decreased from >6 log10 CFU/g initially to between 1 and 2.5 log10 CFU/g after 12 hours in aerobic high RH, microaerophilic low RH, and microaerophilic high RH and 10 hours in aerobic low RH at 20°C. In contrast, Campylobacter was not detected beyond 10 hours when incubated at 25°C and 30°C under any conditions (Fig. 1a–d). The fastest reduction in concentrations was observed in aerobic low RH conditions at 25°C and 30°C, which also saw significant reductions in % moisture in the litter (Fig. 1a). Campylobacter concentrations were significantly higher by 6 hours at 25°C and 30°C in samples stored under microaerophilic conditions compared with aerobic conditions. No significant differences in Campylobacter concentrations were observed at 20°C between samples stored under microaerophilic or aerobic atmospheres at 12 hours in high RH or 10 hours in low RH. However, C. jejuni did persist for 12 hours in microaerophilic low RH compared with 10 hours in corresponding aerobic conditions.
Fig. 1.
Survival of Campylobacter jejuni PA-346 in fresh broiler litter stored under various environmental conditions. (a) Aerobic low RH conditions; (b) aerobic high RH conditions; (c) microaerophilic low RH conditions; (d) microaerophilic high RH conditions.
Campylobacter survival in fresh broiler faeces
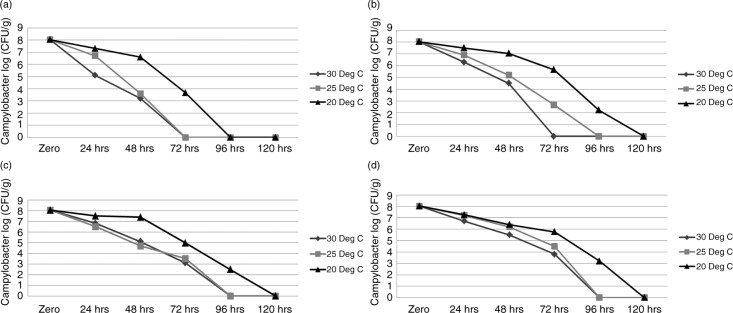
Campylobacter concentrations were significantly higher (P≤0.05) in samples incubated at 20°C under all conditions compared with those incubated at 25°C and 30°C. C. jejuni was not detected beyond 96-hour incubation under aerobic high RH, microaerobic high RH, and microaerophilic low RH conditions and beyond 72 hours in aerobic low RH at 20°C. In contrast, the organism was not detected beyond 72 hours at 25°C and 30°C under aerobic high RH, microaerobic high RH, and microaerobic low RH conditions and beyond 48 hours under aerobic low RH conditions (Fig. 2a–d). C. jejuni survival was significantly higher in samples stored under microaerobic conditions compared to corresponding samples stored under aerobic conditions at all temperatures (P≤0.01). In the low RH conditions, C. jejuni survived 96 hours at 20°C and 72 hours at 25°C and 30°C under microaerophilic conditions (Fig. 2c) compared to 72 hours at 20°C and 72 hours at 25°C and 30°C under aerobic low RH conditions (Fig. 2a). Similarly, in the high RH conditions, C. jejuni survival was higher under microaerophilic conditions, surviving 96 hours at 20°C (3.2 log10 CFU/g in microaerophilic versus 2.23 log10 CFU/g in aerobic) and 72 hours at 25°C (4.5 log10 CFU/g in microaerophilic versus 2.67 log10 CFU/g in aerobic). At 30°C in the high RH conditions, C. jejuni survived for 72 hours under microaerophilic conditions compared with 48 hours under aerobic conditions (Fig. 2d and b). Campylobacter concentrations in faeces decreased by 1–3 log10 CFU/g over a 24-hour period under all environmental conditions.
Fig. 2.
Survival of Campylobacter jejuni PA-346 in fresh broiler faeces stored under various environmental conditions. (a) Aerobic low RH conditions; (b) aerobic high RH conditions; (c) microaerophilic low RH conditions; (d) microaerophilic high RH conditions.
Discussion
Temperature, sample matrix, and atmospheric oxygen concentrations were all important factors affecting Campylobacter survival. RH did not appear to significantly impact Campylobacter survival under all conditions tested. It is possible that the humidity levels in the low (≤70%) RH environment in this study were sufficient to support the survival of Campylobacter. Previous research has demonstrated that the incidence of Campylobacter colonisation in broilers was not impacted by varying RH conditions (60 and 85% RH) (26). This study also noted that predictive models using RH, hours of sunlight, or precipitation did not predict Campylobacter incidence any better than models which used maximum temperature alone. A further study on broiler chickens found no difference in Campylobacter colonisation rates between birds held under high (80%) or low (30%) RH conditions (27), while another study found that C. jejuni inoculated into laying hen faeces persisted for up 72–96 hours when stored at 20°C with 40–60% RH (16).
Campylobacter survived in greater numbers at the lower temperature (20°C) in both sample matrices. The increased survival of Campylobacter at 20°C under all conditions tested could possibly be attributed to the upregulation of survival mechanisms such as the pnp genes at lower temperatures. The pnp genes encode for polynucleotide phosphorylase (PNPase), a 3′–5′ exoribonuclease which has previously been demonstrated to improve the survival of C. jejuni at 4°C and 10°C compared with mutants without the gene (28–30). A review by Lawal et al. noted that upregulation of pnp genes in Escherichia coli was induced with decreasing temperature and that these genes were also responsible for conferring cold growth capabilities to a wide range of pathogenic organisms including C. jejuni (31).
Campylobacter survival was significantly greater under microaerobic conditions compared with aerobic atmospheres in both sample matrices at 25°C and 30°C and less consistently at 20°C. Campylobacters require a microaerophilic environment as atmospheric concentrations of oxygen are toxic. Exposing Campylobacter to oxygen leads to the formation of reactive oxygen intermediates (ROIs) such as superoxide radicals. These ROIs can cause lethal damage to nucleic acids, proteins, and the cell membrane (32). A previous study found that Campylobacter concentrations grew to 3×108 at 17% oxygen compared with 8×108 at 3% oxygen (20). Another study demonstrated that C. jejuni populations survived 2 days longer in stationary water compared to water that was aerated (33). However, the toxic effects of oxygen on Campylobacter are also temperature dependant which may explain the better survival rates at 20°C compared with 25°C and 30°C under aerobic conditions. A study by Garénaux et al. demonstrated C. jejuni was most sensitive to oxygen stress at a temperature of 42°C and most resistant when stored at 4°C (34). This suggests that the increased survival of Campylobacter under aerobic conditions at 4°C was due to lower metabolic activity at 4°C, affecting protein synthesis and decreased catalase activity (34, 35). This demonstrates that temperature can influence the impact of oxygen stress on Campylobacter and that there may be some form of cross resistance between oxidative and temperature stress. A study by Stintzi and Whitworth demonstrated that C. jejuni cold shock responses also gave cross protection to oxygen stress. The study found that C. jejuni overexpressed superoxide dismutases and upregulated sodB and Cj0358 at 4°C, which encode for two proteins that defend against oxidative stress (36). This may explain observations in the current study where fewer differences were seen between microaerobic and aerobic conditions at 20°C.
C. jejuni survived for longer in faeces compared to the litter matrix. This may be due to a number of factors related to the composition of the matrices. For example, previous research has demonstrated that there are large differences in water content between litter and faeces. A study carried out on used broiler litter composed of wood shavings and straw found a dry matter content of 70% (37). In contrast, analysis of fresh poultry faeces demonstrated a dry matter content of approximately 25–30% (38, 39). The higher water content of faeces may protect Campylobacter from the effects of desiccation, thus enhancing its survival (40, 41). Observed differences in survival between the litter and faecal matrices in the current study may be due to differences in the gradient of osmotic pressure between matrices. C. jejuni lacks many osmoadaptive mechanisms, meaning it must rely on other mechanisms to survive hyperosmotic environments (13). In this study, Campylobacter survived in faeces for up to 4 days; a previous study demonstrated that C. jejuni persisted longer (120–144 hours) in naturally contaminated faeces compared with experimentally inoculated faeces (16). Therefore, Campylobacter survival in-vivo may be greater than that observed in this study.
The shorter survival of Campylobacter in litter compared to faeces may also be due to the presence of naturally occurring antimicrobial substances in the litter such as essential oils. Wood shavings are commonly used as bedding material in Irish broiler units and are primarily derived from scots pine and spruce trees. A previous study found that wood from spruce trees contained pinosylvins and phenolic compounds, such as lignans. Other research has shown that pine needles were composed of 30.2% α-terpineol, 24.47% linalool, 14.57% limonene, 14.57% anethole, 3.14% caryophyllene, and 2.14% eugenol. The study found that these essential oils had antioxidant activity by scavenging free radicals and showed antimicrobial activity against a range of food-borne organisms (42). It has been shown that the compounds terpine-4-ol, α-terpineol, and essential oils have strong antimicrobial activity against Campylobacter (43).
It is possible that a proportion of the observed decrease in Campylobacter concentrations over time was due to the organism entering a VBNC state. Campylobacter may enter this state when exposed to environmental stressors such as desiccation, the presence of oxygen, and suboptimal temperatures (33, 35, 44). When in this VBNC state, Campylobacter remains capable of colonising birds (45). A previous study reported that when incubated at 37°C in half-open petri dishes Campylobacter strains became unculturable within 12 hours due to desiccation and oxygen stress (40). This may have consequences for laboratory testing for the detection of Campylobacter in litter or samples from the surrounding farm environment. It also demonstrates that failure to detect Campylobacter using culture-based methods may not prove absence of the organism. A potential solution to overcome this issue may be the use of a BacLight (Thermo Fisher Scientific, Dublin, Ireland) analysis system to detect suspected VBNC state Campylobacter cells. A previous study on Campylobacter survival found that 90% of Campylobacter cells in the experiment that were not detectable using culture (VBNC) were detectable by BacLight analysis (12).
This study has demonstrated the potential effects of various environmental conditions and sample matrices on the survival of C. jejuni. It would be pertinent to carry out any subsequent studies using additional C. jejuni strains given the diversity that exists within this species as other strains could demonstrate different survival characteristics. For example, previous research has demonstrated that the response of C. jejuni to environmental stresses can be strain dependant. Doyle and Roman (18) found inconsistencies in the decline of four C. jejuni populations when they were dried in the presence of skimmed milk at 25°C. In the case of two of the strains, Campylobacter populations declined >7 log10 within 24 hours, whilst another strain took 7 days to decline by 5 log10 CFU (18). Another study by Kaakoush et al. (46) found that whilst most C. jejuni have been shown to be sensitive to the toxic effects of oxygen, some C. jejuni strains can grow in vitro with partial oxygen tensions of up to 21%. The authors noted that the oxygen tolerance of each individual strain was different (46). These studies clearly demonstrate differences in responses among C. jejuni strains to environmental stresses.
The survival of Campylobacter in both sample matrices at lower temperatures may have important implications for broiler litter management. In Ireland, litter is generally removed after depopulation with subsequent cleaning and disinfection of the houses. This can result in contamination of the external farm environment and may risk reintroducing Campylobacter into the broiler houses. A previous study found that Campylobacter-positive flocks were more likely to occur in houses that had an inter-flock period of less than 14 days before restocking (47). A systemic review by Agunos et al. (11) found the greatest risk factors for contamination of flocks was contamination of the farm environment (both inside and outside houses) possibly due to insufficient cleaning and disinfection, followed by insufficient downtime and the presence of multiple houses on farms (11). Furthermore, the study identified the interior and exterior of the house, the air within, drinking water, concrete surrounds, and foot dips as risk factors for Campylobacter.
In conclusion, temperature and sample matrix can have a significant effect on Campylobacter survival in the broiler environment. The study highlights the potential impact that environmental conditions on farm could have on survival and spread of this important enteropathogen. Environmental conditions and sample matrix may vary in broiler farms which may create niches that are favourable to Campylobacter survival.
Acknowledgements
The authors gratefully acknowledge the Food Institutional Research Measure (FIRM) programme administered by the Irish Department of Agriculture, Food and Marine for funding this study (Research grant No. 11SF328). The authors also thank all farmers and stakeholders who participated in this study.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.European Food Safety Authority Panel on Biological Hazards (BIOHAZ) Scientific opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011;9:2105. [Google Scholar]
- 2.European Food Safety Authority & European Centre for Disease Prevention and Control. Trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 2015;13:3991. [Google Scholar]
- 3.European Food Safety Authority. Scientific opinion on quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA J. 2010;8:1437. [Google Scholar]
- 4.Central Statistics Office. Poultry income taken by the Irish state for 2014: agricultural output, input and income. Available from: http://www.cso.ie/px/pxeirestat/Database/eirestat/Agricultural Output Input and Income/Agricultural Output Input and Income_statbank.asp?SP=Agricultural Output, Input and Income&Planguage=0 [cited 6 January 2016]
- 5.Evans SJ, Sayers AR. A longitudinal study of Campylobacter infection of broiler flocks in Great Britain. Prev Vet Med. 2000;46:209–23. doi: 10.1016/s0167-5877(00)00143-4. [DOI] [PubMed] [Google Scholar]
- 6.Shreeve JE, Toszeghy M, Ridley A, Newell DG. The carry-over of Campylobacter isolates between sequential poultry flocks. Avian Dis. 2002;46:378–85. doi: 10.1637/0005-2086(2002)046[0378:TCOOCI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.McDowell SWJ, Menzies FD, McBride SH, Oza AN, McKenna JP, Gordon AW, et al. Campylobacter spp. in conventional broiler flocks in Northern Ireland: epidemiology and risk factors. Prev Vet Med. 2008;84:261–76. doi: 10.1016/j.prevetmed.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Zweifel C, Scheu KD, Keel M, Renggli F, Stephan R. Occurrence and genotypes of Campylobacter in broiler flocks, other farm animals, and the environment during several rearing periods on selected poultry farms. Int J Food Microbiol. 2008;125:182–7. doi: 10.1016/j.ijfoodmicro.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 9.Newell DG, Fearnley C. Sources of Campylobacter colonization in broiler chickens. Appl Environ Microbiol. 2003;69:4343–51. doi: 10.1128/AEM.69.8.4343-4351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damjanova I, Jakab M, Farkas T, Mészáros J, Galántai Z, Turcsányi I, et al. From farm to fork follow-up of thermotolerant campylobacters throughout the broiler production chain and in human cases in a Hungarian county during a ten-months period. Int J Food Microbiol. 2011;150:95–102. doi: 10.1016/j.ijfoodmicro.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Agunos A, Waddell L, Léger D, Taboada E. A systematic review characterizing on-farm sources of Campylobacter spp. for broiler chickens. PLoS One. 2014;9:e104905. doi: 10.1371/journal.pone.0104905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moen B, Oust A, Langsrud Ø, Dorrell N, Marsden GL, Hinds J, Kohler A, Wren B, Rudi K. Explorative multifactor approach for investigating global survival mechanisms of Campylobacter jejuni under environmental conditions. Appl Environ Microbiol. 2005;71:2086–94. doi: 10.1128/AEM.71.4.2086-2094.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cameron A, Frirdich E, Huynh S, Parker CT, Gaynor EC. Hyperosmotic stress response of Campylobacter jejuni . J Bacteriol. 2012;194:6116–30. doi: 10.1128/JB.01409-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moriarty EM, Mackenzie ML, Karki N, Sinton LW. Survival of Escherichia coli, Enterococci, and Campylobacter spp. in sheep feces on pastures. Appl Environ Microbiol. 2011;77:1797–803. doi: 10.1128/AEM.01329-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriarty EM, Weaver L, Sinton LW, Gilpin B. Survival of Escherichia coli, Enterococci and Campylobacter jejuni in Canada Goose Faeces on Pasture. Zoonoses Public Health. 2012;59:490–7. doi: 10.1111/zph.12014. [DOI] [PubMed] [Google Scholar]
- 16. Ahmed MFM, Schulz J, Hartung J. Survival of Campylobacter jejuni in naturally and artificially contaminated laying hen feces. Poultry Sci. 2013;92:364–9. doi: 10.3382/ps.2012-02496. [DOI] [PubMed] [Google Scholar]
- 17.Oosterom J, De Wilde GJA, De Boer E, De Blaauw LH, Karman H. Survival of Campylobacter jejuni during poultry processing and pig slaughtering. J Food Prot. 1983;46:702–6. doi: 10.4315/0362-028X-46.8.702. [DOI] [PubMed] [Google Scholar]
- 18.Doyle MP, Roman DJ. Sensitivity of Campylobacter jejuni to drying. J Food Prot. 1982;45:507–10. doi: 10.4315/0362-028X-45.6.507. [DOI] [PubMed] [Google Scholar]
- 19.Chynoweth RW, Hudson JA, Thom K. Aerobic growth and survival of Campylobacter jejuni in food and stream water. Lett Appl Microbiol. 1998;27:341–4. doi: 10.1046/j.1472-765x.1998.00453.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith MA, Mendz GL, Jorgensen MA, Hazell SL. Fumarate metabolism and the microaerophily of Campylobacter species. Int J Biochem Cell Biol. 1999;31:961–75. doi: 10.1016/s1357-2725(99)00062-x. [DOI] [PubMed] [Google Scholar]
- 21.Koolman L, Whyte P, Bolton DJ. An investigation of broiler caecal counts at first and second thinning. J Appl Microbiol. 2014;117:876–81. doi: 10.1111/jam.12580. [DOI] [PubMed] [Google Scholar]
- 22.International Organisation for Standards. Geneva, Switzerland: ISO; 2006. ISO 10272-1 microbiology of food and animal feeding stuffs. Horizontal method for detection and enumeration of Campylobacter spp-part 1: enrichment method; pp. 1–16. [Google Scholar]
- 23.International Organisation for Standards. Geneva, Switzerland: ISO; 2006. ISO 10272-2 microbiology of food and animal feeding stuffs. Horizontal method for detection and enumeration of Campylobacter spp-part 2: enumeration method; pp. 1–13. [Google Scholar]
- 24.Williams JE, Benson ST. Survival of Salmonella typhimurium in poultry feed and litter at three temperatures. Avian Dis. 1978;22:742–7. [PubMed] [Google Scholar]
- 25.Himathongkham S, Nuanualsuwan S, Riemann H. Survival of Salmonella enteritidis and Salmonella typhimurium in chicken manure at different levels of water activity. FEMS Microbiol Lett. 1999;172:159–63. doi: 10.1111/j.1574-6968.1999.tb13464.x. [DOI] [PubMed] [Google Scholar]
- 26.Patrick ME, Christiansen LE, Waino M, Ethelberg S, Madsen H, Wegener HC. Effects of climate on incidence of Campylobacter spp. in humans and prevalence in broiler flocks in Denmark. Appl Environ Microbiol. 2004;70:7474–80. doi: 10.1128/AEM.70.12.7474-7480.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Line JE. Influence of relative humidity on transmission of Campylobacter jejuni in broiler chickens. Poult Sci. 2006;85:1145–50. doi: 10.1093/ps/85.7.1145. [DOI] [PubMed] [Google Scholar]
- 28.Rosenzweig JA, Chopra AK. The exoribonuclease polynucleotide phosphorylase influences the virulence and stress responses of yersiniae and many other pathogens. Front Cell Infect Microbiol. 2013;3:1–8. doi: 10.3389/fcimb.2013.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haddad N, Tresse O, Rivoal K, Chevret D, Nonglaton Q, Burns CM, et al. Polynucleotide phosphorylase has an impact on cell biology of Campylobacter jejuni . Front Cell Infect Microbiol. 2012;2:1–13. doi: 10.3389/fcimb.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haddad N, Burns CM, Bolla JM, Prévost H, Fédérighi M, Drider D, et al. Long-term survival of Campylobacter jejuni at low temperatures is dependent on polynucleotide phosphorylase activity. Appl Environ Microbiol. 2009;75:7310–18. doi: 10.1128/AEM.01366-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawal A, Jejelowo O, Chopra AK, Rosenzweig JA. Ribonucleases and bacterial virulence. Microbial Biotechnol. 2011;4:558–71. doi: 10.1111/j.1751-7915.2010.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park SF. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int J Food Microbiol. 2002;74:177–88. doi: 10.1016/s0168-1605(01)00678-x. [DOI] [PubMed] [Google Scholar]
- 33.Rollins DM, Colwell RR. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986;52:531–8. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garénaux A, Jugiau F, Rama F, de Jonge R, Denis M, Federighi M, et al. Survival of Campylobacter jejuni strains from different origins under oxidative stress conditions: effect of temperature. Curr Microbiol. 2008;56:293–7. doi: 10.1007/s00284-007-9082-8. [DOI] [PubMed] [Google Scholar]
- 35.Hazeleger WC, Wouters JA, Rombouts FM, Abee T. Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl Environ Microbiol. 1998;64:3917–22. doi: 10.1128/aem.64.10.3917-3922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stintzi A, Whitworth L. Investigation of the Campylobacter jejuni cold-shock response by global transcript profiling. Genome Lett. 2003;2:18–27. [Google Scholar]
- 37.Nicholson FA, Chambers BJ, Smith KA. Nutrient composition of poultry manures in England and Wales. Bioresour Tech. 1996;58:279–84. [Google Scholar]
- 38.Quiroga G, Castrillón L, Fernández-Nava Y, Marañón E. Physico-chemical analysis and calorific values of poultry manure. Waste Manag. 2010;30:880–4. doi: 10.1016/j.wasman.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Kelleher BP, Leahy JJ, Henihan AM, O'Dwyer TF, Sutton D, Leahy MJ. Advances in poultry litter disposal technology – a review. Bioresour Tech. 2002;83:27–36. doi: 10.1016/s0960-8524(01)00133-x. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez H, Vergara M, Tapia F. Desiccation resistance in thermotolerant Campylobacter species. Infection. 1985;13:197. doi: 10.1007/BF01642813. [DOI] [PubMed] [Google Scholar]
- 41.Kusumaningrum HD, Riboldi G, Hazeleger WC, Beumer RR. Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int J Food Microbiol. 2003;85:227–36. doi: 10.1016/s0168-1605(02)00540-8. [DOI] [PubMed] [Google Scholar]
- 42.Zeng W-C, Zhang Z, Gao H, Jia L-R, He Q. Chemical composition, antioxidant, and antimicrobial activities of essential oil from pine needle (Cedrus deodara) J Food Sci. 2012;77:C824–9. doi: 10.1111/j.1750-3841.2012.02767.x. [DOI] [PubMed] [Google Scholar]
- 43.Kurekci C, Padmanabha J, Bishop-Hurley SL, Hassan E, Al Jassim RAM, McSweeney CS. Antimicrobial activity of essential oils and five terpenoid compounds against Campylobacter jejuni in pure and mixed culture experiments. Int J Food Microbiol. 2013;166:450–7. doi: 10.1016/j.ijfoodmicro.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 44.Buswell CM, Herlihy YM, Lawrence LM, McGuiggan JTM, Marsh PD, Keevil CW, et al. Extended survival and persistence of Campylobacter spp. in water and aquatic biofilms and their detection by immunofluorescent-antibody and -rRNA staining. Appl Environ Microbiol. 1998;64:733–41. doi: 10.1128/aem.64.2.733-741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaisowwong W, Kusumoto A, Hashimoto M, Harada T, Maklon K, Kawamoto K. Physiological characterization of Campylobacter jejuni under cold stresses conditions: its potential for public threat. J Vet Med Sci. 2012;74:43–50. doi: 10.1292/jvms.11-0305. [DOI] [PubMed] [Google Scholar]
- 46.Kaakoush NO, Miller WG, De Reuse H, Mendz GL. Oxygen requirement and tolerance of Campylobacter jejuni . Res Microbiol. 2007;158:644–50. doi: 10.1016/j.resmic.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Hald B, Wedderkopp A, Madsen M. Thermophilic Campylobacter spp. in Danish broiler production: a cross-sectional survey and a retrospective analysis of risk factors for occurrence in broiler flocks. Avian Pathol. 2000;29:123–31. doi: 10.1080/03079450094153. [DOI] [PubMed] [Google Scholar]