Abstract
Two new cyclic heptapeptides, stylisin 1(1) and stylisin 2 (2), were isolated and characterized from the Jamaican sponge Stylissa caribica, in addition to the cyclic heptapeptide phakellistatin 13 (3) and the known bromopyrrole alkaloids sceptrin (4), stevensine (5), and oroidin (6). The new structures were assigned on the basis of 1D and 2D NMR spectroscopic data, as well as chemical methods for the elucidation of the absolute configuration of amino acids. The peptides and alkaloids have been evaluated for their antimicrobial, antimalarial, anticancer, anti-HIV-1, anti-Mtb, and antiinflammatory activities.
Peptides are well-established bioactive metabolites from marine invertebrates1,2 and include linear peptides,3,4 depsipeptides,5,6 and cyclic7–10 and bicyclic peptides.11,12 Species of the sponge genera Phakellia and Axinella have yielded several bioactive cyclic peptides13–15 reported to have cytotoxic activity against a number of cancer cell lines. It has been noted that most of these cyclic peptides are rich in proline amino acid moieties, which may be in either cis or trans conformation. There is evidence that some of the sponge cyclic peptides are able to bind undetectable quantities of a very potent antineoplastic substance that can only be revealed through biological assays.16
In this report as part of an investigation of active sponge extracts against infectious diseases two new cyclic heptapeptides, stylisin 1 (1) and stylisin 2 (2), and the known peptide phakellistatin 13 (3) were isolated as well as the known alkaloids sceptrin (4), stevensine (5), and oroidin (6). Here we describe the isolation and structure elucidation of the two new cyclic peptides. Several reports describing the chemistry of different marine sponges belonging to Stylissa spp. have been published including aldisines from Stylissa massa,17 latonduines from Stylissa carteri,18 and massadine from Stylissa aff. massa.19 In a study regarding the chemistry of Stylissa caribica Lehnert & van Soest, 1998 (Halichondrida: Dictyonellidae), a series of bromopyrrole alkaloids have been identified.20 No cyclic peptides have been previously reported from Stylissa species, and these results have suggested a possible microbial or symbiotic origin for these metabolites.
Results and Discussion
The sponge extract was dissolved in ethanol and subjected to silica gel vacuum-liquid chromatography followed by column chromatography, Sephadex LH-20, RP HPLC, and PTLC chromatography to yield two new cyclic heptapeptides, stylisin 1 (1) and stylisin 2 (2), the known cyclic peptide phakellistatin 13 (3), and the known alkaloids sceptrin, stevensine, and oroidin (4–6).
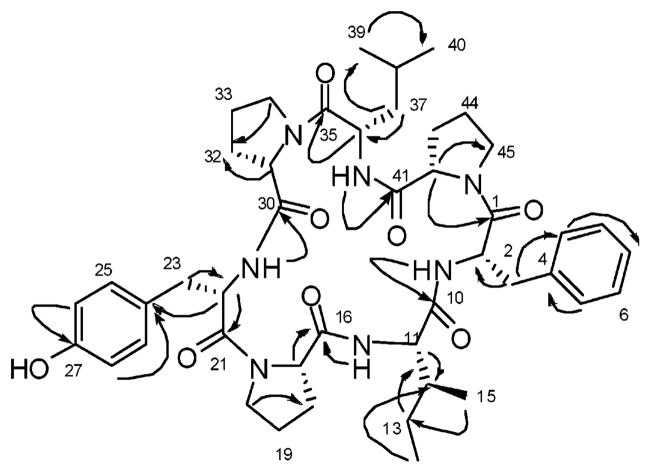
Stylisin 1 (1) (Table 1) was obtained as a white solid with the molecular formula C45H61N7O8 as determined by HRESIMS 850.4428 [M + Na+] (calcd 850.4479). The 13C NMR spectrum contained seven carbonyls, and the 1H NMR showed four secondary amide protons (δ 6.77–8.95) and 19 degrees of unsaturation. All the amino acids were elucidated on the basis of COSY, HMQC, HMBC, and NOESY experiments (Figures 2 and 3). Each amino acid was connected to the adjacent amino acid in a cyclic form based on the HMBC correlations between the αH and NH with the adjacent CO. An HMBC correlation was assigned between the isoleucine NH and CO of Pro1 at δ 171.48 and generated a partial structure for a Pro1-Ile. The amide proton at δ 7.44 of Phe gave an HMBC correlation to CO of Ile at δ 170.5 while the CO of Phe at δ 172.1 showed an HMBC to the αH of Pro3, thus generating a partial structure of four amino acids. The remaining amino acids were assigned through the following HMBC correlations of the residual COs and vicinal NH protons, as well as COSY correlations between NH and αH signals. An HMBC correlation between the amide proton of Leu at δ 8.15 and CO of Pro3 at δ 172.7 provided the partial structure Leu-Pro3-Phe-Ile-Pro1. Pro2 and Tyr yielded the second partial structure through an HMBC correlation between the Tyr amide proton at δ 6.77 and CO of Pro2 at δ 170.2. A NOESY correlation between the αH of Leu at δ 4.71 and αH of Pro2 at δ 4.42 allows the connection of Pro2 to Leu. The last connection between Tyr and Pro1 revealed the cyclic structure with a NOESY correlation between the αH of Tyr at δ 4.55 and αH of Pro1 at δ 4.17. The cis/trans conformation of Pro1, 2, and 3 was determined to be cis on the basis of the Δδβγ (differential value of 13C chemical shifts of Cβ and Cγ in proline),22,23 the value of the three proline moieties, which was >9 ppm, and the NOE correlations between αH of Pro 1, 2, and 3 and the αH of the adjacent amino acid. Marfey’s analysis was used to determine the absolute configuration as L for all the constituent amino acids of stylisin 1.24
Table 1.
13C and 1H NMR Data of Stylisin 1 (1)a
| amino acid | position | δC | δH | HMBC (1H–13C) |
|---|---|---|---|---|
| Phe | C1 | 172.1, qC | ||
| C2 | 55.8, CH | 4.55, m | C1, C3 | |
| C3 | 36.2, CH2 | 3.00, m; 3.05, m | C2, C9 | |
| C4 | 139.7, qC | |||
| C5,5′ | 129.5, CH | 7.37, t; 16.0, 9.0 Hz | C9, C7 | |
| C6,6′ | 128.1, CH | 7.28, t; 15.0, 8.0 Hz | C8, C7 | |
| C7 | 126.1, CH | 7.18, t; 15.0, 7.5 Hz | C6, C5 | |
| NH | 7.44, d; 9.2 Hz | C2, C10 | ||
| Ile | C1 | 170.5, qC | ||
| C2 | 53.9, CH | 4.53, m | C10, C12, C13, C14 | |
| C3 | 38.2, CH | 1.70, m | C13, C14, C15 | |
| C4 | 14.8, CH3 | 0.89, d; 7.0 Hz | C11, C12, C14 | |
| C5 | 24.1, CH2 | 1.20, m; 1.51, m | C12, C13, C15 | |
| C6 | 10.2, CH3 | 0.86, m | C12, C14 | |
| NH | 8.95, d; 8.4 Hz | C16 | ||
| Pro1 (cis) | C1 | 171.5, qC | ||
| C2 | 60.9, CH | 4.17, d; 8.0 Hz | C16, C18, C19 | |
| C3 | 31.5, CH2 | 2.11, m; 2.30, m | C17, C20 | |
| C4 | 21.9, CH2 | 1.61, m; 1.72, m | C17 | |
| C5 | 46.8, CH2 | 3.25, m; 3.41, m | C17, C18 | |
| Tyr | C1 | 169.3, qC | ||
| C2 | 53.0, CH | 4.55, m | C21, C23 | |
| C3 | 36.3, CH2 | 3.27, m; 3.25, m | C21, C22, C29 | |
| C4 | 127.1, qC | |||
| C5,5′ | 131.1, CH | 6.98, d; 8.5 Hz | C26, C27, C29 | |
| C6,6′ | 115.1, CH | 6.78, d; 8.5 Hz | C27, C28, C24 | |
| C7 | 156.5, qC | |||
| NH | 6.77, d; 4.8 Hz | C30 | ||
| Pro2 (cis) | C1 | 170.2, qC | ||
| C2 | 61.9, CH | 4.42, m | C30, C32, C34 | |
| C3 | 31.8, CH2 | 2.11, m | C31 | |
| C4 | 22.2, CH2 | 1.97, m | C31 | |
| C5 | 46.3, CH2 | 3.48, m; 3.62, m | C31 | |
| Leu | C1 | 170.9, qC | ||
| C2 | 52.2, CH | 4.71, m | C37, C35, C38 | |
| C3 | 38.8, CH2 | 1.35, m; 1.62, m | C39, C40 | |
| C4 | 24.9, CH | 1.17, m | C39, C40 | |
| C5 | 20.7, CH3 | 1.02, d; 6.5 Hz | C38, C40 | |
| C6 | 23.4, CH3 | 0.88, d; 6.5 Hz | C38, C40 | |
| NH | 8.15, d; 3.6 Hz | C41 | ||
| Pro3 (cis) | C1 | 172.7, qC | ||
| C2 | 58.6, CH | 4.58, m | C44, C45, C1 | |
| C3 | 30.7, CH2 | 1.85, m; 2.01, m | ||
| C4 | 21.7, CH2 | 1.54, m; 1.51, m | C42 | |
| C5 | 47.12, CH2 | 3.72, m | C42 |
In acetone-d6, 600 MHz for 1H and 150 MHz for 13C NMR. Carbon multiplicities were determined by DEPT experiments. Coupling constants (J) are in Hz.
Figure 2.
Key HMBC correlations of stylisin 1 (1).
Figure 3.
Key NOESY correlations of stylisin 1 (1).
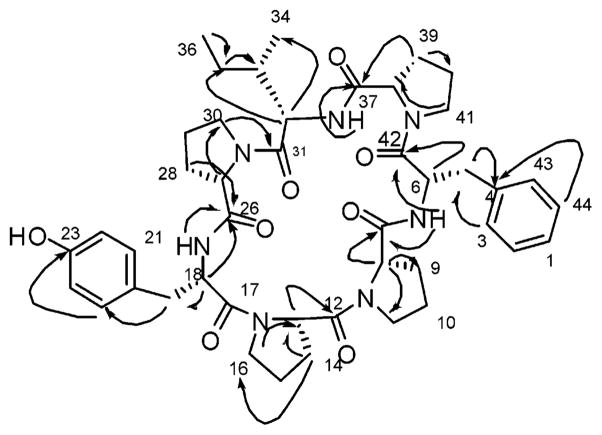
Stylisin 2 (2) (Table 2) was obtained as a white solid with the molecular formula C44H57N7O8, determined by HRESIMS to be 834.4088 [M + Na+] (cald 834.4166). The heptapeptide skeleton was suggested from the presence of seven carbonyl carbons in the 13C NMR spectrum at δ 168.5–172.0. The large number of methylene carbons (14 CH2) and an unsaturation number of 20 suggested a greater number of proline moieties constituting the structural skeleton. Close inspection of the 1D and 2D NMR data [1H, 13C, COSY, HMQC, HMBC and ROESY (Figures 4 and 5)] resulted in three main partial structures, Phe-Pro1, Pro1-Ile, Pro4-Tyr, and a remaining Pro3 moiety. The two aromatic systems of Tyr and Phe were elucidated on the basis of both COSY and HMBC correlations. The Phe amide proton at δ 6.79 showed COSY correlations to the αH of Phe at δ 4.34 and showed an HMBC correlation of the Pro2 carbonyl at δ 171.3, which resulted in the partial structure Phe-Pro2. The HMBC and COSY correlations of the Ile amide proton at δ 8.98 to the Pro1 carbonyl at δ 170.8 and to the αH of Ile at δ 4.18, respectively, allowed the formation of the second partial structure Ile-Pro1. The δH of Pro1 at δ 3.25 showed an HMBC correlation to the Phe carbonyl at δ 168.5 and allowed the connection between the first and second partial structure (Ile-Pro1-Phe-Pro2). An HMBC correlation between the Tyr amide proton at δ 7.62 and the carbonyl carbon of Pro4 at δ 170.7 connected Pro4 and Tyr to form the third partial structure. An HMBC correlation between the carbonyl carbon of the Ile moiety at δ 171.6 and the αH of Pro4 at δ 3.57 allowed the formation of the larger partial structure Tyr-Pro4-Ile-Pro1-Phe-Pro2. Three COSY correlations between the protons at δ 4.22 and 1.90, δ 1.90 and 1.85, and δ 1.85 and 3.50 and an HMBC correlation between the carbonyl at δ 169.4 and the proton at δ 4.22 allowed the construction of the Pro3 moiety, which lacks any HMBC correlations to its carbonyl carbon, α or δH. Seven amide carbonyls along with the presence of 20 degrees of unsaturation including eight for two aromatic systems, four for the proline moieties, and seven for the seven carbonyl carbons gave a total of 19 degrees of unsaturation, leaving one degree of unsaturation for the cyclic structure of stylisin 1. Two key ROESY correlations between γH of Pro3 at δ 1.85 and the βH of Pro2 at δ 1.47 and between the αH of Pro3 at δ 4.22 and the βH of Tyr at δ 2.70 allowed the connection of the Pro3 to Pro2 and Tyr to produce the complete structure of stylisin 2. The amino acid sequence of stylisin 2 was Tyr-Pro4-Ile-Pro1-Phe-Pro2-Pro3. The differential value of Δδβγ was calculated for Pro1 and 4 (6 and 6.2 ppm, respectively), which indicated that Pro1 and Pro4 were in trans conformation. The conformation was cis for Pro2 and Pro3 (Δδβγ are 9.1 and 8.2 ppm, respectively). The ROESY correlations between the αH of Pro2 and 3 and the αH of the adjacent amino acid further support the cis conformation of these two prolines. Using Marfey’s analysis the absolute configuration of the amino acids of stylisin 2 was determined to be L.
Table 2.
13C and 1H NMR Data of Stylisin 2 (2)a
| amino acid | position | δC | δH | HMBC (1H–13C) |
|---|---|---|---|---|
| Phe | C1 | 168.5, qC | ||
| C2 | 53.3, CH | 4.34, m | C42, C5 | |
| C3 | 38.1, CH2 | 3.06, m; 3.21, m | C4, C6, C3 | |
| C4 | 136.9, qC | |||
| C5,5′ | 130.0, CH | 7.11, t; 13.8,7.2 Hz | C1, C2, C5, C43 | |
| C6,6′ | 128.4, CH | 7.27; 14.0, 7.8 Hz | C44, C3, C1 | |
| C7 | 127.1, CH | 7.26, t; 15.0, 7.8 Hz | C2, C3 | |
| NH | 6.79, d; 9.6 Hz | C7 | ||
| Pro2 (cis) | C1 | 171.3, qC | ||
| C2 | 61.3, CH | 3.62, m | C7, C10 | |
| C3 | 31.4, CH2 | 1.47, m | C11, C10 | |
| C4 | 22.3, CH2 | 1.88, m | C8 | |
| C5 | 47.5, CH2 | 3.64, m | C8 | |
| Pro3 (cis) | C1 | 169.4, qC | ||
| C2 | 57.2, CH | 4.22, d, 7.8 Hz | C12 | |
| C3 | 29.7, CH2 | 1.90, m | C16 | |
| C4 | 21.5, CH2 | 1.85, m | C13 | |
| C5 | 47.0, CH2 | 3.50, m, 3.52, m | C14 | |
| Tyr | C1 | 172.0, qC | ||
| C2 | 52.0, CH | 4.62, q, 10.0, 6.0 Hz | C17, C19 | |
| C3 | 38.2, CH2 | 2.70, q, 12.0, 5.5 Hz; 2.83, m | C20, C21 | |
| C4 | 136.9, qC | |||
| C5,5′ | 130.6, CH | 6.95, d; 8.4 Hz | C21, C22 | |
| C6,6′ | 115.5, CH | 6.80, d; 9.0 Hz | C22, C23 | |
| C7 | 156.7, qC | C23, C24 | ||
| NH | 7.62, d; 4.8 Hz | C26 | ||
| Pro4 (trans) | C1 | 170.7, qC | ||
| C2 | 61.3, CH | 3.57, m | C26, C29 | |
| C3 | 28.1, CH2 | 2.19, m | C30 | |
| C4 | 21.9, CH2 | 1.61, m | C27 | |
| C5 | 46.5, CH2 | 3.57, m | C27, C31 | |
| Ile | C1 | 171.6, qC | ||
| C2 | 58.4, CH | 4.18, d, 8.4 Hz | C31, C34 | |
| C3 | 35.5, CH | 2.08, m | C36, C34 | |
| C4 | 15.8, CH3 | 0.80, d; 7.8 Hz | C35, C32 | |
| C5 | 25.8, CH2 | 1.27, m; 1.4, m | C36, C33 | |
| C6 | 10.6, CH3 | 0.82, t; 6.6, 3.0 Hz | C35, C33 | |
| NH | 8.98, d; 3.6 Hz | C37 | ||
| Pro1 (trans) | C1 | 170.8, qC | ||
| C2 | 59.3, CH | 4.44, m | C37, C41 | |
| C3 | 31.3, CH2 | 2.69, q; 5.4, 12.0 Hz | C37, C41 | |
| C4 | 25.6, CH2 | 2.08, m; 2.21, m | C38 | |
| C5 | 46.2, CH2 | 3.25, m | C42 |
In acetone-d6, 600 MHz for 1H and 150 MHz for 13C NMR. Carbon multiplicities were determined by DEPT experiments. Coupling constants (J) are in Hz.
Figure 4.
Key HMBC correlations of stylisin 2 (2).
Figure 5.
Key ROESY correlations of stylisin 2 (2).
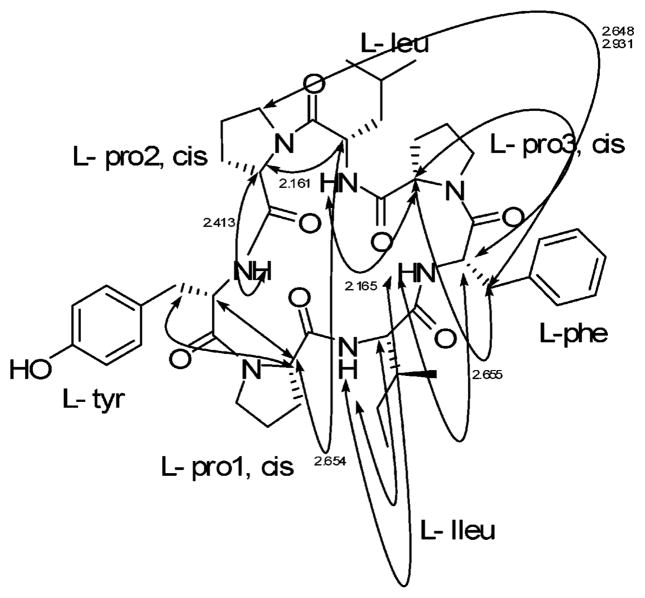
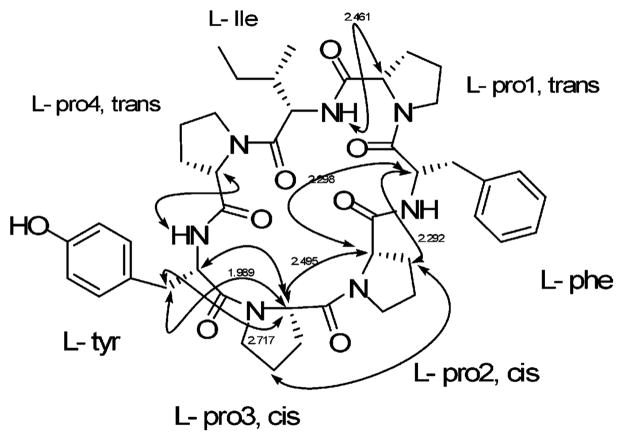
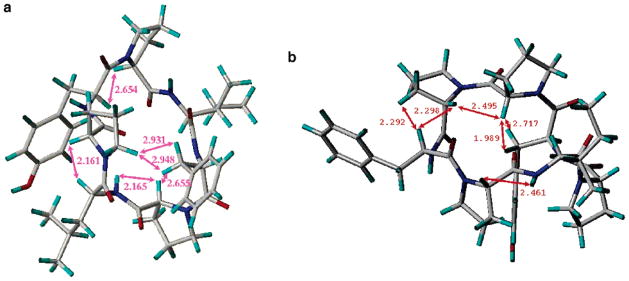
The conformations of stylisin 1 (1) and stylisin 2 (2) were analyzed using molecular modeling combined with NOE NMR experiments (Figure 1a,b). The lowest energy conformers for stylisins 1 and 2 (163.4, 185.0 kcal/mol, respectively) were obtained by simulated annealing and were highly consistent with the experimentally determined NOESY and ROESY data (Figures 3, 5). The distances between the correlated protons, some of which are shown in Figure 1, are less than 3 Å, indicating that the conformation of stylisins 1 and 2 in acetone is highly consistent with the modeled conformers shown in Figure 1.
Figure 1.
(a) Conformation of 1 with minimized energy. (b) Conformation of 2 with minimized energy. The arrows show the NOE correlations, and the data are the distances in Å between correlated protons.
An investigation of active sponge extracts yielded two new cyclic heptapeptides, stylisins 1 and 2, and the known peptide phakellistatin 13 from the sponge S. caribica. Phakellistatin 13 was previously isolated from the sponge Phakellia fusca Thiele and was reported to have potent cytotoxic activity against the human hepatoma BEL-7404 cell line with an ED50 < 0.01 μg/mL.21 Our evaluation of phakellistatin 13 did not show any cytotoxic activity against the NCI tumor cell lines. These results again highlight the problem of lost activity associated with these metabolites, which may be due to changes in the conformation of the cyclic peptide or due to its binding with highly toxic natural products, which can be detected only in the biological assays. The isolated peptides have shown no cytotoxicity against a number of cancer cell lines. They have been tested for their antimicrobial (C. albicans, C. neoformans, S. aureus, MRS, P. aeruginosa, M. intracellualare, and A. fumigatus), antimalarial (P. falciparum, D6 and W2 clone), anti-Mtb, and antiinflammatory activity in rat neonatal microglia and anti-HIV-1 in PBM cells and were inactive in all of the assays. The three alkaloids have been evaluated for their antimalarial activity (P. falciparum, D6 and W2 clone), and only oroidin and stevensine showed marginal activity, with IC50 values of 1200 and 1800 ng/mL against the D6 clone. Oroidin and stevensine showed 71% and 95% inhibition at 128 μg/mL, respectively, against M. tuberculosis, while sceptrin showed no activity at a concentration of >128 μg/mL. The three alkaloids have been tested for their activity against several pathogenic fungi and bacteria, including C. albicans, C. neoformans, S. aureus, MRS, P. aeruginosa, M. intracellulare, and A. fumigatus. Only sceptrin showed activity against C. neoformans, with an IC50 of 3.5 μg/mL. The three alkaloids were not active against Leishmania donovani, nor were they found to be active when assayed against HIV-1 in PBM cells.
In summary the peptides and alkaloids have been evaluated for their antimicrobial, antimalarial, anticancer, anti-HIV-1, anti-Mtb, and antiinflammatory activities. None of the peptides have shown any activity in the stated biological assays. Sceptrin was moderately active against C. neoformans, with an IC50 of 3.5 μg/mL, while oroidin and stevensine were slightly active at 128 μg/mL in the assay against M. tuberculosis.
Experimental Section
General Experimental Procedures
Optical rotations were measured with a JASCO DIP-310 digital polarimeter. UV and IR spectra were obtained using a Perkin-Elmer Lambda 3B UV/vis and AATI Mattson, Genesis Series FTIR spectrophotometer, respectively. The 1H and 13C NMR spectra were recorded in acetone-d6 using NMR spectrometers operating at 500 or 600 MHz for 1H and 125 or 150 MHz for 13C NMR. Chemical shifts, with δ values expressed in parts per million (ppm), are referenced to the residual solvent signals with resonances at δH/δC 2.09/29.91, 206.71 (acetone-d6). The HRMS spectra were measured using a Bioapex FTESI-MS with electrospray ionization. Chromatographic procedures were carried out using normal and reversed-phase (RP) Si gel chromatography, Sephadex LH-20, and C8 and NH2 HPLC (Waters 510 model system, Luna 5 μm, C8(2) 100 Å, 60 × 21.20 mm and Phenomenex Luna 5 μm NH2 100 Å, 250 × 21.29 mm, 5 μm). TLC analysis was carried out on precoated Si gel G254.
Taxonomy, Extraction, and Isolation
This common sponge was collected from a silted reef, between 22 and 30 m deep, at Columbus Park, Jamaica, on November 16, 2002. In life the sponge forms thin fleshy conulose corrugated lamellae emanating from a central thickened region, arising from a rounded stalk. The sponge is deep orange, the texture fleshy and smooth, the sponge flexible and elastic. The skeleton consists of a loose large-meshed reticulation of predominantly angular styles embedded in spongin or free in the choanosome, in a loosely compressed axial region. The external parts of the sponge are fleshy with relatively few spicules. The sponge is closest to Stylissa caribica Lehnert & van Soest 1998 (order Halichondrida, family Dictyonellidae) and can be easily mistaken for Axinella corrugata (George & Wilson, 1919) sensu Alvarez et al. (1998), formerly known as Teichaxinella morchella Wiedenmayer, 1977. A voucher specimen has been deposited in the Natural History Museum, London (BMNH 2003.9.19.1).
Four kilograms of the fresh sponge was freeze-dried and blended with EtOH. The blended material was extracted with EtOH and EtOH/H2O and dried under vacuum. The dried residue was subjected to vacuum-liquid chromatography using Si gel and a gradient of hexanes, EtOAc, EtOH, and H2O to give 11 fractions. Fraction 7, eluted with EtOAc/EtOH (1:1), was dissolved in MeOH. The MeOH-soluble portion was chromatographed using a C18 flash Si column and a Sephadex LH-20 column, followed by RP C8 HPLC, and further purified on a HPLC amino column. Two new peptides, stylisin 1 and stylisin 2, were obtained in addition to phakellistatin 13, which was further purified using PTLC. The 1H NMR and 13C NMR of the insoluble MeOH portion of fraction 7 showed a mixture of small metabolites related to the bromopyrrole alkaloid class, which on further purification by gel chromatography and HPLC produced the three known alkaloids stevensine (5) (1.5 g), oroidin (6) (0.5 g), and sceptrin (4) (100 mg).
The absolute configuration of the stylisins was determined by Marfey’s analysis method. One milligram of stylisin 1 (1) and 0.5 mg of stylisin 2 (2) were subjected to hydrolysis by 6 N HCl in a 1 mL ReactiVial for 24 h at 100 °C. The hydrolyzate was then dried under vacuum and derivatized by Marfey’s reagent (40 μL of FDAA 10% in acetone). Eight microliters of 1 M NaHCO3 was added and heated for 1 h at 40 °C. Then 4 μL of 2 N HCl was added, and the mixture was dried and dissolved in 50 μL of DMSO. The products were analyzed by a HPLC Waters Nova Pack 3.9 × 150 mm column at λ 340 nm, using a gradient of triethylamine phosphate buffer (pH 3.00 ± 0.02) and acetonitrile (90% to 60% TEAP in 1 h). The retention times in minutes of the L and D amino acids were as follows (Thr 22.5, 36.0; Pro 30.7, 36.6; Leu 49.2, 59.3; Ile 49.1, 59.1; Phe 51.1, 58.6; Tyr 63.7, 70.1; alloIle 51.6, 60.3). The retention times of stylisin 1 hydrolyzate were as follows (Leu 49.3, Pro 30.5, Phe 51.0, Ileu 49.1, Tyr 63.9), and those for stylisin 2 hydrolyzate were as follows (Ileu 49.0, Pro 30.6, Phe 51.0, Tyr 64.3).
Molecular modeling was performed using SYBYL 6.8 (Tripos Associates, St. Louis, MO). Initial conformations of the molecule were obtained by 10 rounds of dynamics simulations, in which the molecule was heated to 500 K within 500 fs and then allowed to cool to 200 K within 2000 fs. Twenty lowest energy conformations were selected and refined by molecular mechanics minimization using Powell’s gradient algorithm method with the MMFF94 force field, a constant-dependent dielectric of 4.00, and partial atomic charges, until a root-mean-square deviation of 0.001 kcal/mol·Å was achieved. Finally, from these refined conformations, the conformer with the lowest energy was selected as the global minimum, representing the most likely molecular configuration.
Stylisin 1 (1): white solid (MeOH); [α]D25 −44.9 (c 0.2, MeOH); UV (MeOH) λmax (log ε) 212 (3.12), 278 (1.84) nm; IR (KBr film) νmax 3279, 2960, 1635, 1516, 1447, 1240, 703 cm−1; NMR data, see Table 1; HRESIMS m/z 850.4428 [M + Na+], calcd for C45H61N7O8, 850.4479
Stylisin 2 (2): white solid (MeOH); [α]D25 −39.9 (c 0.2, MeOH); UV (MeOH) λmax (log ε) 214 (3.17), 278 (2.07) nm; IR (KBr film) νmax 3403, 2963, 1642, 1516, 1449, 1348, 703 cm−1; NMR data, see Table 2; HRESMS 834.4088 [M + Na+], calcd for C44H57N7O8, 834.4166.
Phakellistatin 13 (3): pale yellow solid (MeOH); [α]D25 −127 (c 0.09, MeOH), the reported [α]D25 was −136 (c 0.09, MeOH); UV (MeOH) λmax (log ε) 224 (3.22), 280 nm (2.63); IR (KBr film) νmax 3315, 2955, 1641, 1530, 1454, 1082, 745 cm−1; NMR data;21 HRESIMS m/z 821.4514 [M + Na+], calcd for C42H53N8O8.
Sceptrin (4): brownish solid with the molecular formula C22H24N10O2Br2, which was determined by HRESIMS 619.329, 621.425, and 623.526, indicating dibromo substitution. By comparison of ESIMS, 1H NMR, DEPT, and 13C NMR data with the literature25 the compound was identified as sceptrin.
Stevensine (5): yellowish powder with the molecular formula C11H9N5OBr2, which was determined by HRESIMS 385.929, 387.925, and 389.926, indicating dibromo substitution. By comparison of ESIMS, 1H NMR, DEPT, and 13C NMR data with the literature26 the compound was identified as stevensine.
Oroidin (6): yellowish powder with the molecular formula C11H11N5OBr2, which was determined by HRESIMS 387.94, 389.931, and 391.931, indicating dibromo derivatives. By comparison of ESIMS, 1H NMR, DEPT, and 13C NMR data with the literature25 the compound was identified as oroidin.
Chart 1.
Acknowledgments
We are grateful to F. T. Wiggers for acquiring the NMR data, and NIH grant number 1RO1AI36596 (M.T.H.). This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program (C06 RR-14503-01) from the National Center for Research Resources, NIH. The Natural Resource Conservation Authority, Jamaica and Discovery Bay Marine Laboratory (Contribution #718) are greatly acknowledged for assistance with sample collections. We would also like to thank Dr. Larry Walker of the National Center for Natural Products Research for performing antimicrobial and antiparasitic assays, Dr. Scott Franzblau at the University of Illinois at Chicago for Mtb assays, Dr. Raymond Schinazi at Emory University School of Medicine for HIV-1 assays, Dr. Fred Valeriote at the Henry Ford Health System for cytotoxicity assays, and Dr. Alejandro Mayer at Midwestern University for anti-inflammatory assays. R.M. thanks the Egyptian Government for a predoctoral fellowship.
References and Notes
- 1.Matsunaga S, Fusetani N. Curr Org Chem. 2003;7:945–966. [Google Scholar]
- 2.Hamann MT, Scheuer PJ. J Am Chem Soc. 1993;115:5825–5826. [Google Scholar]
- 3.Hamada T, Matsunaga S, Yano G, Fusetani N. J Am Chem Soc. 2005;127:110–118. doi: 10.1021/ja045749e. [DOI] [PubMed] [Google Scholar]
- 4.Nakao Y, Masuda A, Matsunaga S, Fusetani N. J Am Chem Soc. 1999;121:2425–2431. [Google Scholar]
- 5.Ford PW, Gustafson KR, McKee TC, Shigematsu N, Maurizi LK, Pannell LK, Williams DE, Dilip de Silva E, Lassota P, Allen TM, Van Soest R, Andersen RJ, Boyd MR. J Am Chem Soc. 1999;121:5899–5909. [Google Scholar]
- 6.Reese MT, Gulavita NK, Nakao Y, Hamann MT, Yoshida WY, Coval SJ, Scheuer PJ. J Am Chem Soc. 1996;118:11081–11084. [Google Scholar]
- 7.Renner MK, Shen YC, Cheng XC, Jensen PR, Frankmoelle W, Kauffman CA, Fenical W, Emil CJ. J Am Chem Soc. 1999;121:11273–11276. [Google Scholar]
- 8.Clark WD, Corbett T, Valeriote F, Crews P. J Am Chem Soc. 1997;119:9285–9286. [Google Scholar]
- 9.Nakao Y, Yeung BKS, Yoshida WY, Scheuer PJ, Kelly-Borges M. J Am Chem Soc. 1995;117:8271–8272. [Google Scholar]
- 10.Fusetani N, Sugawara T, Matsunaga S, Hirota H. J Am Chem Soc. 1991;113:7811–7812. [Google Scholar]
- 11.Matsunaga S, Fusetani N, Hashimoto K, Walchli M. J Am Chem Soc. 1989;111:2582–2588. [Google Scholar]
- 12.Ireland C, Scheuer PJ. J Am Chem Soc. 1980;102:5688–5691. [Google Scholar]
- 13.Pettit GR, Cichacz Z, Barkoczy J, Dorsaz AC. J Nat Prod. 1993;56:260–267. doi: 10.1021/np50092a011. [DOI] [PubMed] [Google Scholar]
- 14.Pettit GR, Srirangam JK, Herald DL, Xu J. J Org Chem. 1995;60:8257–8361. [Google Scholar]
- 15.Pettit GR, Gao F, Cerny RL, Doubek DL. J Med Chem. 1994;37:1165–1168. doi: 10.1021/jm00034a014. [DOI] [PubMed] [Google Scholar]
- 16.Pettit GR, Taylor SR. J Org Chem. 1996;61:2322–2325. [Google Scholar]
- 17.Tasdemir D, Mallon R, Greenstien M, Ireland CM. J Med Chem. 2002;45:529–532. doi: 10.1021/jm0102856. [DOI] [PubMed] [Google Scholar]
- 18.Linington RG, Williams DE, Tahir A, van Soest R, Andersen RJ. Org Lett. 2003;5:2735–2738. doi: 10.1021/ol034950b. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura S, Matsunaga S, Shibazaki M, Suzuki K, Furihata K, Fusetani N. Org Lett. 2003;13:2255–2257. doi: 10.1021/ol034564u. [DOI] [PubMed] [Google Scholar]
- 20.Assmann M, Vansoest RWM, Kock M. J Nat Prod. 2001;64:1345–1347. doi: 10.1021/np000482s. [DOI] [PubMed] [Google Scholar]
- 21.Li WL, Yi YH, Wu HM, Xu QZ, Tang HF, Zhou DZ, Lin HW, Wang ZH. J Nat Prod. 2003;66:146–148. doi: 10.1021/np020223y. [DOI] [PubMed] [Google Scholar]
- 22.Siemion IZ, Wieland T, Pook KH. Angew Chem, Int Ed Engl. 1975;14:702–703. doi: 10.1002/anie.197507021. [DOI] [PubMed] [Google Scholar]
- 23.Tan LT, Sitachitta N, Gerwick WH. J Nat Prod. 2003;66:764–771. doi: 10.1021/np020492o. [DOI] [PubMed] [Google Scholar]
- 24.Marfey P. Carlesberg Res Commun. 1984;49:591–596. [Google Scholar]
- 25.Walker RP, Faulkner DJ. J Am Chem Soc. 1981;103:6772–6773. [Google Scholar]
- 26.Albizati KF, Faulkner DJ. J Org Chem. 1985;50:4163–4164. [Google Scholar]