1. Introduction
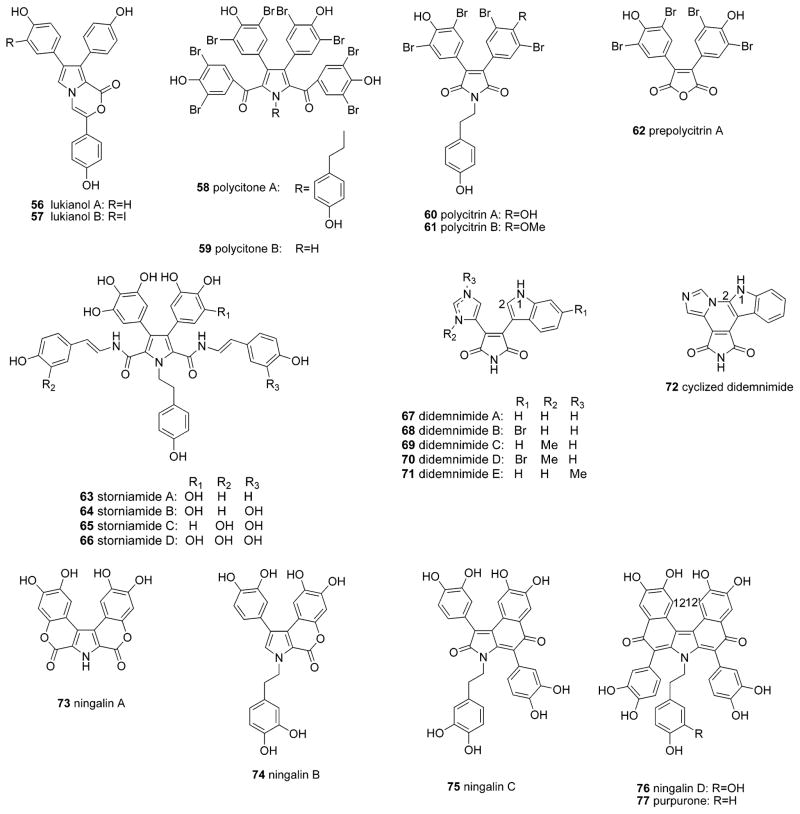
Marine organisms have proven to be rich sources of unique alkaloids. In earlier surveys we comprehensively reviewed several intriguing groups of marine alkaloids, including the manzamine- and bromotyrosine-derived alkaloids.1,2 The number of reported marine alkaloids continues to grow at an increasing rate, and in recent years, the lamellarins and related pyrrole-derived alkaloids (lukianols, polycitrins, polycitones, storniamides, didemnimides, ningalins, and purpurone, Table 1)3–24 have been regarded as a group of marine alkaloids with promising biological activities. As a result, these compounds have been the subject of several previous reviews regarding their chemistry and pharmacology.25–30 In addition, the lamellarins and related pyrrole-derived alkaloids have also provoked a great deal of interest in regard to their synthesis (Table 2).21,31–75
Table 1.
Lamellarins and Related Pyrrole-Derived Alkaloids from Marine Organisms
| lamellarin and related alkaloids | marine sources | collection localities | ref |
|---|---|---|---|
| lamellarins A–D (1, 32, 3, 35) | prosobranch mollusc Lamellaria sp. | peduliaes headland on Ngarol Island at the entrance to Malakal Harbor, Palau | 3 |
| lamellarins C, U (3, 22) | prosobranch mollusc Coriocella hibyae | around the Feydhoo Islands in the Maldives | 4 |
| lamellarins E–H (6–8, 37) | marine ascidian Didemnum chartaceum | Indian Ocean on the atoll of Aldabra, Republic of the Seychelles | 5 |
| lamellarins I, J–M, triacetate of lamellarin N, lamellarins A–C, the triacetate of lamellarin D (10–12, 15, 38, 41, 1, 32, 3, 36) | marine ascidian Didemnum sp. | South West Cay, Lihou Reef, off the North Queensland coast/Little Broadhurst Reef on the Great Barrier Reef | 6 |
| lamellarins O, P (52, 53) | southern Australian marine sponge Dendrilla cactos | Bass Strait, Australia | 7 |
| lamellarins Q, R (54, 55) | Australian marine sponge, Dendrilla cactos | coast off Durras, New South Wales, Australia | 8 |
| lamellarins S, K (18, 12) | Australian tunicate Didemnum sp. | near Durras, New South Wales, Australia | 9 |
| lamellarins T–X, N, lamellarin T 20-sulfate, lamellarin U 20-sulfate, lamellarin V 20-sulfate, lamellarin Y 20-sulfate (19, 22, 24, 42, 43, 40, 21, 23, 25, 27) | unidentified ascidian | near Trivandrum coast in the Arabian Sea, India | 10 |
| lamellarin Z, lamellarin B 20-sulfate, lamellarin C 20-sulfate, lamellarin L 20-sulfate, lamellarin G 8-sulfate, lamellarins A–C, E, G, L, lamellarin D triacetate, lamellarin N triacetate (28, 34, 5, 17, 9, 1, 32, 3, 6, 8, 15, 36, 41) | Great Barrier Reef ascidian, Didemnum chartaceum | near Friget Cay in the Swains Reef group, Great Barrier Reef | 11 |
| lamellarin α 20-sulfate (46) | unidentified ascidian | Arabian Sea near Trivandrum, Indian | 12 |
| lamellarins β, G, L (29,8,15) | purple unidentified encrusting marine ascidian Didemnum sp. | Indian Ocean | 13 |
| lamellarins γ, α, ε, lamellarins C-diacetate, I, K, | Indian ascidian Didemnum obscurum | Tiruchandur coast in the Gulf of Mannar, | 14 |
| K-diacetate, K-triacetate, M, U, X-triacetate (30, 45, 48, 4, 10, 12–14, 38, 22, 44) | Tamilnadu, India | ||
| lamellarins ζ, η, ϕ, χ, lamellarins F, I, J, K, lamellarin K triacetate, lamellarin L triacetate, lamellarin T diacetate (49–51, 31, 7, 10–12, 14, 16, 20) | red colonial ascidian Didemnum obscurum | Tiruchandur coast, Tamilnadu, India | 15 |
| lukianols A, B (56, 57) | unidentified Pacific tunicate | lagoon of the Palmyra atoll | 16 |
| polycitone A, polycitrins A, B (58, 60, 61) | Indo Pacific ascidian Polycitor sp. | Sodwana Bay, South Africa | 17 |
| polycitone B, prepolycitrin A (59, 62) | Indo Pacific asdidian Polycitor africanus | lagoon of Toliara, Madagascar, or in the lagoon of Mayotte, Comoros Islands, northwest of Madagascar | 18 |
| storniamides A–D (63–66) | Patagonian sponge Cliona sp. | Punta Verde, near San Antonio Oeste, Rio Negro, Argentina | 19 |
| didemnimides A–D (67–70) | Caribbean mangrove ascidian Didemnum conchyliatum | blades of the seagrass Thalassia testudinum in the mangrove channels of Sweetings Cay, near Grand Bahama Island, Bahamas | 20 |
| didemnimides A, D, E (67, 70, 71) | ascidian Didemnum granulatum | rocky shore of Araca beach, Sao Sebastiao (Sao Paulo state, southeastern Brazil) | 21 |
| cyclized didemnimide (72) | Caribbean ascidian Didemnum conchyliatum | blades of the seagrass T. testudinum in the mangrove channels of Sweetings Cay, Grand Bahama Island, Bahamas | 22 |
| ningalins A–D (73–76) | western Australian ascidian of the genus Didemnum | western Australia near Ningaloo Reef | 23 |
| purpurone (77) | marine sponge Iotrochota sp. | Koror island, Palau | 24 |
Table 2.
Synthesis of Lamellarins and Related Pyrrole-Derived Alkaloids
| lamellarin and related alkaloids | research group | synthesis strategy | ref |
|---|---|---|---|
| lamellarins D, H (35, 37) | Iwao, Ishibashi | N-ylide-mediated pyrrole formation | 31 |
| lamellarin D (35) | Albericio, Álvarez | sequential and regioselective bromination/Suzuki cross-coupling procedure | 32 |
| lamellarin D analogues | Albericio, Álvarez | sequential and regioselective bromination/palladium-catalyzed Suzuki cross-coupling procedure | 33 |
| lamellarins D, L, N (35, 15, 40) | Iwao, Ishibashi | Hinsberg-type pyrrole synthesis and palladium-catalyzed Suzuki–Miyaura coupling reactions | 34 |
| lamellarin H (37) and its derivatives | You | intermediate for lamellarin | 35 |
| lamellarins I, K (10, 12) | Guitián | intramolecular [3 + 2] cycloadditon pathway | 36 |
| lamellarins G, K, lukianol A, ningalin B (8, 12, 56, 74) | Steglich | biomimetic synthesis | 37 |
| lamellarin G trimethyl ether | Steglich | biomimetic synthesis | 38 |
| lamellarin G trimethyl ether | Ruchirawat | N-ylide-mediated pyrrole formation | 39 |
| lamellarin G trimethyl ether, permethyl storniamide A, ningalin B (74) | Iwao, Ishibashi | Hinsberg-type pyrrole synthesis and palladium-catalyzed Suzuki–Miyaura coupling reactions | 40 |
| lamellarin G trimethyl ether | Handy | three iterative halogenation/cross-coupling reactions | 41 |
| lamellarin K (12) | Banwell | intramolecular [3 + 2] cycloadditon pathway | 42 |
| lamellarins K, L (12,15) | Ruchirawat | convergent synthetic approach; Michael addition/ring-closure reaction | 43 |
| lamellarin L (15) | Steglich | biomimetic total synthesis | 44 |
| lamellarins U, L (22,15) | Albericio, Álvarez | solid-phase total synthesis; Baeyer–Villiger reaction and an intramolucular [3 + 2] cycloaddition | 45 |
| lamellarins U, L, O, Q (22, 15, 52, 54) | Albericio, Álvarez | solid-phase total synthesis; intramolucular [3 + 2] cycloaddition, two successive and selective cross-coupling reactions | 46 |
| lamellarins O, Q, lukianol A (52, 54, 56) | Banwell | convergent synthesis; Stille, Suzuki, or Negishi cross-coupling reactions | 47 |
| lamellarins Q, O (54,52) | Albericio, Álvarez | solid-phase synthesis; Negishi/Suzuki cross-coupling reactions | 48 |
| lamellarin T hybrids | Banwell | Negishi cross-coupling reaction | 49 |
| lamellarin α, lamellarin α 13,20-disulfate, lamellarin H (45,47,37) | Faulkner | intramolecular [3 + 2] cycloaddition pathway | 50 |
| lamellarin α 20-sulfate (46) | Iwao, Ishibashi | Hinsberg-type pyrrole synthesis and Suzuki–Miyaura coupling reaction | 51 |
| lamellarins C, E–G, I–L, T, U, Y, χ (3, 6–8, 10–12, 15, 19, 22, 26, 31) | Ruchirawat | Michael addition/ring-closure reaction | 52 |
| lukianol A (56), lamellarin O dimethyl ether | Fürstner | titanium-mediated formation | 53 |
| lukianol A (56), lamellarin O dimethyl ether | Gupton | vinylogous iminium chemisty | 54 |
| lukianol A (56) | Wong | palladium-catalyzed Sonogashira, Heck, and Suzuki cross-coupling reactions | 55 |
| lukianol A (56) | Steglish | biomimetic synthesis | 56 |
| polycitrin A (60) | Steglish | biomimetic total synthesis | 57 |
| polycitrin A (60), prepolycitrin A (62) | Correia | palladium-catalyzed Heck diarylation | 58 |
| polycitrin A (60) | Steglich | biomimetic synthesis | 59 |
| polycitrin B (61) | Beccalli | palladium-catalyzed cross-coupling reaction | 60 |
| polycitones A, B (58, 59) | Steglich | total synthesis; Friedel–Crafts reaction | 61 |
| polycitones A, B (58, 59) | Gupton | vinylogous iminium chemisty | 62 |
| storniamide A nonamethyl ether | Steglich | biomimetic synthesis | 63 |
| permethyl storniamide A | Fürstner | palladium-catalyzed Suzuki and Negishi cross-coupling reactions | 64 |
| didemnimide A (67) | Berlinck, Piers, Andersen | biomimetic synthesis | 21 |
| didemnimides A, B (67, 68) | Cava | K+t-BuO−-catalyzed condensation | 65 |
| didemnimide C (69) | Steglich | Stille coupling reaction | 66 |
| didemnimide C (69) | Piers | biomimetic synthesis | 67 |
| ningalin A (73), lamellarin O (52), lukianol A (56), permethyl storniamide A | Boger | Diels–Alder reaction/ring contraction pathway | 68 |
| ningalin B (74) | Boger | heterocyclic azadiene Diels–Alder reaction | 69 |
| ningalin B (74) and its analogs | Boger | heterocyclic azadiene Diels–Alder strategy | 70 |
| ningalin B (74) | Bullington | regioselective synthesis | 71 |
| ningalin B hexamethyl ether | Gupton | vinylogous iminium chemistry | 72 |
| ningalin C, purpurone (75,77) | Steglich | biomimetic total synthesis | 73 |
| ningalin C (75) | Ruchirawat | one-pot reaction | 74 |
| ningalin D (76) | Boger | Heterocyclic azadiene Diels–Alder reaction | 75 |
Lamellarins are a group of DOPA-(2-amino-3-(3′,4′-dihydroxyphenyl)propionic acid)-derived pyrrole alkaloids that were first isolated from the prosobranch mollusc Lamellaria sp. in 1985 by Faulkner and co-workers.3 Since then, more than 70 different lamellarins and related naturally occurring pyrrole-derived alkaloids have been reported (Table 1). Most of the lamellarins, lukianols, polycitrins, polycitones, storniamides, and ningalins possess a 3,4-diarylated pyrrole 2-carboxylic acid ester or amide moiety as the common structural subunit. They have been isolated from widely varying locations and diverse marine organisms, such as marine prosobranch molluscs,3,4 ascidians (tunicates),5,6,9–18,20–23 and sponges.7,8,19,24 The diversity of the producing organisms suggests a potential microbial link to their biosynthesis.
The lamellarins and related pyrrole-derived alkaloids have shown a diverse range of bioactivities such as cytotoxicity and antitumor activity,4,6,10,12,13,15–17,50,75–80 reversal of multidrug resistance (MDR),64,68,69,72,75,79,81–83 HIV-1 integrase inhibition,12,50,84,85 antibiotic activity,19 human aldose reductase (h-ALR2) inhibition,86 cell division inhibition,3 immunomodulation,6,87 antioxidant activity,14 and feeding deterrence,20 making these compounds a particularly important subject for research. In this review we summarize the isolation, structure determination, biological activities, and synthetic studies of lamellarins and related pyrrole-derived alkaloids including lukianols, polycitrins, polycitones, storniamides, didemnimides, ningalins, and purpurone from different marine organisms since the first report of lamellarins in 1985 until April of 2007.
2. Isolation and Structure Determination of Lamellarins and Related Pyrrole-Derived Alkaloids from Marine Organisms
The lamellarin skeleton was first isolated and identified in 1985 from the Palauan marine prosobrach mollusc Lamellaria sp.3 Later in 1988 four additional members, lamellarins E–H 6–8 and 37, were found in the didemnid ascidian Didemnum chartaceum collected from the Republic of the Seychelles.5 In 1992 the related alkaloids lukianols A 56 and B 57 were recovered from an unidentified Pacific tunicate,16 while in 1993 lamellarins I–M 10–12, 15, and 38 and the triacetate of lamellarin N 41 were isolated from a Great Barrier Reef colonial ascidian, Didemnum sp.6 In 1994, lamellarins O 52 and P 53 were isolated from a southern Australian marine sponge Dendrilla cactos.7 Lamellarins Q 54 and R 55 were also isolated from a geographically distinct re-collection of Dendrilla cactos.8
The lamellarins are a growing family of marine alkaloids, and to date, over 70 lamellarins and related pyrrole-derived alkaloids have been isolated and identified (Tables 1, 3, and 4; Figures 1 and 2). The lamellarins and related compounds have a pyrrole ring as a core component of their skeleton. The molecules fall into two structural groups depending on whether the central pyrrole ring is fused (Group I, lamellarins 1–51) or unfused (Group II, lamellarins O–R 52–55). Group I could be further divided into two subsections, viz., compounds possessing an olefin at C-5 and C-6 (Figure 1, Table 4, lamellarins 32–51) and those in which this olefin is saturated (Figure 1, Table 3, 1–31).
Table 3.
Lamellarins (Type 1a)
| no. | lamellarin | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A | OH | OMe | H | OH | OMe | OMe | OMe | OMe | OH |
| 2 | A triacetate | OAc | OMe | H | OAc | OMe | OMe | OMe | OMe | OAc |
| 3 | C | OH | OMe | H | OH | OMe | OMe | OMe | OMe | H |
| 4 | C diacetate | OAc | OMe | H | OAc | OMe | OMe | OMe | OMe | H |
| 5 | C 20-sulfate | OSO3− | OMe | H | OH | OMe | OMe | OMe | OMe | H |
| 6 | E | OH | OMe | H | OMe | OH | OMe | OMe | OH | H |
| 7 | F | OH | OMe | H | OMe | OMe | OMe | OMe | OH | H |
| 8 | G | OMe | OH | H | OMe | OH | OMe | OH | H | H |
| 9 | G 8-sulfate | OMe | OH | H | OMe | OH | OMe | OSO3− | H | H |
| 10 | I | OH | OMe | H | OMe | OMe | OMe | OMe | OMe | H |
| 11 | J | OH | OMe | H | OMe | OMe | OMe | OH | H | H |
| 12 | K | OH | OMe | H | OH | OMe | OMe | OMe | OH | H |
| 13 | K diacetate | OAc | OMe | H | OAc | OMe | OMe | OMe | OH | H |
| 14 | K triacetate | OAc | OMe | H | OAc | OMe | OMe | OMe | OAc | H |
| 15 | L | OH | OMe | H | OMe | OH | OMe | OH | H | H |
| 16 | L triacetate | OAc | OMe | H | OMe | OAc | OMe | OAc | H | H |
| 17 | L 20-sulfate | OSO3− | OMe | H | OMe | OH | OMe | OH | H | H |
| 18 | S | OH | OH | H | OH | OH | OMe | OH | H | H |
| 19 | T | OH | OMe | H | OMe | OH | OMe | OMe | OMe | H |
| 20 | T diacetate | OAc | OMe | H | OMe | OAc | OMe | OMe | OMe | H |
| 21 | T 20-sulfate | OSO3Na | OMe | H | OMe | OH | OMe | OMe | OMe | H |
| 22 | U | OH | OMe | H | OMe | OH | OMe | OMe | H | H |
| 23 | U 20-sulfate | OSO3Na | OMe | H | OMe | OH | OMe | OMe | H | H |
| 24 | V | OH | OMe | H | OMe | OH | OMe | OMe | OMe | OH |
| 25 | V 20-sulfate | OSO3Na | OMe | H | OMe | OH | OMe | OMe | OMe | OH |
| 26 | Y | OH | OMe | H | OMe | OH | OH | OMe | H | H |
| 27 | Y 20-sulfate | OSO3Na | OMe | H | OMe | OH | OH | OMe | H | H |
| 28 | Z | OMe | OH | H | OH | OH | OMe | OH | H | H |
| 29 | β | OH | OH | H | OMe | OH | OH | OH | H | H |
| 30 | γ | OH | OMe | OMe | H | OMe | OMe | OMe | OH | H |
| 31 | χ | OAc | OMe | H | OAc | OMe | OMe | OAc | H | H |
Table 4.
Lamellarins (Type 1b)
| no. | lamellarin | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|---|
| 32 | B | OH | OMe | OH | OMe | OMe | OMe | OMe |
| 33 | B diacetate | OAc | OMe | OAc | OMe | OMe | OMe | OMe |
| 34 | B 20-sulfate | OSO3− | OMe | OH | OMe | OMe | OMe | OMe |
| 35 | D | OH | OMe | OH | OMe | OMe | OH | H |
| 36 | D triacetate | OAc | OMe | OAc | OMe | OMe | OAc | H |
| 37 | H | OH | OH | OH | OH | OH | OH | H |
| 38 | M | OH | OMe | OH | OMe | OMe | OMe | OH |
| 39 | M triacetate | OAc | OMe | OAc | OMe | OMe | OMe | OAc |
| 40 | N | OH | OMe | OMe | OH | OMe | OH | H |
| 41 | N triacetate | OAc | OMe | OMe | OAc | OMe | OAc | H |
| 42 | W | OH | OMe | OMe | OH | OMe | OMe | OMe |
| 43 | X | OH | OMe | OMe | OH | OMe | OMe | OH |
| 44 | X triacetate | OAc | OMe | OMe | OAc | OMe | OMe | OAc |
| 45 | α | OH | OMe | OMe | OH | OMe | OMe | H |
| 46 | α 20-sulfate | OSO3Na | OMe | OMe | OH | OMe | OMe | H |
| 47 | α 13,20-disulfate | OSO3Na | OMe | OMe | OSO3Na | OMe | OMe | H |
| 48 | ε | OH | OMe | OMe | OMe | OMe | OMe | OH |
| 49 | ζ | OH | OMe | OMe | OMe | OMe | OMe | OMe |
| 50 | η | OH | OMe | OMe | OMe | OMe | OMe | H |
| 51 | ϕ | OAc | OMe | OAc | OMe | OAc | OMe | OMe |
Figure 1.
Structure of lamellarins 1a–c.
Figure 2.
Structures of lamellarin-related pyrrole-derived alkaloids.
2.1. Isolation and Structure Determination of Lamellarins
2.1.1. Lamellarins A–D
In 1985, Faulkner and colleagues reported the isolation and structural elucidation of four aromatic metabolites, which were obtained from the marine prosobranch mollusc Lamellaria sp. (family Lamellariidae) collected in the waters of Palau. These compounds comprised a new class of marine natural product and were named lamellarins A–D 1, 32, 3, and 35. Lamellarin A 1 is an optically inactive pale yellow prism that occurred as an inseparable racemic mixture of two geometrical isomers due to restricted rotation about the C-1 and C-11 bond. Its structure was determined by a single-crystal X-ray crystallographic study, while the structures of lamellarins B–D 32, 3, and 35 were assigned by spectroscopic data.3 Lamellarin C 3 was also isolated from the prosobranch mollusc Coriocella hibyae.4 Lamellarins A 1, B 32, and C 3 and the triacetate of lamellarin D 36 were found from both prosobranch molluscs and the marine ascidians.6,11
2.1.2. Lamellarins E–H
In 1988, Fenical’s group reported four new alkaloids of the lamellarin class, lamellarins E–H 6–8 and 37. They were isolated from the Indian Ocean marine colonial didemnid ascidian Didemnum chartaceum (Sluiter, 1909, Didemnidae, Ascidiacea).5 The structure of lamellarin E 6 was elucidated by spectroscopic and X-ray crystallographic methods, while lamellarins F–H 7, 8, and 37 were determined by interpretation of NMR spectral data.5 Advanced 2D NMR techniques mostly detailed HMBC correlations were utilized in 1996 during the structure assignment of lamellarin H 37.88
2.1.3. Lamellarins I–M and the Triacetate of Lamellarin N
Bowden’s group reported six new lamellarin-class alkaloids, lamellarins I 10, J 11, K 12, L 15, and M 38 and the triacetate of lamellarin N 41 obtained from an Australian colonial ascidian Didemnum sp.6 The structures were deduced by 13C–1H shift-correlated 2D NMR and NOE correlations.6 Lamellarins A 1, B 32, and C 3 and the triacetate of lamellarin D 36 were also isolated from this Pacific ascidian. Moreover, extraction of the same ascidian species collected from Little Broadhurst Reef also yielded lamellarins A 1, B 32, C 3, I 10, K 12, and M 38.6 These isolation studies suggested that lamellarins A–D 1, 32, 3, and 35, obtained from Lamellaria sp., were likely sequestered from the ascidian diet of this mollusc.5,6
2.1.4. Lamellarins O–R
In 1994, two new unstable, oily lamellarin members, lamellarins O 52 and P 53, were isolated from the marine sponge Dendrilla cactos collected during a trawling operation in Bass Strait, southern Australia, by Capon et al.7 Both metabolites 52 and 53 readily decomposed with 53 being slightly more stable than 52. The increased stability was assumed to be a product of hydrogen bonding in 53. No biological data could be obtained due to their reported instability and the small amounts of these two alkaloids available from natural sources. Later in 1995 two new stable oily alkaloids, lamellarins Q 54 and R 55, were obtained by the same research group from a geographically distinct recollection of the Australian marine sponge D. cactos harvested by SCUBA off the coast of New South Wales.8 The structures of 52–55 were elucidated by spectroscopic analysis and chemical transformation.7,8 The structure of lamellarin O 52 was confirmed later by single-crystal X-ray analysis.47
2.1.5. Lamellarin S
In 1996, Urban and Capon isolated lamellarin S 18 along with the known lamellarin K 12 from an Australian tunicate Didemnum sp. collected by SCUBA near Durras, New South Wales. The structure was determined by spectroscopic analysis. Lamellarin S 18 was the first example that demonstrated atropisomerism.9 The C-1 phenyl substituent in the lamellarins is generally rotationally restricted and capable of producing atropisomerism. No other lamellarins, however, have been reported to possess optical properties. Lamellarin S 18 initially displayed a positive optical rotation, indicating it is at least enantiomerically enriched. Repeated optical rotation measurements of 18 over several months revealed slow racemization with a half-life calculated to be ca. 90 days.9 A Serena software molecular modeling experiment indicated that the lowest energy barrier to rotation for 18 was 84 kJ/mol with both atropisomers being of comparable energy.9
2.1.6. Lamellarins N and T–X, Lamellarin T 20-Sulfate, Lamellarin U 20-Sulfate, Lamellarin V 20-Sulfate, and Lamellarin Y 20-Sulfate
In 1997, nine new alkaloids of the lamellarin class, lamellarins T 19, U 22, V 24, W 42, and X 43 and 20-sulfates of lamellarins T 21, U 23, V 25, and Y 27 together with the known lamellarin N 40 were isolated from an unidentified ascidian obtained from the Trivandrum coast of India.10 The structures of lamellarins T–X 19, 22, 24, 42, and 43 and the four 20-sulfate derivatives of lamellarins T 21, U 23, V 25, and Y 27 were identified by interpretation of spectroscopic data. This was the first example of lamellarin sulfates. Lamellarin U 22 was also isolated from the prosobranch mollusc Coriocella hibyae in 1999.4
2.1.7. Lamellarin Z, Lamellarin B 20-Sulfate, Lamellarin C 20-Sulfate, Lamellarin L 20-Sulfate, and Lamellarin G 8-Sulfate
Five novel lamellarin members, lamellarin Z 28, the 20-sulfated derivatives of lamellarins B 34, C 5, and L 17, and lamellarin G 8-sulfate 9 along with the known lamellarins A 1, B 32, C 3, E 6, G 8, and L 15 plus the triacetate derivatives of lamellarins D 36 and N 41 were isolated from a Great Barrier Reef ascidian Didemnum chartaceum Sluiter (Didemnidae).11 They were identified by interpretation of spectroscopic data. Among them, lamellarin Z 28 is the first example of a dimethoxylated lamellarin, while the 8-sulfated derivative of lamellarin G 9 is the first example of this class of compounds sulfated at the C-8 position.11
2.1.8. Lamellarin α 20-Sulfate
In 1999, the lamellarin α 20-sulfate 46 was isolated from an unidentified ascidian collected from the Arabian Sea coast of India. The structure was determined by 1H and 13C NMR data and high-resolution FAB mass measurement.12 The spectral data indicated that lamellarin α 20-sulfate 46 differs from lamellarin U 20-sulfate 2310 by addition of a double bond at C-5 and C-6.12
2.1.9. Lamellarin β
A novel lamellarin alkaloid, lamellarin β 29, along with previously reported lamellarins G 8 and L 15 were obtained from a purple unidentified encrusting marine ascidian Didemnum sp. (other than D. chartaceum) collected in the Indian Ocean.13 The structure of lamellarin β 29 was elucidated by spectroscopic methods including 2D NMR experiments.13 Similar to lamellarin S 18, energy minimization of 29 was studied using Serena and pcmodel, which adopted an MMX force field.13 The rotational barrier of the bond between C-1 and C-11 was in excess of 80 kcal/mol. Due to the highly restricted rotation of the catechol ring, the molecule has the potential to adopt a chiral configuration. However, 29 is racemic, as observed in all previously isolated lamellarins (except for lamellarin S 18) and suggests thermal racemization is a facile process.9,13
2.1.10. Lamellarins γ, α, ε, ζ, η, ϕ, and χ
In 2004, lamellarins γ 30, α 45, and ε 48 along with eight known lamellarin alkaloids, lamellarins I 10, K 12, M 38, U 22, C-diacetate 4, K-diacetate 13, K-triacetate 14, and X-triacetate 44, were isolated from the Indian red colonial ascidian Didemnum obscurum F. Monniot, 1969 (Didemnidae) collected from the Indian Tiruchandur coast during February 2002.14 One year later, in 2005, the same research group reported four new lamellarin alkaloids, lamellarins ζ 49, η 50, ϕ 51, and χ 31 along with seven known lamellarins, lamellarins F 7, I 10, J 11, K 12, lamellarin K triacetate 14, lamellarin L triacetate 16, and lamellarin T diacetate 20 isolated from the same ascidian collected from the same location but in August 2002.15 Lamellarin α 45 was synthesized in 2002.50 Structures were established using NMR spectroscopic techniques.14,15 The structure of lamellarin K-triacetate 14 was further confirmed by X-ray crystallographic analysis and is optically inactive. The X-ray structure revealed that the crystal was racemic with equal amounts of each atropisomer.14
2.2. Isolation and Structure Determination of Pyrrole-Derived Alkaloids Related to Lamellarins
2.2.1. Lukianols A and B
Lukianols A 56 and B 57 were isolated from an unidentified encrusting Pacific tunicate collected by SCUBA in the lagoon of the Palmyra atoll by Scheuer’s group in 1992.16 Their structures were elucidated by spectral methods. Lukianol B 57 is an iodo-substituted lukianol A 56 which may be considered as the ring-closed analogue of lamellarin O 52. The basic structural feature of the lukianols is an N-alkylpyrrolecarboxylic acid, which is quite rare among natural products.16
2.2.2. Polycitones A and B, Prepolycitrin A, and Polycitrins A and B
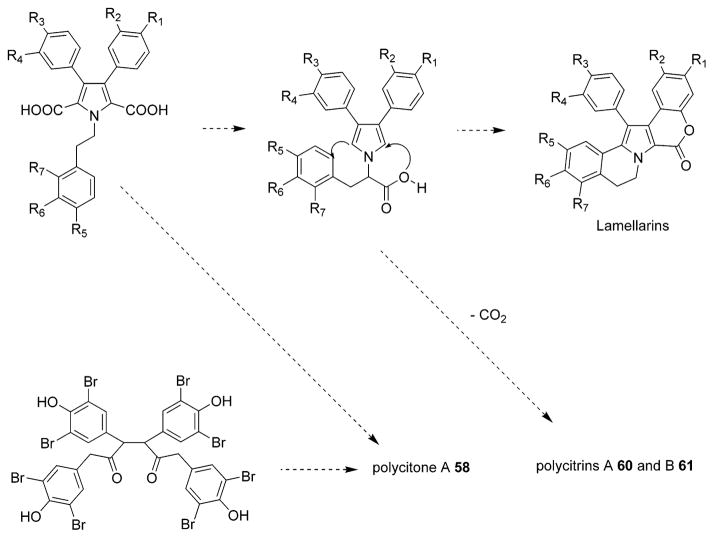
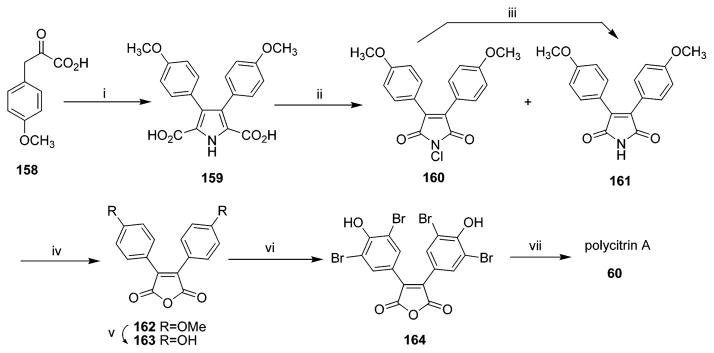
Three alkaloids, polycitone A 58 and polycitrins A 60 and B 61, were isolated in 1994 from the Indo-Pacific marine ascidian Polycitor sp. collected in Sodwana Bay, South Africa.17 The Polycitor sp. is a translucent white colonial tunicate. These three compounds were the first examples of two new classes of marine alkaloids with unprecedented skeletons that maybe biogenetically related to the lamellarins.17 Six years later, in 2000, two additional related alkaloids, polycitone B 59 and prepolycitrin A 62, were isolated from the marine ascidian Polycitor africanus (order Aplousobranchia, family Polycitoridae).18 P. africanus is also a translucent white or bluish colonial tunicate collected by hand using SCUBA in the lagoon of Toliara, Madagascar, or the lagoon of Mayotte, Comoros Islands.18 The structures of these compounds were determined using NMR and HREIMS data. Both polycitrins A 60 and B 61 contain a maleimide unit.17 The structure of polycitone A 58 was confirmed by single-crystal X-ray diffraction analysis. In fact, the crystallographic analysis was performed on the penta-O-methyl derivative of 58 with both 58 and the derivative yielding poorly diffracting crystals of highly anisotropic shape.17 Polycitone B 59 is a highly unsaturated aromatic structure with 21 units of unsaturation. Interestingly, pre-polycitrin A 62 was previously synthesized as an intermediate in a biomimetic synthesis of polycitrin A 60.18,89 Isolation of prepolycitrin A 62 could support the earlier suggestion that it might be the bioprecursor of polycitrin A 60.18 The structure relationship between the N-substituted polycitone A 58 and polycitone B 59 resembles the relationship between lamellarins O 52 and Q 54.7,8,18 According to the description of the animal material Polycitor sp.17,18 and the co-occurrence of the metabolites of 58, 59, and 62 from P. africanus,18 the unidentified species collected in July 199217 might be taxonomically identical to the investigated P. africanus that was first identified by Monnitor in 1998.18
2.2.3. Storniamides A–D
Storniamides A–D 63–66, a new family of peptide alkaloids, were isolated from a Patagonian sponge Cliona sp. (family Clionidae) collected during low tide (−0.5 m) at Punta Verde, near San Antonio Oeste province of Rio Negro, Argentina, in 1996.19 The crude ethanolic extract of the marine sponge showed antibiotic activity against Gram-positive bacteria. Purification of the individual constituents was accomplished by bioassay-guided fractionation and purification to reveal storniamides A–D 63–66. The structures of storniamides A–D 63–66 were determined by HRFABMS and a combination of 1D and 2D NMR techniques (COSY, NOESY, homonuclear 2DJ, HETCOR, and COLOC). Storniamide A 63 was a key for the structure elucidation of this family of compounds since the substitution pattern of the aromatic rings in 63 was extremely simple. Characterization showed that each storniamide differs only in the oxygenation pattern within the peripheral aromatic rings.19
2.2.4. Didemnimides A–E and Cyclized Didemnimide
In 1997, four predator-deterrent alkaloids possessing a novel indole–maleimide–imidazole carbon skeleton, didemnimides A–D 67–70, were isolated from the Caribbean ascidian Didemnum conchyliatum (Sluiter, 1898, family Didemnidae) collected from the blades of the seagrass Thalassia testudinum in the mangrove channels of Sweetings Cay, near Grand Bahama Island.20 The structure of didemnimide A 67 was identified by a single-crystal X-ray diffraction analysis, while didemnimides B–D 68–70 were determined by combined spectral methods, especially 1D and 2D NMR methods. The didemnimides 67–70 were the first examples of a new alkaloid structural class and add to a relatively small group of naturally occurring maleimides.20 In 1998, didemnimide E 71 was isolated from the ascidian Didemnum granulatum collected in Brazil.21
A less polar and deep purple cyclized didemnimide alkaloid 72 was isolated from the same Caribbean mangrove ascidian in 1999.22 Compound 72 is the cyclization product of didemnimide A 67 formed via a C-2 indole condensation with the imidazole nitrogen and represented the first alkaloid to be isolated with this cyclized indole–maleimide–imidazole structure.22
2.2.5. Ningalins A–D
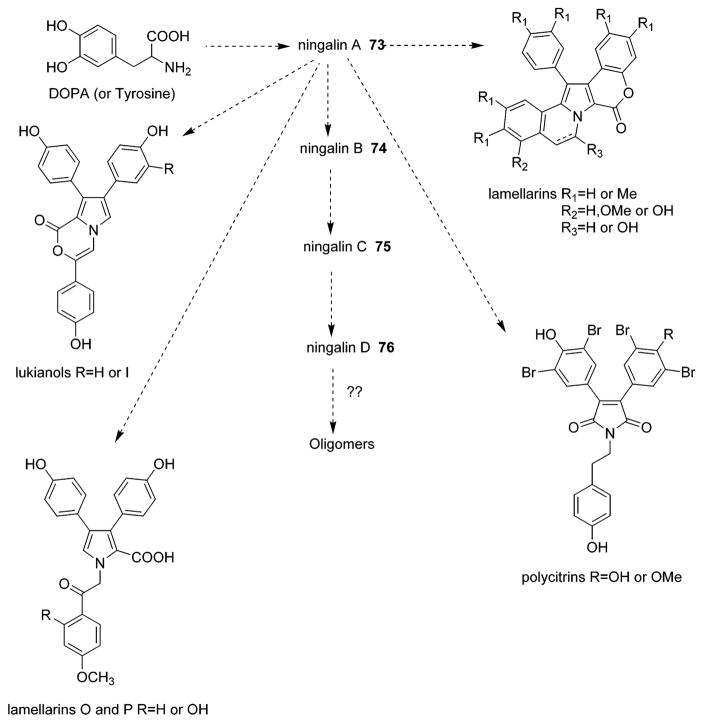
Four aromatic alkaloids, ningalins A–D 73–76, were isolated from an unidentified Western Australian ascidian of the genus Didemnum collected in Western Australia near Ningaloo Reef by Fenical’s group in 1997.23 The ningalins are a class of natural alkaloids that have a 3,4-dihydroxy-phenylalanine (DOPA)-derived o-catechol moiety. The four polar and highly colored ningalins A–D 73–76 are composed of several condensed aromatic systems with the unified theme that all appear derived via condensation of DOPA amino acid units, three of which possess highly unsaturated, new carbon skeletons.23 Their structures were identified by interpretation of spectral data, especially 2D NMR analysis. In the highly condensed alkaloids, steric crowding forces several catechol rings into twisted configurations, resulting in unexpected shielding and deshielding of several proton shifts in their NMR spectra.23 NMR analyses and subsequently computer modeling studies illustrate that phenyl rings in ningalins B–D 74–76 adopt low-energy conformations at approximately 90° to the planes of the molecules. Although this conformation appears to restrict rotation, creating the possibility of optical activity, the molecules appear to be racemic. Similar to most of the lamellarins, the energy barriers to rotation and helical racemization are low and the racemization is a facile process. Accordingly, ningalins A–D 73–76 showed zero rotation at the sodium D line.23
2.2.6. Purpurone
Purpurone 77, whose name reflects the purple color of the natural product, was isolated from the marine sponge Iotrochota sp. collected in September 1983 at the Koror Island, Palau.24 The structures of purpurone 77 and ningalin D 76 are very similar (Figure 2). Purpurone 77 was reported 4 years earlier than that of ningalin D 76.23,24 Purpurone 77 cannot adopt a planar geometry due to the steric interaction between H-12 and H-12′ (Figure 2).24 This could result in an optically active material; however, no optical rotation was observed for 77.
3. Biological Activities
At present, more than 70 lamellarins and related pyrrole-derived alkaloids have been isolated and identified. They not only possess unique structural features but also exhibit interesting and significant biological properties, including cytotoxicity and antitumor activity,4,6,10,12,13,15–17,50,75–80 multidrug resistance (MDR),64,68,69,72,75,79,81–83 HIV-1 integrase inhibition,12,50,84,85 antibiotic activity,19 human aldose reductase (h-ALR2) inhibition,86 cell division inhibition,3 immunomodulatory activity,6,87 antioxidant activity,14 and feeding deterrence.20
3.1. Biological Activities of Lamellarins and Related Pyrrole-Derived Alkaloids
3.1.1. Cytotoxicity and Antitumor Activity
A considerable number of lamellarins and related pyrrole-derived alkaloids have been found to be cytotoxic to a wide range of cancer cell lines.25 The most potent of these compounds (lamellarins D 35, K 12, and M 38) exhibited cytotoxicity values in the mid-to-high nanomolar range (38–110 nM).79 Lamellarins C 3 and U 22 demonstrated potent cytotoxicity against 10 human tumor cell lines (A549, HCT-116, LOX IMVI, MALME-3M, MCF-7, MOLT-4, OVCAR-3, PC-3, SF-295, UO-31) with IC50’s ranging from 0.4 to 19.4 nM.4 Lamellarin D 35 has potent cytotoxic activity against various tumor cells, especially to human prostate cancer cells (DU-145, LNCaP) and leukemia cells (K562).78 Lamellarin D 35 is a potent inhibitor of DNA topoisomerase I90,91 and a potent pro-apoptotic agent with cytotoxic action that is fully maintained in MDR cells compared to the sensitive parental cell line.91 Lamellarin D 35 maintained a marked cytotoxicity toward cell lines resistant to the reference topoisomerase I poison camptothecin. Bailly’s group hypothesized that topoisomerase I is not the only cellular target for camptothecin, and lamellarin D 35 acts on cancer cell mitochondria to induce apoptosis.92 Such a mechanism would reinforce the pharmacologic interest of the lamellarins and define lamellarin D 35 as a lead in the search for treatments against chemoresistant cancer cells.
Lamellarins I 10, K 12, and L 15 exhibited comparable and significant cytotoxicity against P388 and A549 cultured cancer cell lines (IC50 ≈ 0.5 nM against each cell line).6 The tri-O-acetyl derivative of lamellarin K showed remarkable selectivity against the A549 human lung cancer cell line (IC50 ≈ 7.6 nM).79,93 In the NCI 60 cell-line panel, lamellarin N 40 showed some selectivity toward the melanoma cell lines SK-MEL-5 (LC50 = 1.87 × 10−7 M) and UACC-62 (LC50 = 9.88 × 10−6 M).10 Compounds lamellarin ζ 49, lamellarin χ 31, lamellarin L triacetate 16, and lamellarin F 7 have shown excellent activities against colorectal cancer cells (COLO-205).15 Cytotoxicity toward HeLa cells of lamellarin H 37, lamellarin α 45, and lamellarin α 13, 20-disulfate 47 was measured using the MTT [3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide] dye as an indicator of cell survival with LD50s of 5.7, 5.1, and 29 μM, respectively.12,50 The MTT assay of lamellarin α-20 sulfate 46 toward HeLa cells displayed the least toxicity with an LD50 of 274 μM. Lamellarin H 37 and lamellarin α 45 were tested against a panel of eight human tumor cell lines. Both compounds exhibited good potency and selectivity (37, mean IC50 = 4 μM, min/max IC50 ratio = 20; 45, mean IC50 = 2.9 μM, min/max IC50 ration = 10) although the selectivity patterns differed.50 Lamellarin β 29 showed cytotoxicity against human promyelocytic leukemia HL-60 cells with an IC50 of 11.6 nM.13 The lamellarin analogues were tested for activity against erythroleukemia, lung carcinoma, malignant melanoma, colon adenocarcinoma, prostate carcinoma, breast adenocarcinoma, ovary adenocarcinoma, cervix epithelioid carcinoma, and pancreatic epitheloid carcinoma in 2004 by Bailly et al.80
Lukianols A 56 and B 57 exhibited moderate activity in the standard bioassay against a cell line derived from human epidermaoid carcinoma (KB) with an MIC of 2.4 and 185.9 nM, respectively.16 A derivative of polycitone A, penta-O-methyl polycitone A, was found to inhibit the growth of SV40 transformed fibroblast at concentrations of 7.6 nM.17 Didemnimides A 67, D 70, and E 71 were all proved to be inactive as cell cycle checkpoint inhibitors. Compounds that inhibit the G2 checkpoint might be valuable in cancer therapy.21,94,95 Ningalin D 76, permethyl ningalin D, and ningalin D decaacetate were found to exhibit modest cytotoxic activity against a murine leukemia cell line (L1210, IC50 = 1–7 μM) and a parental human colon cancer cell line (HCT116, IC50 = 1–70 μM).75
Lamellarin O 52 and lukianol A 56 proved to be effective cytotoxic agents in a number of leukemia and lymphoma screens. They were also active in human HeLa–S3 uterine and glioma screens. Lukianol A 56 afforded selective activity against SW-480 colon adenocarcinoma growth, but it in general was less active against the growth of solid tumors. The principal biochemical effect of lamellarin O 52 and lukianol A 56 was inhibition of L1210 lymphocytic leukemia DNA synthesis with less effect on RNA and protein synthesis. They demonstrate a therapeutic profile that is very similar to current clinically used anticancer agents.76,77
3.1.2. Multidrug Resistance Reversal (MDR) Activity
Multidrug resistance (MDR) is a persistent problem limiting the effectiveness of a wide variety of anticancer drugs, antibiotics, and protease inhibitors. Some lamellarins and related pyrrole-derived alkaloids were reported to act as nontoxic inhibitors of acquired MDR in various cancer cell lines, revealing equally effective cytotoxic activity against MDR cell lines and demonstrating that even at noncytotoxic concentrations they reverse MDR by inhibiting P-glycoprotein (P-gp)-mediated drug efflux.64,79,91,96 It has been claimed that lamellarins I 10 and K 12 not only exhibit potent cytotoxic activity in vitro against MDR tumor cell lines but also reverse MDR at noncytotoxic concentrations,69,79,97,98 and the results of in vivo tests of lamellarin K 12 are consistent with these claims.93 Experiments suggested that lamellarin I 10 reversed MDR by directly inhibiting the P-glycoprotein-mediated drug efflux at nontoxic doses and that the potency of lamellarin I 10 as an MDR modulator was 9–16-fold higher than that of approved anticancer agent verapamil, resensitizing the resistant malignant cells to front-line therapeutics.79
Permethyl storniamide A, permethyl ningalin B, and much simpler 3,4-diarylpyrrole derivatives also exhibited similar MDR reversal activity at nontoxic concentrations.68,69 Lamellarin O 52 and lukianol A 56 were found in Boger’s group to exhibit modest cytotoxic activity against both wild-type and MDR tumor cell lines, suggesting they may serve as new leads for development of antitumor agents insensitive to MDR.68 Permethyl storniamide A and ningalin B 74 were shown to potently reverse MDR phenotype, resensitizing a resistant human colon cancer cell line (HCT116/VM46) to vinblastine and doxorubicin at lower doses than the prototypical agent verapamil. Permethyl storniamide A and ningalin B 74 may constitute the initial members of a new class of MDR reversal agents.68,69 Ningalin B hexamethyl ether was also found to have good MDR activity when used in conjunction with vinblastine or doxorubicin as compared to use of the standard MDR reversal agent verapamil.72
In 2003, Soenen and his colleagues made a further study on synthetic derivatives of the ningalins, and permethyl ningalin B dimethylamide was discovered as a potent MDR reversal agent that hypersensitizes P-glycoprotein-resistant tumor cell lines to front-line conventional therapeutic agents.81 Amide derivatives of permethyl ningalin B were also found as multidrug resistance reversal agents.82 Ningalin D 76, permethyl ningalin D, and ningalin D decaacetate were examined against a resistant HCT116 cell line that embodied the MDR phenotype through overexpression of P-gp. This cell line, HCT116/VM46, which is resistant to both vinblastine (IC50 = 100 vs 7 nM for parental HCT116) and doxorubicin (IC50 = 900 vs 70 nM for parental HCT116), was treated with each of the ningalin derivatives at 1 μM, and the IC50 was measured for co-administered vinblastine or doxorubicin. Under these conditions, ningalin D 76 and its decaacetate were inactive, whereas permethyl ningalin D effectively resensitized the resistant HCT116/VM46 cell line to vinblastine (IC50 = 8.5 nM, 80% reversion) and doxorubicin (IC50 = 100 nM, 70% reversion).75 Ningalin derivatives have been evaluated for their properties of potent reversal of MDR and use in drug combinations against human colon carcinoma xenograft in nude mice.83 The profound enhancement of antitumor cytotoxicity of vinblastine and taxol in vitro by ningalin derivatives may have multiple mechanisms including the MDR-reversing effects.
3.1.3. Inhibition of HIV-1 Integrase and MCV Topoisomerase
Human immunodeficiency virus (HIV) encodes three enzymes of reverse transcriptase, protease, and integrase. Anti-HIV drugs targeting the first two enzymes have been successfully employed for the treatment of acquired immune deficiency syndrome (AIDS). HIV-1 integrase is a promising target for anti-retroviral chemotherapy. Faulkner and coworkers reported that lamellarin α-20 sulfate 46 is a selective inhibitor of HIV-1 integrase both in vitro and in vivo.12,50 Compound 46 inhibited the integrase terminal cleavage activity with an IC50 of 16 μM, strand transfer activity with an IC50 of 22 μM, and growth of the HIV-1 virus in cell culture with an IC50 of 8 μM.12 Unlike most other natural product integrase inhibitors, inhibition was not completely regulated by the core domain but also partially by the N-and/or C-terminal domains (IC50 for disintegration for the catalytic core 64 μM, for the intact enzyme 7 μM), indicating that some form of unique multisite binding might be responsible for this inhibition.41 Compound 46 could inhibit viral replication in cultured cells by acting on part of the viral life cycle consistent with inhibition of integration. Firm data arguing for inhibition of integrase in cultured cells awaited the isolation of viral mutants with reduced sensitivity to 46. Mapping of such mutations to the integrase coding region could argue strongly for inhibition of integrase during viral replication.12
Lamellarin H 37, lamellarin α 45, and lamellarin α 13,20-disulfate 47 were also studied for their HIV-1 integrase inhibition.50 The integrase inhibition assay measured the reduction of accumulation of strand transfer products that result from incubation of purified HIV-1 integrase with target DNA and DNA mimicking one end of the unintegrated viral DNA.50 Lamellarin H 37, lamellarin α 13,20-disulfate 47, and lamellarin α-20 sulfate 46 were also screened for inhibition of the type 1B topoisomerase of the Molluscum contagiosum virus (MCV) with IC50 (μM) of 0.23, 70, and 170, respectively. This assay, which measured DNA cleavage and religation, was used as a counter-screen to detected nonselective inhibitors. Lamellarin H 37 exhibited very potent inhibition (IC50 = 1.3 μM) of HIV-1 integrase but unfortunately was even more active in the MCV topo-isomerase counter-screen (IC50 = 0.23 μM).50 Lamellarin α 13,20-disulfate 47 was a less effective inhibitor (IC50 = 49 μM) of HIV-1 integrase than was lamellarin α-20 sulfate 46 (IC50 = 22 μM). Lamellarin α 13,20-disulfate 47’s inhibition of HIV-1 integrase was also restrictive due to inhibiting MCV topoisomerase at about the same concentration (IC50 = 70 μM). Lamellarin H 37 is a more potent inhibitor of HIV-1 integrase but lacked the specificity required to be clinically useful.50 Polycitone A 58 was found to be a potent inhibitor of retroviral reverse transcriptases (i.e., HIV-1) and cellular DNA polymerases.84
3.1.4. Antibiotic Activity and Antibacterial Activity
Storniamides A–D 63–66 showed antibiotic activity against Gram-positive bacteria (Staphylococcus aureus, Bacillus subtilis, and Micrococcus luteus) at 50 μg/disk.19 Lamellarins Q 54 and R 55 have been shown to have no antibiotic activity.8 Lamellarins A 1, B 32, and C 3, the triacetate of lamellarin D 36, I 10, J 11, K 12, L 15, and M 38, and the triacetate of lamellarin N 41 were also found to have no antibacterial activity.6
3.1.5. Inhibition of ATP-Citrate Lyase and Human Aldose Reductase (h-ALR2)
Purpurone 77 is a potent inhibitor of ATP-citrate lyase (ACL).24 It inhibited ACL in a dose-dependent manner and has an IC50 of 7 μM. When tested for cytotoxicity, purpurone 77 was found to be nontoxic to Hep G2 cells, showing no reduction in cellular ATP levels at 143.3 nM. Preliminary biological data indicated that 77 was able to reduce fatty acid but not cholesterol biosynthesis.24 In 2006, lukianol B 57 was found to be the most active aldose reductase inhibitor (ARI) among all the phenolic derivatives assayed.86
3.1.6. Cell Division Inhibition and Immunomodulatory Activity
Lamellarins D 35 and C 3 were shown to inhibit cell division of sea urchin egg at concentrations of 19 μg/mL with 78% and 15% inhibition, respectively, while lamellarins A 1 and B 32 were inactive during the same bioassay.3 Lamellarins K 12 and L 15 have been observed to possess moderate immunomodulatory effects in the micromolar range (for 12 and 15, LcV = MLR 147 and 98, respectively).6 In 2006, there was a patent filed that claimed ningalin compounds for use in modulating immune responses to infection, injury, allergy, and/or transplantation.87
3.1.7. Antioxidant Activity
Lamellarins γ 30, K 12, U 22, I 10, and C-diacetate 4 were evaluated for their antioxidant activity, and the results showed that all of them demonstrated antioxidant properties only at millimolar range compared with standard antioxidants, which were active in the micromolar range. On the basis of the values of IC50 and the Trolox equivalent antioxidant capacity (TEAC), the rank order from the most active to the least active compound was 10 > 30 > 12 > 4 > 22.14
3.1.8. Feeding Deterrent
Didemnimides A–D 67–70 were reported to be potent feeding deterrents against a natural assemblage of mangrove-specific carnivorous fish.20,99 Ecologically relevant field and laboratory experiments have been designed to test the hypothesis that didemnimides function as predator deterrents. Didemnimides A–D 67–70 were found to constitute a significant chemical defense against carnivorous fish in mangrove habitats. Didemnimide D 70, the most active compound, deterred feeding of carnivorous wrasse Thalassoma bifasciatum at natural concentrations in aquarium assays. When tested in the mangrove environment itself, using an established method with control and treated foods, didemnimide D 70 alone significantly reduced consumption relative to controls.20 The relationship between antipredatory activity and the structure was the subject of additional studies. Fenical et al. found that the activity of the didemnimides was highly dependent on structure. Didemnimide D 70, the only compound that inhibited feeding in both lab and field assays, bears both a bromine on the indole ring and a methyl group on the imidazole ring.100
3.2. Structure–Activity Relationship (SAR) Studies
The first reported study on SAR of lamellarins and related pyrrole-derived alkaloids concerned lamellarin D 35,97 one of the most potent lead compounds for the control of cancer.33 The double bond at C-5 and C-6 in lamellarin D 35 seemed to be an essential element for the antitopoisomerase I activity.27,101 A library of open lactone analogues of lamellarin D was generated and tested in a panel of three human tumor cell lines, MDA-MB-231 (breast), A-549 (lung), and HT-29 (colon), to evaluate their cytotoxic potential. From these data it was concluded that more than 75% of the open-chain lamellarin D analogues tested showed cytotoxicity in a low micromolar GI50 range.33 The hydroxyl groups at C-8, C-14, and C-20 in lamellarin D 35 were also important for cytotoxic activity and DNA-topoisomerase I inhibition.33,101 Interestingly, derivatization of the these phenolic hydroxyl groups with amino acids, which preserved the hydrogen-bonding capacity at these sites, afforded potent compounds, whereas acylation with various carboxylic acids resulted in a considerable decrease in potency.33,101
Lamellarin D 35, which displayed potent cytotoxic activities against MDR tumor cell lines and was highly cytotoxic to prostate cancer cells, has a pentacyclic planar chromophore, whereas its synthetic 5,6-dihydro analogue, LAM-501, has a significantly tilted structure.90 DNA binding measurements by absorbance, fluorescence, and electric linear dichroism spectroscopy showed that lamellarin D 35 was a weak DNA binder that intercalated between base pairs of the double helix. In contrast, the nonplanar LAM-501 did not bind to DNA. LAM-501 was therefore found to be inactive against topoisomerase I and considerably less cytotoxic than the natural product lamellarin D 35, indicating that the planarity of the hexacyclic chromophore was essential for DNA binding and topoisomerase I inhibition.90
Lamellarins B 32, H 37, M 38, N 40, W 42, and X 43 also possess a double bond at C-5 and C-6 in the B ring and showed potent inhibition of human topoisomerase I.102 Ten derivatives of lamellarin D were synthesized with varying substituents on the aromatic rings and evaluated for cytotoxicity against a HeLa cell line to examine their SAR. The study showed that the presence of hydroxyl groups at the C-8 and C-20 positions would be essential for their cytotoxicity, while the hydroxyl group at C-14 and the two methoxy groups at C-13 and C-21 appeared to be less important for the cytotoxic activity.103 Further studies on the SAR of eight lamellarin derivatives were reported in 2005.104 The derivatives have been studied as topoisomerase I (Top1) inhibitors. Molecular models of the ternary complexes formed between the DNA-Top1 ensemble and lamellarin D 35 fully intercalated into the duplex DNA have been built and studied by means of nanosecond molecular dynamics simulations in aqueous solution. The results showed that the C-8 and C-20 hydroxyl groups in the lamellarin series would be the major determinants of both Top1 cleavable complex stabilization and the cytotoxic action.104 Another report also showed that the absence of a hydroxyl group at C-8 in lamellarins γ 30, K 12, U 22, I 10, and C-diacetate 4 decreased the potency of antioxidant activity of these compounds, while the presence of different functional groups at C-14 did not produce marked changes in the antioxidant activity.14,103
Faulkner and co-workers studied the SAR of lamellarin α 45 and its 20-sulfate 46 and 13,20-disulfate derivatives 47 for inhibition of HIV-1 integrase. Lamellarin α 45 showed no inhibition of HIV-1 integrase at concentrations up to 1.6 mM, while its sulfate derivatives 46 and 47 had significant IC50s of 22 and 49 μM, respectively. These results showed that the presence of sulfate on the periphery could greatly influence selectivity in HIV-1 integrase inhibition.50 The results were unexpected because other non-sulfated lamellarins inhibited HIV-1 integrase with IC50s in the low micromolar range.12 It did however support the idea that the activity of lamellarin α 20-sulfate 46 results from the compound acting as a mimic of the terminal unit of the viral DNA with the sulfate group binding to a site that normally binds the terminal phosphate of the DNA. Lamellarin α 13,20-disulfate 47 was considered as a nonselective HIV-1 integrase inhibitor. Faulkner and co-workers proposed that the nonselective inhibitors with two or more sulfate groups bind to sites that normally bind to two consecutive phosphate units in intact DNA.50 Cytotoxicity toward HeLa cells of lamellarin H 37, lamellarin α 45, lamellarin α 20-sulfate 46, and lamellarin α 13,20-disulfate 47 was measured, and the results showed that protection of the phenolic hydroxyl group as the sulfate could reduce the cytotoxicity of the parental lamellarins in general.12,50,51
The didemnimides A 67, D 70, and E 71 were inactive as cell cycle checkpoint inhibitors. In order to initiate a quantitative structure–activity relationship (QSAR) study on granulatimide (inhibitor of the G2 cell cycle checkpoint of mammalian cancer cells) and didemnimides as cell cycle checkpoint inhibitors, Trsic et al. performed molecular orbital calculations for these compounds. The electronic features and structural parameters were compared with inhibition of the G2 checkpoint. The hydrogen atom attached to the nitrogen atom of the indole moiety has a higher value of electronic charge in the biologically active compounds than in the inactive compounds. The inactive didemnimides A 67, D 70, and E 71 were more lipophilic than the active molecules due to the high electron density in the central regions that enhance their hydrophobicity.95
4. Synthesis
In light of the fascinating novel structures, intriguing biological properties and the difficulty in obtaining large quantities from the natural sources, the lamellarins and related pyrrole-derived alkaloids have attracted great interest as challenging natural product targets for total synthesis. Several research groups, including Ishibashi,31,34,40,51,103 Steglich,37,38,44,56,57,59,61,63,66,73 Ruchirawat,39,43,52,74,105 Banwell,42,47,49,106,107 Faulkner,50 Gupton,54,62,72 Boger,68–70,75,108 and Handy,41 have reported synthesis of lamellarins and related pyrrole-derived alkaloids. A number of elegant synthetic strategies have been employed in which highly functionalized precursors are assembled into the pyrrole core by means of intramolecular ylide cycloaddition,31,39,42,45,52 azadiene Diels–Alder cycloaddition,68 oxidative dimerization,38,44 or double-barreled Heck cyclization.106 The postulated biogenetic pathway for the alkaloids thought to be DOPA-derived is shown in Figure 3.23 A possible biogenetic relationship between the lamellarins and polycitone A 58 and polycitrins A 60 and B 61 was described by Kashman et al. (Figure 4).17
Figure 3.
Possible biogenetic pathways for several apparently DOPA-derived alkaloids from marine ascidians. (Reprinted with permission from ref 23. Copyright 1997 American Chemical Society.)
Figure 4.
Possible biogenetic relationship between the lamellarins, polycitone A (58) and polycitrins A (60) and B (61). (Reprinted with permission from ref 17. Copyright 1994 American Chemical Society.)
Solid-phase combinatorial synthesis is one of the most useful techniques for the preparation of a large number of structurally related molecules. This technique provides fast access to larger collections of products that may possess great diversity and incorporate optimized physical and pharmacological properties into the structures of lamellarins.46,48,109–113 Synthetic studies of lamellarins and related pyrrole-derived alkaloids have been summarized in Table 2. Several typical synthetic approaches leading to lamellarins and related compounds are presented in Schemes 1–11.
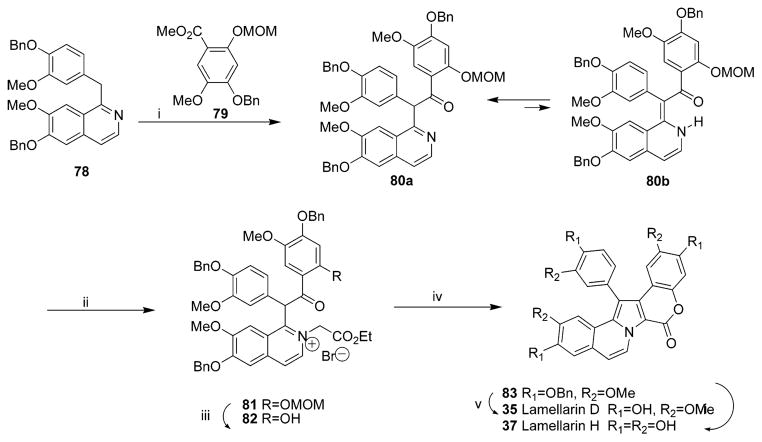
Scheme 1.
Synthesis of Lamellarins D (35) and H (37)a
a Reagents and conditions: (i) LDA, THF, 79, 63%; (ii) BrCH2CO2Et; (iii) cat. HCl, MeOH; (iv) Et3N, CH2Cl2, 34% (3 steps); (v) H2, Pd(OH)2/C, EtOAC, 82%; (vi) BBr3, CH2Cl2, 68%. (Reprinted with permission from ref 31. Copyright 1997 Elsevier B.V.)
Scheme 11.
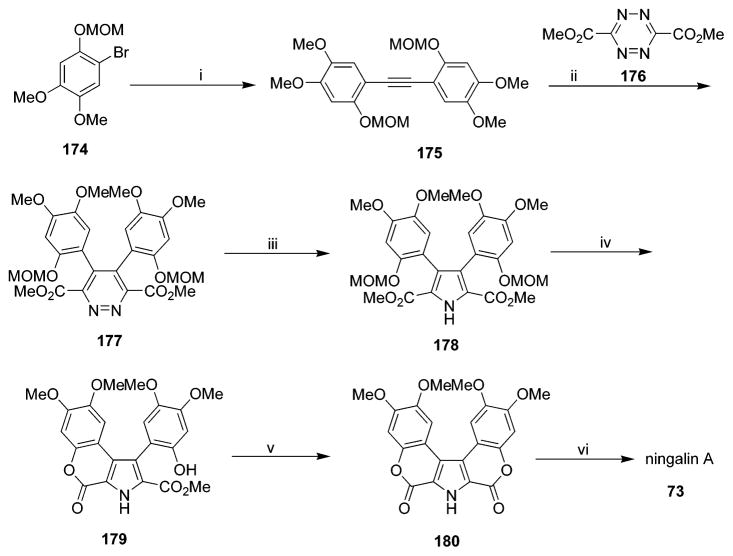
Synthesis of Ningalin A (73)a
a Reagents and conditions: (i) Bu3SnCCSnBu3,(Ph3P)4Pd, 79%; (ii) 176, 87%; (iii) Zn, HOAC, 63%; (iv) HCl·EtOAc, 94%; (v) DBU, 100%; (vi) BBr3, 96%. (Reprinted with permission from ref 68. Copyright 1999 American Chemical Society.)
4.1. Synthesis of Lamellarins
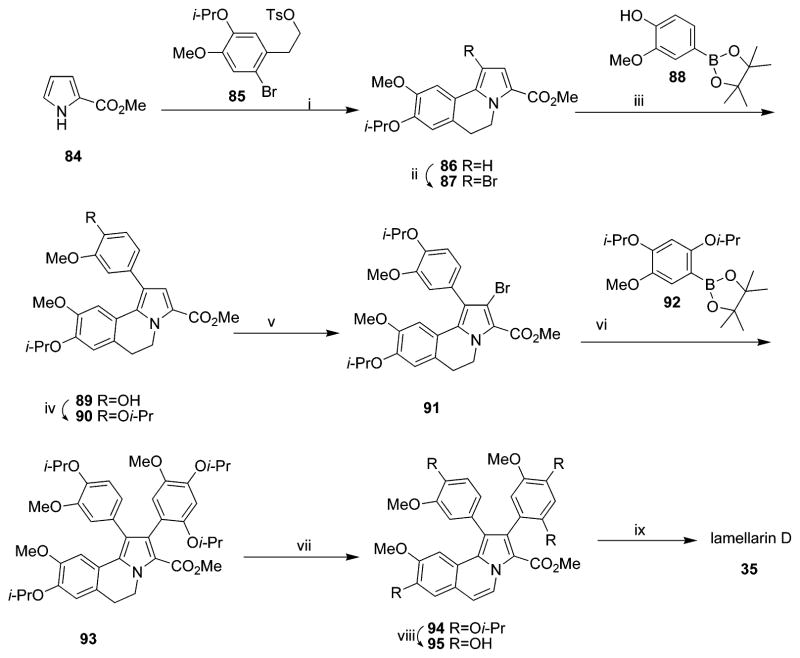
4.1.1. Synthesis of Lamellarins D, H, and N
In 1997, Ishibashi’s group accomplished the first total synthesis of lamellarin-class natural alkaloids, lamellarin D 35 and H 37, using N-ylide-mediated cyclization of a benzylisoquinoline derivative as the key ring construction procedure in five steps; yields of 35 and 37 were 18% and 15%, respectively (Scheme 1).31 In 2005, an efficient and highly convergent total synthetic route to lamellarin D 35 was achieved in eight steps with an overall yield of 18% through sequential and regioselective bromination/Suzuki cross-coupling, microwave-assisted oxidation, phenol group deprotection, and subsequent lactonization (Scheme 2).32 This synthetic strategy can be exploited for the preparation of other open-chain lamellarins. A library of open lactone analogues of lamellarin D was then prepared from the scaffold of methyl 5,6-dihydropyrrolo [2,1-a] isoquinoline-3-carboxylate by introducing various aryl groups through sequential and regioselective bromination followed by Pd (0)-catalyzed Suzuki cross-coupling chemistry in a 24–44% overall yield.33
Scheme 2.
Synthesis of Lamellarin D (35)a
a Reagents and conditions: (i) (1) 85, NaH, DMF, (2) PdCl2(pph3)2, PPh3, K2CO3; (ii) NBS, THF; (iii) 88, Pd(PPh3)4, Na2CO3, DMF; (iv) i-PrBr, K2CO3, DMF; (v) NBS, THF; (vi) 92, Pd(PPh3)4, K3PO4, DMF; (vii) DDQ, CHCl3, MW; (viii) AlCl3, CH2Cl2; (ix) NaH, THF. (Reprinted with permission from ref 32. Copyright 2005 American Chemical Society.)
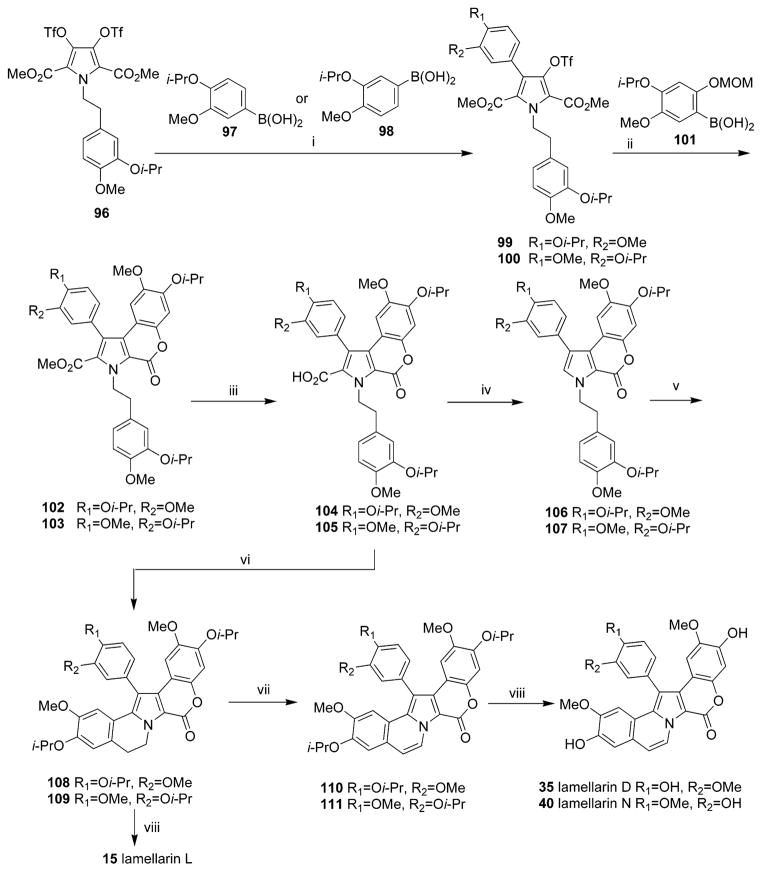
In 2006, lamellarins D 35, L 15, and N 40 were efficiently generated in total yields of 54%, 58%, and 50%, respectively, using Hinsberg-type pyrrole synthesis and palladium-catalyzed Suzuki–Miyaura coupling of the common intermediate 3,4-dihydroxypyrrole bistriflate as the key reactions (Scheme 3).34 Convergence of this synthetic strategy could enable the synthesis of a wide range of natural or non-natural lamellarins by simple structural modification of the starting bistriflate and arylboronic acids in relatively short steps.34
Scheme 3.
Synthesis of Lamellarins D (35), L (15), and N (40)a
a Reagents and conditions: (i) 97 or 98 (1.0 equiv), Pd(PPh3)4 (2 mol %), THF, reflux, 4 h (99, 76%; 100, 77%); (ii) (1)101 (2.0 equiv), Pd(PPh3)4 (8 mol %), THF, reflux, 18 h, (2) concd HCl, MeOH, reflux 1 h (102, 98%; 103, 95%); (iii) (1) 40% KOH–EtOH (1/1), reflux, 3 h, (2) cat. p-TsOH, CH2Cl2, reflux, 1 h (104, 90%; 105, 91%); (iv) Cu2O, quinoline, 220 °C, 7 min (106, 94%; 107, 99%); (v) Phl(OCOCF3)2, BF3·OEt2, CH2Cl2, −40 °C, 1.5 h (108, 88%; 109, 90%); (vi) Pd(OAc)2 (1.1 equiv), CH3CN, reflux, 12 h (110, 65%; 106, 14%; 109, 65%; 107, 15%); (vii) DDQ (1.5 equiv), toluene, reflux, 18 h (110, 97%; 111, 96%); (viii) BCl3, CH2Cl2, −78 °C, 0.5 h, then rt, 3 h (35, quant; 15, 98%; 40, 88%). (Reprinted with permission from ref 34. Copyright 2006 Elsevier B.V.)
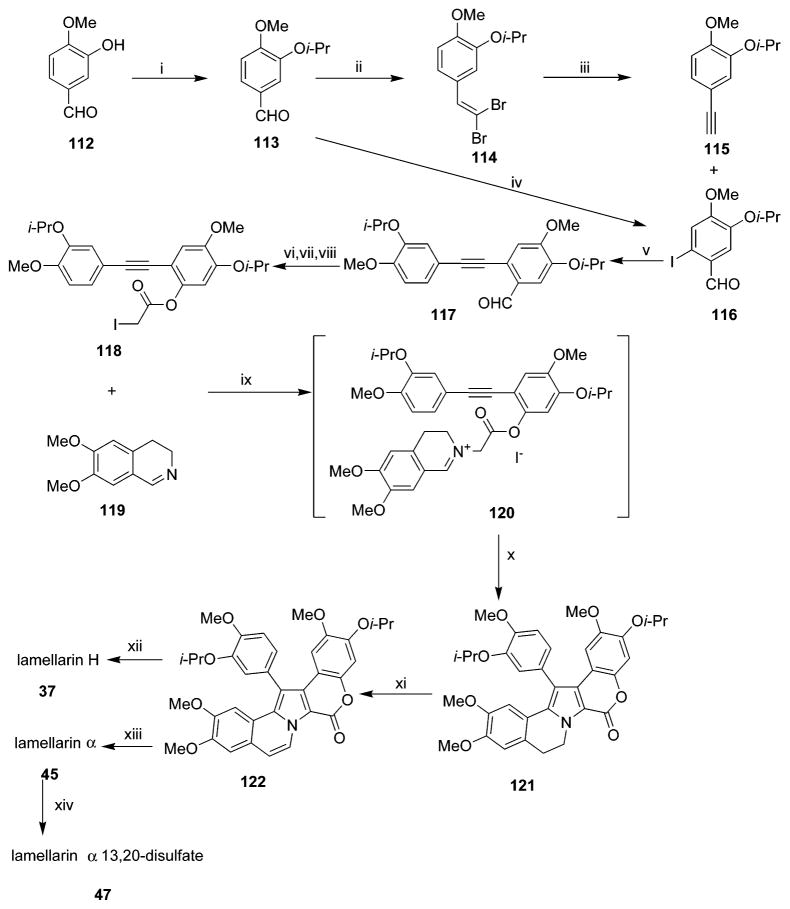
The total synthesis of lamellarin H 37 was first reported in 1997.31 In 2002, lamellarin H 37 was also synthesized by Faulkner and co-workers using a different route (Scheme 4).50 The two routes were complex and accomplished by more than 10 steps of chemical synthesis. In 2005, a new and simple route was developed to synthesize lamellarin H 37 and its derivatives. The fabricated intermediate, 2,4,5-trimethoxy-α-chloroacetophenone, was the key starting material for this synthesis.35 The next year the same group reported the synthesis of the second intermediate of lamellarin H 37, 1-(3,4-dimethoxyphenyl)-8,9-dimethoxy-2-(2,4,5-trimethoxyphenyl)-pyrrolo-[2,1-α]-isoquinoline.114
Scheme 4.
Synthesis of Lamellarin H (37), Lamellarin α (45), and Lamellarin α 13,20-Disulfate (47)a
a Reagents and conditions: (i) i-PrBr, K2CO3, DMF, rt, 48 h; (ii) CBr4, Zn, PPh3, CH2Cl2, 0–25 °C, 4 h; (iii) n-BuLi, THF, −78 to −25 °C, 2 h; (iv) AgOCOCF3, I2, CH2Cl2, reflux, 12 h; (v) Pd(PPh3)4, CuI, NEt3, 45 °C, 5 h; (vi) MCPBA, KHCO3, CH2Cl2, rt,1 h; (vii) NH3, CH2Cl2/MeOH (1:1), rt, 2 h; (viii) ICH2COOH, DCC, DMAP, CH2Cl2, rt, 5 h; (ix) ClCH2CH2Cl, rt, 24 h; (x) Hunig’s base, reflux, 30 h; (xi) DDQ, CH2Cl2/EtOH (1:1), reflux, 48 h; (xii) BCl3, CH2Cl2, 0 °C, 6 h; (xiii) DMF–SO3, DMF/pyridine (4:1), 65 °C, 2 h; (xiv) BBr3, CHCl3, 55 °C, 24 h. (Reprinted with permission from ref 50. Copyright 2002 Elsevier B.V.)
4.1.2. Synthesis of Lamellarin G Trimethyl Ether and Lamellarin G
In 1997, Steglich’s group described a biomimetic synthesis of lamellarin G trimethyl ether in 33% overall yield. The key step was the oxidative dimerization of an arylpyruvic acid and condensation of the resulting 1,4-dicarbonyl compound with a suitable 2-arylethylamine. This method could be applied to the synthesis of other lamellarins, especially to those with nonsymmetrically substituted aryl groups.38 In 2001, a general and efficient synthesis of lamellarin G trimethyl ether was achieved by Ruchirawat et al. in Thailand.39 The synthesis involved formation of the core pyrrole [2,1-α] isoquinoline followed by formation of the lactone ring. Later on, in 2003, Ruchirawat et al. further developed the synthesis of lamellarins using direct metal–halogen exchange of 2-bromopyrrole carbonate derivatives with tert-butyllithium followed by intramolecular lactonization of the resulting 2-pyrrole anion onto the carbonate to generate the corresponding lamellarins in moderate to good yields.52 The synthesis of lamellarin G trimethyl ether, starting from a symmetric 3,4-dihydroxypyrrole bis-triflate derivative, was reported by Iwao and co-workers in 2003. This short and flexible route was developed using two key reactions: Hinsberg-type condensation and palladium-catalyzed Suzuki cross-coupling starting from a symmetric bis-trifloxy pyrrole.40 More recently, Handy et al. reported a modular synthesis of the lamellarin G trimethyl ether that was based on application of three iterative sequential and regioselective halogenation/Suzuki cross-coupling events. This route accessed the target structure in 11 steps with 9% overall yield.41
In 2006, lamellarin G 8 was synthesized in 57% overall yield by the key step of formation of 3,4-diarylpyrrole-2,5-dicarboxylic acids from arylpyruvic acids and 2-arylethyl-amines.37
4.1.3. Synthesis of Lamellarins I, K, L, and U
Banwell’s group reported the total synthesis of lamellarin K 12 in 1997. The pivotal step is construction of the central pyrrole ring by an intramolecular 1,3-dipoar cycloaddition between an azomethine ylide and an alkyne.42 Banwell’s synthesis of lamellarin K 12 was particularly interesting because it was convergent, versatile, and high yielding. The highly convergent nature of the reaction sequence would suggest that this approach could be readily adapted to the preparation of lamellarins A–N 1, 32, 3, 35, 6–8, 37, 10–12, 15, 38, and 40 and S–X 18, 19, 22, 24, 42, and 43 as well as many analogues thereof. In 2001, lamellarins I 10 and K 12 were synthesized by a related approach based on a [3 + 2] cycloaddition of a nitrone to an alkyne. The key cycloaddition yielded an isoxazoline which was rear-ranged to afford the central pyrrole ring.36 In 2004, lamellarins K 12 and L 15 were successfully obtained by Ruchirawat et al. in three steps from benzyldihydroisoquinolines with ester nitrostyrene in 65% and 61% overall yields, respectively. The key step was the Michael addition/ring-closure reaction of benzyldihydroisoquinoline derivatives with ethoxycarbonyl-β-nitrostyrenes (Scheme 5).43 The basic building blocks for the lamellarins are simple and easily prepared substituted-benzaldehyde derivatives. Each lamellarin could be analyzed to consist of three such building blocks: two in the benzyldihydroisoquinoline derivative and the other in the ester nitrostyrene. This convergent synthetic approach could allow easy incorporation of all aryl groups on the lamellarin skeleton without the need for complex protecting group strategies. The benzyl group was chosen as the only necessary hydroxyl protecting group since all the benzyl groups could be removed in the same step by simple palladium-catalyzed hydrogenolysis.43 In 2006, bio-mimetic syntheses of lamellarin K 12 were developed by Steglich et al. in four steps and 37% overall yield.37 In the same year, 28 natural (lamellarins C 3, E 6, F 7, G 8, I 10, J 11, K 12, L 15, T 19, U 22, Y 26, and χ 31) and unnatural lamellarins with either a saturated or an unsaturated D ring were synthesized by Ruchirawat et al. The key step involved the Michael addition/ring closure (Mi-RC) of the benzyldihydroisoquinoline and α-nitrocinnamate derivatives, which provided the 2-carboethoxypyrrole intermediates in moderate to good yields (up to 78% yield).52
Scheme 5.
Synthesis of Lamellarins K (12) and L (15)a
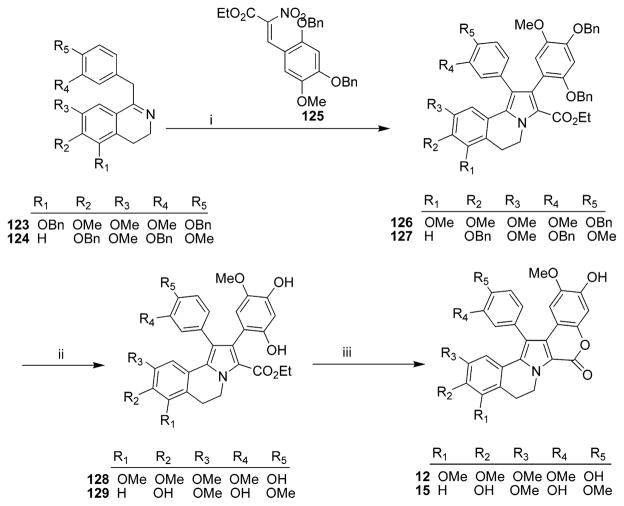
a Reagents and conditions: (i) NaHCO3, 125, CH3CN, reflux, 70% (126), 70% (127); (ii) H2, Pd/C, EtOAC; (iii) NaH, THF, 93% (12, over 2 steps), 87% (15, over 2 steps). (Reprinted with permission from ref 43. Copyright 2004 by John Wiley & Sons, Inc.)
Biomimetic total synthesis of the nonsymmetrical lamellarin L 15 was developed in 2000 by reaction of the ethyl 3-arylpyruvate with the methyl 2-bromo-3-arylpyruvate in the presence of the 2-arylethylamine, which afforded the pyrrole derivative and was transformed into lamellarin L 15 in five steps.44 The synthesis proceeded with 38% overall yield and mimicked the probable biosynthesis of the analogues of lamellarin L.44 Lamellarin L 15 and lamellarin U 22 were generated via a total solid-phase synthesis.45,46 The key step of this approach was the solid-phase conversion of an aldehyde group into a formate by a Baeyer–Villiger reaction and an intramolecular [3 + 2] cycloaddition of a 3,4-dihydroisoquinolinium salt over a triple bond.45 This solid-phase synthetic strategy would enable the construction of related compound libraries for biological evaluation. Lamellarins U 22 and L 15 were generated in 10% and 4% overall yield, respectively, after eight-step solid-supported synthesis (Scheme 6).45 Lamellarin L 15 was also synthesized by Iwao et al. in 2006 (Scheme 3).34
Scheme 6.
Synthesis of Lamellarin U (22) and Lamellarin L (15)a
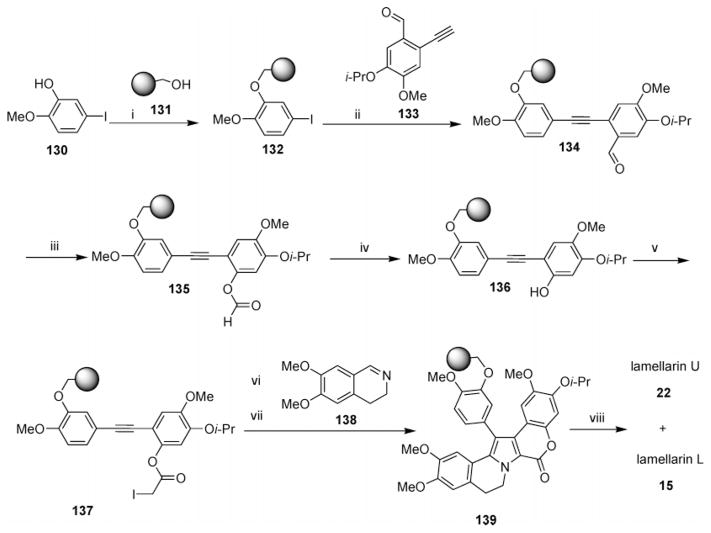
a Reagents and conditions: (i) DEAD, PPh3, DIEA, THF, 0 °C, 10 min, rt, 3 h, loading of Merrifield resin = 0.68 mmol/g; (ii) 2-ethynyl-5-isopropoxy-4-methoxy-benzaldehyde (3 equiv), CuI (0.6 equiv), Pd [(Ph3)2Cl2] (0.3 equiv), THF–Et3N (3:1), N2, 20 h; (iii) mCPBA (6 equiv), NaHCO3 (12 equiv), CH2Cl2, 0–20 °C, 15 h; (iv) 2 M KOH, THF–MeOH, rt, 5 h; (v) ICH2CO2H (10 equiv), DMAP (15%), DIP (10 equiv), DMF, rt, 12 h; (vi) 3,4-dihydro-6,7-dimethoxyisoquinoline (6 equiv), CH2ClCH2Cl, rt, 24 h; (vii) DIEA (7 equiv), 83 °C, 24 h; (viii) AlCl3 (15 equiv), CH2Cl2, rt, 12 h. (Reprinted with permission from ref 45. Copyright 2003 American Chemical Society.)
4.1.4. Synthesis of Lamellarins O and Q and Lamellarin O Dimethyl Ether
In 1997, lamellarins O 52 and Q 54 were synthesized by Banwell et al. using Stille, Suzuki, or Negishi cross-coupling reactions as the key step (Scheme 7).47 Total synthesis of lamellarin O 52 has also been reported utilizing a common heterocyclic azadiene Diels–Alder reaction by Boger et al.68 In 2004, Albericio’s group described an efficient solid-phase strategy for synthesis of lamellarins O 52 and Q 54 using Merrifield resin and N-protected methyl 3,4-dibromopyrrole-2-carboxylate as a scaffold.48 The process involved incorporation of the appropriately substituted pyrrole ring onto a p-alkoxy iodophenyl resin through a Negishi cross-coupling reaction. This step was followed by a Suzuki cross-coupling reaction to introduce the second substituted phenyl ring and N-alkylation of the pyrrole. Final cleavage was achieved using Lewis acid to give the desired product. Diversity can be introduced in each step of the synthetic process as well as during the final cleavage using the appropriate Lewis acid conditions, which could generate a compound library of lamellarins O 52 and Q 54. Lamellarins O 52 and Q 54 were also generated via a solid-phase synthetic approach that was applied to the preparation of lamellarins U 22 and L 15 at the same time.46
Scheme 7.
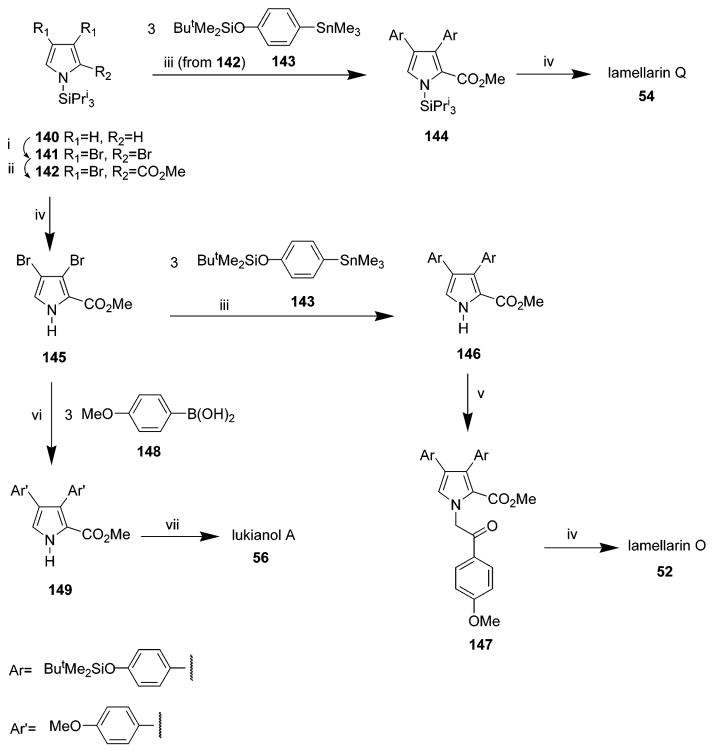
Convergent Synthesis of Lamellarins O (52) and Q (54) and Lukianol A (56)a
a Reagents and conditions: (i) NBS (3 equiv), THF, −78 °C, 1 h then 20 °C for 4 h; (ii) PhLi (1 equiv), −78 °C, 0.16 h then ClCO2Me (1.05 equiv), −78 to 20 °C, 1 h; (iii) Pd(PPh3)2Cl2 (10 mol %), 1,4,-dioxane, 101 °C, 14 h; (iv) Bu4NF (10 mol % excess), THF, 20 °C, 1 h then 0.5 M aq.HCl; (v) p-MeOC6H4COCH2Br (3 equiv.), K2CO3 (5 equiv.), Bu4NCl (20 mol %), THF, 66 °C, 7 h; (vi) Pd(PPh3)4 (5 mol %), satd aq. Na2CO3 (6 equiv), DMF, 153 °C, 23 h; (vii) see ref 53. (Reprinted with permission from ref 47. Copyright Royal Society of Chemisty 1997.)
In 1995, a titanium-induced cyclization of readily accessible amido–enones to substituted pyrrole derivatives was applied to the total synthesis of lamellarin O dimethyl ether (Scheme 8).53 After that, lamellarin O dimethyl ether was also synthesized by Gupton’s group in 1999. The authors demonstrated that the vinylogous amide, the chloropropeniminium salt, and the β-chloroenal react successfully with glycine esters in a regiocontrolled and efficient fashion to produce 2,3,4-trisubstututed pyrroles that lead to lamellarin O dimethyl ether.54
Scheme 8.
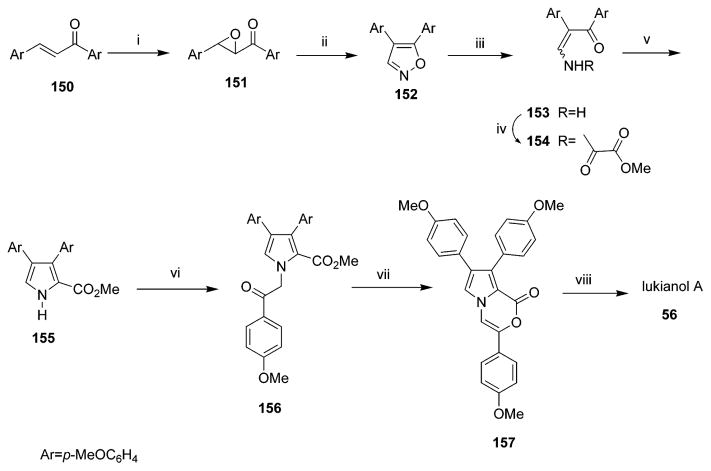
Synthesis of Lukianol A (56)a
a Reagents and conditions: (i) H2O2, NaOH, EtOH/H2O, 0 °C, 98%; (ii) (1) BF3·Et2O, Et2O, reflux; (2) NH2OH·HCl, pyridine, EtOH, reflux, 67% (over 2 steps); (iii) H2 (1 atm), Pd (5%)/C, THF, 94%, (Z):(E) = 1:1; (iv) ClOCCO2Me, pyridine, THF, 73%, (Z):(E) = 2.5:1; v, Ti–graphite (TiCl3:C8K = 1:2), DME, reflux, 52%; (vi) p-MeO–C6H4COCH2Br, K2CO3, acetone, reflux, 91%; (vii) (1) KO–tBu, H2O, Et2O, 0 °C; (2) Ac2O, NaOAC, reflux, 59% (over 2 steps); (viii) BBr3, CH2Cl2, −78 °C, 99%. (Reprinted with permission from ref 53. Copyright 1995 American Chemical Society.)
4.1.5. Synthesis of Lamellarin T Hybrids
The lamellarin T hybrids were synthesized by Banwell et al. in 2006. The key steps included selective lithium-for-halogen exchange, Negishi, and Suzuki–Miyaura cross-coupling reactions.49
4.1.6. Synthesis of Lamellarin α, Lamellarin α 13,20-Disulfate, and Lamellarin α 20-Sulfate
In 2002, a synthetic approach to lamellarin α 45 and lamellarin α 13,20-disulfate 47 was reported (Scheme 4).50 Lamellarin α 45 was generated relying on palladium-mediated and other coupling reactions, and lamellarin α 13,20-disulfate 47 was semi-synthesized by treatment of lamellarin α 45 with the DMF complex of sulfur trioxide in DMF/pyridine.50 The first total synthesis of lamellarin α 20-sulfate 46 has been just recently developed in 2006 in 14 steps from 2-(3,4-dimethoxyphenyl) ethylamine with an overall yield of 24%.51 The lamellarin α core in which 13-OH and 20-OH were differentially protected by isopropyl and benzyl groups, respectively, was constructed using Hinsberg-type pyrrole synthesis and Suzuki–Miyaura coupling as the key reactions. The 20-sulfate was prepared by a sequence including debenzylation of 20-OBn, 2,2,2-trichloroethylsulfation of the resulting 20-OH, deprotection of 13-Oi-Pr, and final reductive cleavage of the 2,2,2-trichloroethyl ester. This synthesis could pave the way to generation of diverse sulfated lamellarins.51
4.2. Synthesis of Pyrrole-Derived Alkaloids Related to Lamellarins
4.2.1. Synthesis of Lukianol A
Seven independent total synthetic routes of lukianol A 56 have been reported since 1995.37,47,53–56,68 Structurally, lukianol A 56 possesses a unique methyl 3,4-diarylpyrrole-2-carboxylate skeleton. In 1995, a concise titanium-mediated approach to pyrroles was accomplished, and this method was applied to the first total synthesis of lukianol A 56 (Scheme 8) in eight steps with 12% overall yield starting from commercially available 4,4′-dimethoxychalcone.53 Lukianol A 56 was also convergently synthesized using Stille, Suzuki, or Negishi cross-coupling reactions as the key step.47 In 1999, Boger’s group reported obtaining lukianol A 56 by utilizing a common heterocyclic azadiene Diels–Alder reaction.68 In the same year, Gupton et al. reported an efficient relay synthesis of lukianol A 56 and related compounds utilizing vinylogous iminium salt derivatives as key intermediates for the regioselective synthesis of heterocyclic compounds.54 In 2000, a formal total synthesis of lukianol A 56 was completed by Wong’s group. The target alkaloid was prepared through a strategy that utilized the β effect of a trimethylsilyl group to a highly regioselective synthesis of 2,3,4-trisubsti-tuted 1H-pyrroles as the pivotal step.55 In 2006, Steglich et al. developed a biomimetic approach to synthesize lukianol A 56 in which the lukianol skeleton is assembled in a single step.37 The following year, they reported that simple 3,4-diaryl- and 3,4-diindolylpyrrole-2,5-carboxylic acids can be easily obtained from aryl(indolyl)pyruvic acids and ammonia in a reaction that can be applied to a short synthesis of lukianol A 56.56
4.2.2. Synthesis of Polycitrins A and B and Polycitones A and B
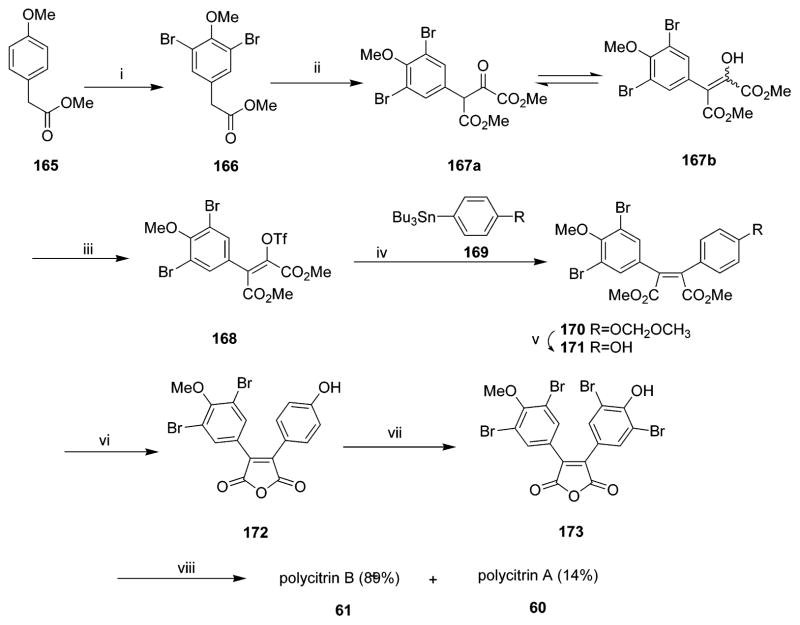
Both polycitrins A 60 and B 61 contain a maleimide unit in their structures. Biomimetic total synthesis of polycitrin A 60 was described by Steglich and co-workers in 1995.57 The synthesis was based on formation of 3,4-bisarylpyrrole-2,5-dicarboxylic acids from 3-arylpyruvic acids by oxidative coupling and consecutive pyrrole ring formation. The pyrrole dicarboxylic acids were then converted into 3,4-bisaryl maleimides by treatment with hypochlorite. The synthesis was then completed by bromination and introduction of the N-alkyl substituent. Polycitrin A 60 was thus obtained in six steps from 3-(4-methoxyphenyl) pyruvic acid with 26% overall yield (Scheme 9).57 After 11 years the same group reported a considerably shorter synthetic route to polycitrin A 60 starting from 3-(3,4-dibromo-4-methoxyphenyl) pyruvic acid in three steps and in 43% overall yield.59 Polycitrin A 60 and prepolycitrin A 62 were also reported by Correia’s group in 2006. Polycitrin A 60 was obtained from maleic anhydride and 4-methoxybenzenediazonium tetrafluoroborate by palladium-catalyzed Heck diarylation of maleic anhydride in four steps.58
Scheme 9.
Biomimetic Total Synthesis of Polycitrin A (60)a
a Reagents and conditions: (i) (1) THF, n-BuLi, −78 °C, (2) 0.5 equiv, I2, −78 to 25 °C, (3) NH3, TiCl4, 72%; (ii) NaOCl,95%; (iii) NaHSO3; (iv) (1) KOH, (2) HCl, 75%; (v) BBr3, 78%; (vi) Br2, AcOH,83%; (vii) tyramine, diisopropylethylamine, PhOH (melt), 160 °C, 78%. (Reprinted with permission from ref 57. Copyright 1995 Elsevier B.V.)
In 2000, Italian scientists reported the first total synthesis of polycitrin B 61 based on a palladium-catalyzed cross-coupling reaction (Scheme 10) and starting from 3,5-dibromo-4-methoxy-phenylacetic acid methyl ester.60 Polycitrin B 61 was obtained in a good yield and crystallized from CH2Cl2–pentane as yellow crystals.
Scheme 10.
Total Synthesis of Polycitrin B (61)a
a Reagents and conditions: (i) Br2,FeBr2, 80%; (ii) MeONa, MeOH, (CO2Me)2, 99%; (iii) (CF3SO2)2O, iPr2NEt,CH2Cl2, 84%; (iv) 169, Pd2dba3, Ph3P,DMF, 81%; (v) Me3SiCl, NaI, MeCN, 92%; (vi) NaOH, H2O, MeOH, 77%; (vii) Br2,AcOH, 89%; (viii) tyramine, iPr2NEt, phenol, molecular sieves, 140 °C. (Reprinted with permission from ref 60. Copyright 2000 Elsevier B.V.)
Polycitones A 58 and B 59 are polybrominated lamellarin-related alkaloids. Both polycitones A 58 and B 59 were first synthesized in 2002 in Steglich’s group. Polycitone B 59 was obtained in four steps from pyrrole dicarboxylic acid, including Friedel–Crafts reaction of the corresponding acid chloride with anisole.61 Conversion of polycitone B 59 into polycitone A 58 was then achieved in two steps via Mitsunobu alkylation of the pyrrolic NH group. Polycitone A 58 was obtained in eight steps from 3-(4-methoxyphenyl) pyruvic acid with 22% overall yield. The synthesis is very flexible and can be easily adapted to the preparation of analogues with various substituents on the pyrrole ring.61 In 2006, Gupton’s group demonstrated a new and efficient relay strategy to synthesize polycitones A 58 and B 59. Their approach relied on formation of 2,4-disubstituted pyrroles from vinamidinium salts followed by electrophilic substitution at the 5-position of the pyrrole and halogenation and a microwave-accelerated Suzuki coupling at the 4-position to produce the tetrasubstituted heterocycle efficiently with complete control of regiochemistry.62 It is important to note that each pyrrole substituent in this strategy was introduced independently and can be easily varied so as to accommodate in-depth SAR studies for polycitones A and B analogues.
4.2.3. Synthesis of Storniamide A Nonamethyl Ether and Permethyl Storniamide A
In 1998, storniamide A nonamethyl ether was concisely obtained by Steglich et al. from 3-(3,4,5-trimethoxyphenyl) pyruvic acid and 2-(4-hydroxyphenyl) ethylamine in three steps.63 The applied biomimetic methodology is very similar to that of the synthesis of polycitrin A 60.57 Storniamide A nonamethyl ether was first obtained as a mixture of the (E,E)-, (E,Z)-, and (Z,Z)-isomers in a ratio of 3:4:1. Isomerization of the mixture with iodine in CHCl3 enriched the (E,E)-isomer, giving a ratio of 6:5:1. The three isomers could be separated by chromatography on a silica gel column to yield the desired isomer of (E,E)-storniamide A nonamethyl ether with an overall yield of 19%. Experiments to convert (E,E)-storniamide A nonamethyl ether into the natural product storniamide A 63 were as yet unsuccessful.63 Permethyl storniamide A was synthesized utilizing a common heterocyclic azadiene Diels–Alder reaction in 1999.68 On the basis of palladium-catalyzed Suzuki and Negishi cross-coupling reactions, permethyl storniamide A was synthesized in 2002 by Fürstner et al.64 In 2003, a formal syntheses of permethyl storniamide A was reported, which was based on a highly efficient route to 3,4-diarylpyrrole marine alkaloids.40
4.2.4. Synthesis of Didemnimides A–C
In 1998, the total syntheses of didemnimides A 67 and B 68 were first reported by Cava and Hughes. They applied the methodology for the synthesis of unsymmetrical bisin-dolylmaleimides via a K+t-BuO−-catalyzed condensation of indole-3-acetamide with methyl indolyl-3-glyoxylates.65 The total synthesis of didemnimide A 67 was also developed by Piers et al.21 In the same year, didemnimide C 69 was synthesized by Steglich’s group in four steps in 29% overall yield. The key step in the synthesis is the Stille coupling between the urethane-protected 3-bromo-4-(indol-3-yl)-methylmaleimide and 5-tributylstannyl-1-methylimidazole.66 Two years later, Piers and co-workers also reported the synthesis of didemnimide C 69 in three steps from 1-methyl-2-phenylthioimidazole in 28% overall yield. It is a shorter synthesis than those previously reported.67
4.2.5. Synthesis of Ningalins A–D
The first total syntheses of ningalins A 73 and B 74 were achieved in Boger’s lab in 1999 and 2000, respectively.68,69 The syntheses were based on a common heterocyclic azadiene Diels–Alder strategy (1,2,4,5-tetrazine → 1,2-diazine → pyrrole) (Scheme 11).68–70 The main feature of this strategy is the unusually effective [4 + 2] cycloaddition of the electron-deficient 1,2,4,5-tetrazine with an unsymmetrical and electron-rich alkyne, which was also applied to the synthesis of lamellarin O 52, lukianol A 56, and permethyl storniamide A at the same time.68 Moreover, the methodology may be suitable for construction of other densely functionalized pyrrole cores.
In 2002, Bullington et al. first regioselectively prepared the key intermediate used to generate DOPA-derived o-catechol alkaloids, the Fürstner intermediate. Ningalin B 74 was then concisely synthesized in a 51% overall yield.71 On the basis of the two key reactions, Hinsberg-type pyrrole synthesis and palladium-catalyzed Suzuki cross-coupling of the 3,4-dihydroxypyrrole bis-triflate derivatives, a formal synthesis of ningalin B 74 was reported in 2003.40 In 2006, a biomimetic synthesis of ningalin B 74 was developed by Steglich et al., starting with the oxidative coupling of two equivalents of 3-(3,4-dimethoxyphenyl) pyruvic acid and in situ reaction of the resulting 1,4-diketone with one equivalent of 2-(3,4-dimethoxyphenyl)ethylamine and obtained in four steps in 52% overall yield.37 Application of vinylogous iminium salt derivatives to a convenient and efficient synthesis of ningalin B hexamethyl ether was reported by Gupton et al. in 2003.72 This methodology was an extension from an earlier strategy that was used in the synthesis of lukianol A 56.54
In 2000, Steglich’s lab reported the first total synthesis of ningalin C 75 using an intramolecular Friedel–Crafts acylationfrom1-[2-(3,4-dimethoxyphenyl)ethyl]-3,4-bis(3,4-dimeth-oxyphenyl)-1H-pyrrole and methyl 2-diazo-2-(3,4-dimethoxyphenyl) acetate in five steps with a total yield of 19%.73 After that, a new and efficient method for the synthesis of permethyl ningalin C was accomplished with a key step involving formation of a pyrrolinone from an aminoquinone in one pot. Ningalin C 75 was then obtained through demethylation of its permethyl precursor.74 Ningalin D 76, the most complicated member of the ningalins, was synthesized in 2005 in Boger’s lab in nine steps based on a key 1,2,4,5-tetrazine → 1,2-diazine → pyrrole Diels–Alder strategy to assemble a fully substituted pyrrole core central to its structure.75 The overall yield of the total synthesis was 19%.75
5. Conclusion
Over the past two decades, more than 70 lamellarins and related pyrrole-derived alkaloids have been isolated from taxonomically quite distinct marine organisms (ascidians, molluscs, and sponges) collected in the Indian or Pacific oceans by SCUBA diving (−5 to −32 m). Among these marine organisms the ascidian Didemnum (Didemnidae) is byfarthemostproductivemarinegenusforlamellarins.5,6,9,11,13–15 Purification was accomplished with chromatography on silica gel, Sephadex LH-20, or HPLC. The isolated metabolites were elucidated by NMR, HRMS, single-crystal X-ray, and other spectroscopic methods.
Often lamellarins have been reported to be isolated from several marine sources. For example, lamellarins A 1, B 32, and C 3 were found in both prosobranch mollusc Lamellaria sp.3 and marine ascidians.6,11 The re-isolation of these compounds from other sources indicate that these molluscs likely sequester the compounds from a didemnid ascidian food source.6 The structures of lamellarins and other pyrrole-derived alkaloids are related, and they likely have a common biogenetic origin. Their common structural feature is a pyrrole ring substituted at positions 3 and 4 by polyhydroxy-or methoxyphenyl groups, a combination was not found outside of this class. The aromatic ring is rotationally highly restricted (600 kcal/mol, MM2 calculation) and exists essentially orthogonal to the plane of the pentacyclic ring. Thus, these molecules should exist as chiral forms due to the atropisomerism. Interestingly, however, all examples (except for lamellarin S 18) so far isolated were racemic. Faulkner’s group even attempted to separate the atropisomers of lamellarin α 45 using chiral reagents such as Mosher’s reagent or D-(+)-10-camphorsulfonyl chloride as well as HPLC or chiral TLC but were unsuccessful.50
The unique structures and biological activities of the lamellarins and related pyrrole-derived alkaloids have attracted significant attention from synthetic chemists. As the core skeleton of lamellarins and related compounds, pyrroles and pyrrole-containing heterocycles attract numerous approaches for synthetic studies.114–139 These studies have contributed to the development of elegant synthetic routes of lamellarins and related pyrrole-derived alkaloids as well as their key intermediates.
Although numerous lamellarins and related pyrrole-derived alkaloids have been identified and their biological activities studied, very little is known about the mechanism of action at the cellular and molecular level. Further studies could contribute to a better understanding of the mechanism of lamellarins and analogues and will be beneficial for the ongoing development and lead optimization of this class. Development of synthetic methods for lamellarins and related pyrrole-derived alkaloids has provided quantities for study of their mechanism of action and the structure–activity relationship (SAR). At present a few SAR studies have been reported, especially focusing on lamellarin D 35. Additional lamellarin SAR studies will be useful to fully utilize the lamellarins as leads for the treatment of numerous diseases.
Acknowledgments
The preparation of this review was supported, in part, by NSFC grants 90713040 and 30640068, a NCET grant, STCSM grants 06DZ19002, 06PJ14033, and 07DZ22006 (JFH), and NIH grant 1RO1AI36596 (M.T.H.). J.F.H. thanks his research group for their assistance in the preparation of this review.
Biographies

Hui Fan was born in Langfang, Hebei Province, China, in 1977. She received her B.Sc. (2000) and M.Sc. (Botany, 2003) degrees from Hebei Agriculture University and Beijing Forestry University, respectively. After graduating, she worked as a research assistant at Key Laboratory of Brain Functional Genomics, Ministry of Education & Shanghai Key Laboratory of Brain Functional Genomics (MOE & SBFG). She is currently working toward her Ph.D. degree with Professor Jin-Feng Hu at East China Normal University, where she is carrying out research on isolation, elucidation, and biological activity studies of natural products.

Jiangnan Peng is a Research Scientist at the Department of Pharmacognosy at the University of Mississippi. He received his B.Sc. and M.Sc. degrees in Pharmacy from the Beijing University of Chinese Medicine in 1987 and 1990, respectively. He received his Ph.D. degree in Natural Products Chemistry in 1995 from the Institute of Materia Medica, Academy of Medical Sciences & Peking Union Medical School, under the guidance of Professors Xiao-Zhang Feng and Xiao-Tian Liang. Then he worked as Assistant Professor and Associate Professor in Beijing Institute of Radiation Medicine studying traditional Chinese medicine. He has focused on marine natural products since he moved to Dr. Mark T. Hamann’s laboratory in 2000. During his research career, he has published over 35 scientific papers, book chapters, and patents. His interests lie in the isolation and structure elucidation of bioactive compounds from marine and terrestrial organisms, determination of the relative and absolute configuration of complex natural products using spectroscopic methods in combination with molecular modeling, and optimization of marine natural products through semi-synthesis or biotransformation. Currently he is working on the characterization of molecules associated with harmful algal blooms and marine natural products with activity against infectious diseases.

Mark T. Hamann is a Professor of Pharmacy and Chemistry & Biochemistry as well as a Research Professor with the National Center for Natural Products Research at the University of Mississippi and an Adjunct Professor with the Center of Marine Biotechnology (COMB), University of Maryland Biotechnology Institute (UMBI). He received his B.Sc. degree in Chemistry & Biology from Bemidji State University in Minnesota in 1985. He has several years of experience in GMP pharmaceutical manufacturing at Solvay Pharmaceuticals in Baudette, Minnesota, and then completed his Ph.D. degree in Marine Natural Products Chemistry in 1992 at the University of Hawaii, Chemistry Department, under the guidance of the late Professor Paul Scheuer, a pioneer in the development of marine products. During his research career, he has published over 100 scientific papers, reviews, and book chapters and currently serves as an editor for Biochimica et Biophysica Acta. His group is actively involved in the isolation, structure determination, and synthetic optimization of marine natural products and marine toxins. His group is currently working on marine natural products with activity against infectious diseases, cancer, and neuropsychiatric disorders.

Jin-Feng Hu studied organic chemistry at Lanzhou University, where he obtained his B.Sc. (1990) and Ph.D. (1996) degrees in Natural Organic Chemistry under the supervision of Professor Zhong-Jian Jia. He was a postdoctoral fellow (1996–1998) in the Department of Natural Products Chemistry, Institute of Materia Medica (Chinese Academy of Medical Sciences and Peking Union Medical College), with Professor Xiao-Zhang Feng. He joined the Hans-Knoell-Institute for Natural Products Research as a BMBF & DLR fellow (1998–2000) with Professors Susanne Grabley and Ralf Thiericke. He moved to the United States as a postdoctoral associate at the Department of Pharmacognosy, the University of Mississippi, with Professor Mark T. Hamann (2000–2001) and at the Genomics Institute of the Novartis Research Foundation, The Scripps Research Institute, with Professor Peter G. Schultz (2001–2002). He left his position as a Principal Scientist in Natural Products Chemistry from Sequoia Sciences, Inc. (San Diego, CA), where he worked from 2002 to 2006, and since has been a Professor at East China Normal University. From plants, microorganisms, and marine invertebrates, he has so far isolated and characterized more than 700 natural products, and over 200 of them are novel with broad bioactivities. He is currently engaged in the application of high-throughput natural products chemistry methods for parallel production and analysis of large natural products libraries for drug discovery and has undertaken the miniaturization of structure elucidation of mass-limited natural products as an initiative at Sequoia Sciences and the Key Laboratory of Brain Functional Genomics in Shanghai. During his research career, he has published over 50 scientific papers, reviews, book chapters, and patents. He is an invited reviewer for Journal of Natural Products and currently serves as a member of the Editorial Board of Chemistry-An Indian Journal. His group is focusing on natural products as small molecular probes for functional genomics studies that lead to discoveries of new diagnostic markers and neuropharmacological agents for treatment of obesity, age-related memory disorders, and CNS diseases.
References
- 1.Hu J-F, Hamann MT, Hill R, Kelly M. In: The Manzamine Alkaloids. The Alkaloids: Chemistry and Biology. Cordell GA, editor. Vol. 60. Elsevier Academic Press; Boston: 2003. p. 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng J-N, Li J, Hamann MT. In: The Marine Bromotyrosine Derivatives. The Alkaloids: Chemistry and Biology. Cordell GA, editor. Vol. 61. Elsevier Academic Press; Boston: 2005. p. 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen RJ, Faulkner DJ, He C-H, Van Duyne GD, Clardy J. J Am Chem Soc. 1985;107:5492. [Google Scholar]
- 4.Cantrell CL, Groweiss A, Gustafson KR, Boyd MR. Nat Prod Lett. 1999;14:39. [Google Scholar]
- 5.Lindquist N, Fenical W, Van Duyne GD, Clardy J. J Org Chem. 1988;53:4570. [Google Scholar]
- 6.Carroll AR, Bowden BF, Coll JC. Aust J Chem. 1993;46:489. [Google Scholar]
- 7.Urban S, Butler MS, Capon RJ. Aust J Chem. 1994;47:1919. [Google Scholar]
- 8.Urban S, Hobbs L, Hooper JNA, Capon RJ. Aust J Chem. 1995;48:1491. [Google Scholar]
- 9.Urban S, Capon RJ. Aust J Chem. 1996;49:711. [Google Scholar]
- 10.Reddy MVR, Faulkner DJ, Venkateswarlu Y, Rao MR. Tetrahedron. 1997;53:3457. [Google Scholar]
- 11.Davis RA, Carroll AR, Pierens GK, Quinn RJ. J Nat Prod. 1999;62:419. doi: 10.1021/np9803530. [DOI] [PubMed] [Google Scholar]
- 12.Reddy MVR, Rao MR, Rhodes D, Hansen MST, Rubins K, Bushman FD, Venkateswarlu Y, Faulkner DJ. J Med Chem. 1999;42:1901. doi: 10.1021/jm9806650. [DOI] [PubMed] [Google Scholar]
- 13.Ham J, Kang H. Bull Korean Chem Soc. 2002;23:163. [Google Scholar]
- 14.Krishnaiah P, Reddy VLN, Venkataramana G, Ravinder K, Srinivasulu M, Raju TV, Ravikumar K, Chandrasekar D, Ramakrishna S, Venkateswarlu Y. J Nat Prod. 2004;67:1168. doi: 10.1021/np030503t. [DOI] [PubMed] [Google Scholar]
- 15.Reddy SM, Srinivasulu M, Satyanarayana N, Kondapi AK, Venkateswarlu Y. Tetrahedron. 2005;61:9242. [Google Scholar]
- 16.Yoshida WY, Lee KK, Carroll AR, Scheuer PJ. Helv Chim Acta. 1992;75:1721. [Google Scholar]
- 17.Rudi A, Goldberg I, Stein Z, Frolow F, Benayahu Y, Schleyer M, Kashman Y. J Org Chem. 1994;59:999. [Google Scholar]
- 18.Rudi A, Evan T, Aknin M, Kashman Y. J Nat Prod. 2000;63:832. doi: 10.1021/np9905158. [DOI] [PubMed] [Google Scholar]
- 19.Palermo JA, Brasco MFR, Seldes AM. Tetrahedron. 1996;52:2727. [Google Scholar]
- 20.Vervoort HC, Richards-Gross SE, Fenical W, Lee AY, Clardy J. J Org Chem. 1997;62:1486. [Google Scholar]
- 21.Berlinck RGS, Britton R, Piers E, Lim L, Roberge M, Da Rocha RM, Andersen RJ. J Org Chem. 1998;63:9850. [Google Scholar]
- 22.Vervoort HC, Fenical W, Keifer PA. J Nat Prod. 1999;62:389. doi: 10.1021/np980409q. [DOI] [PubMed] [Google Scholar]
- 23.Kang H, Fenical W. J Org Chem. 1997;62:3254. doi: 10.1021/jo962132+. [DOI] [PubMed] [Google Scholar]
- 24.Chan GW, Francis T, Thureen DR, Offen PH, Pierce NJ, Westley JW, Johnson RK, Faulkner DJ. J Org Chem. 1993;58:2544. [Google Scholar]
- 25.Urban S, Hickford SJH, Blunt JW, Munro MHG. Curr Org Chem. 2000;4:765. [Google Scholar]
- 26.Bowden BF. Stud Nat Prod Chem. 2000;23:233. [Google Scholar]
- 27.Bailly C. Curr Med Chem-Anti-Cancer Agents. 2004;4:363. doi: 10.2174/1568011043352939. [DOI] [PubMed] [Google Scholar]
- 28.Cironi P, Albericio F, Alvarez M. Prog Heterocycl Chem. 2004;16:1. [Google Scholar]
- 29.Yang G, Wang AL, Chen HL, You YC. Chin J Org Chem. 2005;25:641. [Google Scholar]
- 30.Handy ST, Zhang YA. Org Prep Proced Int. 2005;37:411. [Google Scholar]
- 31.Ishibashi F, Miyazaki Y, Iwao M. Tetrahedron. 1997;53:5951. [Google Scholar]
- 32.Pla D, Marchal A, Olsen CA, Albericio F, Álvarez M. J Org Chem. 2005;70:8231. doi: 10.1021/jo051083a. [DOI] [PubMed] [Google Scholar]
- 33.Pla D, Marchal A, Olsen CA, Francesch A, Cuevas C, Albericio F, Álvarez M. J Med Chem. 2006;49:3257. doi: 10.1021/jm0602458. [DOI] [PubMed] [Google Scholar]
- 34.Fujikawa N, Ohta T, Yamaguchi T, Fukuda T, Ishibashi F, Iwao M. Tetrahedron. 2006;62:594. [Google Scholar]
- 35.You YC, Yang G, Wang AL, Li DP. Curr Appl Phys. 2005;5:535. [Google Scholar]
- 36.Díaz M, Guitián E, Castedo E. Synlett. 2001:1164. [Google Scholar]
- 37.Peschko C, Winklhofer C, Terpin A, Steglich W. Synthesis. 2006:3048. [Google Scholar]
- 38.Heim A, Terpin A, Steglich W. Angew Chem, Int Ed Engl. 1997;36:155. [Google Scholar]
- 39.Ruchirawat S, Mutarapat T. Tetrahedron Lett. 2001;42:1205. [Google Scholar]
- 40.Iwao M, Takeuchi T, Fujikawa N, Fukuda T, Ishibashi F. Tetrahedron Lett. 2003;44:4443. [Google Scholar]
- 41.Handy ST, Zhang YN, Bregman H. J Org Chem. 2004;69:2362. doi: 10.1021/jo0352833. [DOI] [PubMed] [Google Scholar]
- 42.Banwell MG, Flynn BL, Hockless DCR. Chem Commun. 1997:2259. [Google Scholar]
- 43.Ploypradith P, Mahidol C, Sahakitpichan P, Wongbundit S, Ruchirawat S. Angew Chem, Int Ed. 2004;43:866. doi: 10.1002/anie.200352043. [DOI] [PubMed] [Google Scholar]
- 44.Peschko C, Winklhofer C, Steglich W. Chem Eur J. 2000;6:1147. doi: 10.1002/(sici)1521-3765(20000403)6:7<1147::aid-chem1147>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- 45.Cironi P, Manzanares I, Albericio F, Álvarez M. Org Lett. 2003;5:2959. doi: 10.1021/ol0351192. [DOI] [PubMed] [Google Scholar]
- 46.Fernández D, Ahaidar A, Danelón G, Cironi P, Marfil M, Pérez O, Cuevas C, Albericio F, Joule JA, Álvarez M. Monatsh Chem. 2004;135:615. [Google Scholar]
- 47.Banwell MG, Flynn BL, Hamel E, Hockless DCR. Chem Commun. 1997:207. [Google Scholar]
- 48.Marfil M, Albericio F, Álvarez M. Tetrahedron. 2004;60:8659. [Google Scholar]
- 49.Banwell MG, Hamel E, Hockless DCR, Verdier-Pinard P, Willis AC, Wong DJ. Bioorg Med Chem. 2006;14:4627. doi: 10.1016/j.bmc.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 50.Ridley CP, Reddy MVR, Rocha G, Bushman FD, Faulkner DJ. Bioorg Med Chem. 2002;10:3285. doi: 10.1016/s0968-0896(02)00237-7. [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi T, Fukuda T, Ishibashi F, Iwao M. Tetrahedron Lett. 2006;47:3755. [Google Scholar]
- 52.Ploypradith P, Petchmance T, Sahakitpichan P, Litvinas ND, Ruchirawat S. J Org Chem. 2006;71:9440. doi: 10.1021/jo061810h. [DOI] [PubMed] [Google Scholar]
- 53.Fürstner A, Weintritt H, Hupperts A. J Org Chem. 1995;60:6637. [Google Scholar]
- 54.Gupton JT, Krumpe KE, Burnham BS, Webb TM, Shuford JS, Sikorski JA. Tetrahedron. 1999;55:14515. [Google Scholar]
- 55.Liu JH, Yang QC, Mak TCW, Wong HNC. J Org Chem. 2000;65:3587. doi: 10.1021/jo9915224. [DOI] [PubMed] [Google Scholar]
- 56.Hinze C, Kreipl A, Terpin A, Steglich W. Synthesis. 2007:608. [Google Scholar]
- 57.Terpin A, Polborn K, Steglich W. Tetrahedron. 1995;51:9941. [Google Scholar]
- 58.Burtoloso ACB, Garcia ALL, Miranda KC, Correia CRD. Synlett. 2006:3145. [Google Scholar]
- 59.Winklhofer C, Terpin A, Peschko C, Steglich W. Synthesis. 2006:3043. [Google Scholar]
- 60.Beccalli EM, Clerici F, Marchesini A. Tetrahedron. 2000;56:2699. [Google Scholar]
- 61.Kreipl AT, Reid C, Steglich W. Org Lett. 2002;4:3287. doi: 10.1021/ol026555b. [DOI] [PubMed] [Google Scholar]
- 62.Gupton JT, Miller RB, Krumpe KE, Clough SC, Banner EJ, Kanters RPF, Du KX, Keertikar KM, Lauerman NE, Solano JM, Adams BR, Callahan DW, Little BA, Scharf AB, Sikorski JA. Tetrahedron. 2005;61:1845. [Google Scholar]
- 63.Ebel H, Terpin A, Steglich W. Tetrahedron Lett. 1998;39:9165. [Google Scholar]
- 64.Fürstner A, Krause H, Thiel OR. Tetrahedron. 2002;58:6373. [Google Scholar]
- 65.Hughes TV, Cava MP. Tetrahedron Lett. 1998;39:9629. [Google Scholar]
- 66.Terpin A, Winklhofer C, Schumann S, Steglich W. Tetrahedron. 1998;54:1745. [Google Scholar]
- 67.Piers E, Britton R, Andersen RJ. J Org Chem. 2000;65:530. doi: 10.1021/jo9914723. [DOI] [PubMed] [Google Scholar]
- 68.Boger DL, Boyce CW, Labroli MA, Sehon CA, Jin Q. J Am Chem Soc. 1999;121:54. [Google Scholar]
- 69.Boger DL, Soenen DR, Boyce CW, Hedrick MP, Jin Q. J Org Chem. 2000;65:2479. doi: 10.1021/jo9916535. [DOI] [PubMed] [Google Scholar]
- 70.Boger DL. 2001064635. WO. 2001; PCT Int Appl. 2001:38. [Google Scholar]
- 71.Bullington JL, Wolff RR, Jackson PF. J Org Chem. 2002;67:9439. doi: 10.1021/jo026445i. [DOI] [PubMed] [Google Scholar]
- 72.Gupton JT, Clough SC, Miller RB, Lukens JR, Henry CA, Kanters RPF, Sikorski JA. Tetrahedron. 2003;59:207. [Google Scholar]
- 73.Peschko C, Steglich W. Tetrahedron Lett. 2000;41:9477. [Google Scholar]
- 74.Namsa-aid A, Ruchirawat S. Org Lett. 2002;4:2633. doi: 10.1021/ol026074s. [DOI] [PubMed] [Google Scholar]
- 75.Hamasaki A, Zimpleman JM, Hwang I, Boger DL. J Am Chem Soc. 2005;127:10767. doi: 10.1021/ja0526416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burnham BS, Gupton JT, Krumpe K, Webb T, Shuford J, Bowers B, Warren AE, Barnes C, Hall IH. Arch Pharm Pharm Med Chem. 1998;331:337. doi: 10.1002/(sici)1521-4184(199811)331:11<337::aid-ardp337>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 77.Gupton JT, Burham BS, Byrd BD, Krumpe KE, Stokes C, Shuford J, Winkle S, Webb T, Warren AE, Barnes CR, Henry J, Hall IH. Pharmazie. 1999;54:691. [PubMed] [Google Scholar]
- 78.Vanhuyse M, Kluza J, Tardy C, Otero G, Cuevas C, Bailly C, Lansiaux A. Clin Cancer Res. 2003;9(Suppl):6109S. [Google Scholar]
- 79.Quesada AR, Grávalos MDG, Puentes JLF. Br J Cancer. 1996;74:677. doi: 10.1038/bjc.1996.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bailly C, Francesch SA, Mateo U, Maria C, Jimenez GJA, Pastor DCA, Cuevas MC. 2004014917. WO. 2004; PCT Int Appl. 2004:222. [Google Scholar]
- 81.Soenen DR, Hwang I, Hedrick MP, Boger DL. Bioorg Med Chem Lett. 2003;13:1777. doi: 10.1016/s0960-894x(03)00294-4. [DOI] [PubMed] [Google Scholar]
- 82.Tao HC, Hwang I, Boger DL. Bioorg Med Chem Lett. 2004;14:5979. doi: 10.1016/j.bmcl.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 83.Chou T-C, Guan YB, Soenen DR, Danishefsky SJ, Boger DL. Cancer Chemother Pharmacol. 2005;56:379. doi: 10.1007/s00280-005-1019-y. [DOI] [PubMed] [Google Scholar]
- 84.Loya S, Rudi A, Kashman Y, Hizi A. Biochem J. 1999;344:85. [PMC free article] [PubMed] [Google Scholar]
- 85.Hwang Y, Rhodes D, Bushman F. Nucleic Acids Res. 2000;28:4884. doi: 10.1093/nar/28.24.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Manzanaro S, Salvá J, De la Fuente JA. J Nat Prod. 2006;69:1485. doi: 10.1021/np0503698. [DOI] [PubMed] [Google Scholar]
- 87.Chou T-C. 2006026496. WO. 2006; PCT Int Appl. 2006:52. [Google Scholar]
- 88.Köck M, Reif B, Fenical W, Griesinger C. Tetrahedron Lett. 1996;37:363. [Google Scholar]
- 89.Kashman Y, Koren-Goldshlager G, Gravalos MDG, Schleyer M. Tetrahedron Lett. 1999;40:997. [Google Scholar]
- 90.Facompré M, Tardy C, Bal-Mahieu C, Colson P, Perez C, Manzanares I, Cuevas C, Bailly C. Cancer Res. 2003;63:7392. [PubMed] [Google Scholar]
- 91.Vanhuyse M, Kluza J, Tardy C, Otero G, Cuevas C, Bailly C, Lansiaux A. Cancer Lett. 2005;221:165. doi: 10.1016/j.canlet.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 92.Kluza J, Gallego M-A, Loyens A, Beauvillain J-C, Sousa-Farco J-MF, Cuevas C, Marchetti P, Bailly C. Cancer Res. 2006;66:3177. doi: 10.1158/0008-5472.CAN-05-1929. [DOI] [PubMed] [Google Scholar]
- 93.Fernandez Puentes JL, Garcia, Gravalos MD, Quesada A. 9701336. PCT Int Appl WO. 1996; Chem Abstr. 1996;126:166474. [Google Scholar]
- 94.Roberge M, Berlinck RGS, Xu L, Anderson HJ, Lim LY, Curman D, Stringer CM, Friend SH, Davies P, Vincent I, Haggarty SJ, Kelly MT, Britton R, Piers E, Andersen RJ. Cancer Res. 1998;58:5701. [PubMed] [Google Scholar]
- 95.Camargo AJ, Oliveira JHHL, Trsic M, Berlinck RGS. J Mol Struct. 2001;559:67. [Google Scholar]
- 96.Puentes JLF, Gravalos DG, Quesada AR. 9701336. WO. 1997; PCT Int Appl. 1997:24. [Google Scholar]
- 97.Puentes JLF, Gravalos DG, Quesada AR. 5,852,033. US Patent. 1995
- 98.Banwell MG, Flynn BL. 9850365. WO. 1998; PCT Int Appl. 1998:75. [Google Scholar]
- 99.Hill RA, Pitt AR. Nat Prod Rep. 1997;14:iii. [PubMed] [Google Scholar]
- 100.Vervoort HC, Pawlik JR, Fenical W. Mar Ecol: Prog Ser. 1998;164:221. [Google Scholar]
- 101.Tardy C, Facompré M, Laine W, Baldeyrou B, García-Gravalos D, Francesch A, Mateo C, Pastor A, Jiménez JA, Manzanares I, Cuevas C, Bailly C. Bioorg Med Chem. 2004;12:1697. doi: 10.1016/j.bmc.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 102.Dias N, Vezin H, Lansiaux A, Bailly C. Top Curr Chem. 2005;253:89. [Google Scholar]
- 103.Ishibashi F, Tanabe S, Oda T, Iwao M. J Nat Prod. 2002;65:500. doi: 10.1021/np0104525. [DOI] [PubMed] [Google Scholar]
- 104.Marco E, Laine W, Tardy C, Lansiaux A, Iwao M, Ishibashi F, Bailly C, Gago F. J Med Chem. 2005;48:3796. doi: 10.1021/jm049060w. [DOI] [PubMed] [Google Scholar]
- 105.Ploypradith P, Jinaglueng W, Pavaro C, Ruchirawat S. Tetrahedron Lett. 2003;44:1363. [Google Scholar]
- 106.Banwell MG, Flynn BL, Hockless DCR, Longmore RW, Rae AD. Aust J Chem. 1998;52:755. [Google Scholar]
- 107.Banwell MG, Flynn BL, Stewart SG. J Org Chem. 1998;63:9139. [Google Scholar]
- 108.Soenen DR, Zimpleman JM, Boger DL. J Org Chem. 2003;68:3593. doi: 10.1021/jo020713v. [DOI] [PubMed] [Google Scholar]
- 109.Cironi P, Cuevas C, Albericio F, Álvarez M. Tetrahedron. 2004;60:8669. [Google Scholar]
- 110.Ploypradith P, Kagan RK, Ruchirawat S. J Org Chem. 2005;70:5119. doi: 10.1021/jo050388m. [DOI] [PubMed] [Google Scholar]
- 111.Marfil M, Albericio F, Álvarez M. Lett Org Chem. 2005;2:371. [Google Scholar]
- 112.Petchmanee T, Ploypradith P, Ruchirawat S. J Org Chem. 2006;71:2892. doi: 10.1021/jo052337v. [DOI] [PubMed] [Google Scholar]
- 113.Alvarez M, Albericio F, Cironi P, Cuevas C, Francesch A, Marfil M. 2004073598. WO. 2004; PCT Int Appl. 2004:89. [Google Scholar]
- 114.You Y-C, Wang A-L, Li D-P, Yang G. Biomed Mater. 2006;1:L7. doi: 10.1088/1748-6041/1/3/L01. [DOI] [PubMed] [Google Scholar]
- 115.Gupton JT, Burham BS, Krumpe K, Du K, Sikorski JA, Warren AE, Barnes CR, Hall IH. Arch Pharm Pharm Med Chem. 2000;333:3. doi: 10.1002/(sici)1521-4184(200001)333:1<3::aid-ardp3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 116.Kel’in AV, Sromek AW, Gevorgyan V. J Am Chem Soc. 2001;123:2074. doi: 10.1021/ja0058684. [DOI] [PubMed] [Google Scholar]
- 117.Kim S, Son S, Kang H. Bull Korean Chem Soc. 2001;22:1403. [Google Scholar]
- 118.Ghosez L, Franc C, Denonne F, Cuisinier C, Touillaux R. Can J Chem. 2001;79:1827. [Google Scholar]
- 119.Paulus O, Alcaraz G, Vaultier M. Eur J Org Chem. 2002:2565. [Google Scholar]
- 120.Fujita R, Watanabe N, Tomisawa H. Chem Pharm Bull. 2002;50:225. doi: 10.1248/cpb.50.225. [DOI] [PubMed] [Google Scholar]
- 121.Palacios F, Herran E, Rubiales G. Heterocycles. 2002;58:89. [Google Scholar]
- 122.Zhu SZ, Liu XY, Wang SW. Tetrahedron. 2003;59:9669. [Google Scholar]
- 123.Handy ST, Bregman H, Lewis J, Zhang XL, Zhang YN. Tetrahedron Lett. 2003;44:427. [Google Scholar]
- 124.Shibata I, Kato H, Kanazawa N, Yasuda M, Baba A. Synlett. 2004:137. doi: 10.1021/ja038712n. [DOI] [PubMed] [Google Scholar]
- 125.Holub JM, O’Toole-Colin K, Getzel A, Argenti A, Evans MA, Smith DC, Dalglish GA, Rifat S, Wilson DL, Taylor BM, Miott U, Glersaye J, Lam KS, McCranor BJ, Berkowitz JD, Miller RB, Lukens JR, Krumpe K, Gupton JT, Burnham BS. Molecules. 2004;9:135. doi: 10.3390/90300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Balme G. Angew Chem, Int Ed. 2004;43:6238. doi: 10.1002/anie.200461073. [DOI] [PubMed] [Google Scholar]
- 127.Shaabani A, Teimouri MB, Arab-Ameri S. Tetrahedron Lett. 2004;45:8409. [Google Scholar]
- 128.Rao HSP, Jothilingam S, Scheeren HW. Tetrahedron. 2004;60:1625. [Google Scholar]
- 129.Baldoli C, Cremonesi G, Croce PD, La Rosa C, Licandro E. Heterocycles. 2004;64:491. [Google Scholar]
- 130.Azizian J, Karimi AR, Kazemizadeh Z, Mohammadi AA, Mohammadizadeh MR. Synthesis. 2005:1095. doi: 10.1021/jo0486692. [DOI] [PubMed] [Google Scholar]
- 131.Azizian J, Karimi AR, Kazemizadeh Z, Mohammadi AA, Mohammadizadeh MR. J Org Chem. 2005;70:1471. doi: 10.1021/jo0486692. [DOI] [PubMed] [Google Scholar]
- 132.Naud S. Synlett. 2004:2836. [Google Scholar]
- 133.Mathew P, Asokan CV. Tetrahedron Lett. 2005;46:475. [Google Scholar]
- 134.Olsen CA, Parera N, Albericio F, Álvarez M. Tetrahedron Lett. 2005;46:2041. [Google Scholar]
- 135.Nyerges M, Töke L. Tetrahedron Lett. 2005;46:7531. [Google Scholar]
- 136.Blaszykowski C, Aktoudianakis E, Bressy C, Alberico D, Lautens M. Org Lett. 2006;8:2043. doi: 10.1021/ol060447y. [DOI] [PubMed] [Google Scholar]
- 137.Handy ST, Sabatini JJ. Org Lett. 2006;8:1537. doi: 10.1021/ol0530981. [DOI] [PubMed] [Google Scholar]
- 138.Smith JA, Ng S, White J. Org Biomol Chem. 2006;4:2477. doi: 10.1039/b604692d. [DOI] [PubMed] [Google Scholar]
- 139.Banwell MG, Flynn BL. 9967250. WO. 1999; PCT Int Appl. 1999:57. [Google Scholar]