Abstract
During 2001–2002, research on the pharmacology of marine chemicals continued to be global in nature involving investigators from Argentina, Australia, Brazil, Canada, China, Denmark, France, Germany, India, Indonesia, Israel, Italy, Japan, Mexico, Netherlands, New Zealand, Pakistan, the Philippines, Russia, Singapore, Slovenia, South Africa, South Korea, Spain, Sweden, Switzerland, Thailand, United Kingdom, and the United States. This current article, a sequel to the authors’ 1998, 1999 and 2000 marine pharmacology reviews, classifies 106 marine chemicals derived from a diverse group of marine animals, algae, fungi and bacteria, on the basis of peer-reviewed preclinical pharmacology. Anthelmintic, antibacterial, anticoagulant, antifungal, antimalarial, antiplatelet, antiprotozoal, antituberculosis or antiviral activities were reported for 56 marine chemicals. An additional 19 marine compounds were shown to have significant effects on the cardiovascular, immune and nervous system as well as to possess anti-inflammatory and antidiabetic effects. Finally, 31 marine compounds were reported to act on a variety of molecular targets and thus may potentially contribute to several pharmacological classes. Thus, during 2001–2002 pharmacological research with marine chemicals continued to contribute potentially novel chemical leads for the ongoing global search for therapeutic agents for the treatment of multiple disease categories.
Keywords: Chemical, Marine, Metabolites, Natural, Pharmacology, Products, Review, Toxicology
1. Introduction
The purpose of this article is to review the 2001–2002 primary literature on pharmacological and toxicological studies with marine natural products using a similar format to the one used in our previous reviews of the marine pharmacology peer-reviewed literature (Mayer and Lehmann, 2000; Mayer and Hamann, 2002, 2004). Consistent with our previous reviews, only those articles reporting on the bioactivity and/or pharmacology of 106 marine chemicals whose structures have been published are included in the present review. We have used the same chemical classification as our previous reviews (Schmitz et al., 1993) to assign each marine compound to a major chemical class, namely, polyketides, terpenes, nitrogen-containing compounds or polysaccharides. Those publications reporting on anthelmintic, antibacterial, anticoagulant, antifungal, antimalarial, antiplatelet, antiprotozoal, antituberculosis or antiviral properties of 56 marine chemicals have been tabulated in Table 1 with the corresponding structures shown in Fig. 1. The articles reporting on 19 marine compounds affecting the cardiovascular, immune and nervous systems, as well as those with anti-inflammatory and antidiabetic effects, are grouped in Table 2 and the structures presented in Fig. 2. Finally 31 marine compounds targeting a number of distinct cellular and molecular targets and mechanisms are shown in Table 3 and their structures depicted in Fig. 3. Publications on the biological and/or pharmacological activity of marine extracts or as yet structurally uncharacterized marine compounds have been excluded from the present review, though several promising reports were published during 2001–2002 (Duarte et al., 2001; Ermakova et al., 2001; Kaji et al., 2002; Liu et al., 2002b; Matou et al., 2002; Mohapatra et al., 2002; Preeprame et al., 2001; Suput et al., 2001; Trento et al., 2001).
Table 1.
Marine pharmacology in 2001–2002: marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities
| Drug class | Compound/organisma | Chemistry | Pharmacologic activity | MMOAb | Countryc | References |
|---|---|---|---|---|---|---|
| Anthelmintic | Nafuredin (1)/fungus | Polyketided | Inhibition of helminth NADH-fumarate reductase | Competes for the quinone-binding site in complex 1 | GER, JAPN | Omura et al., 2001 |
| Onnamide F (2)/sponge | Polyketided | H. contortus inhibition | Undetermined | AUS | Vuong et al., 2001 | |
| Antibacterial | Arenosclerins A, B, C and haliclonacyclamine E (3, 4, 5, and 6)/sponge | Alkaloidse | S. aureus inhibition | Bis-piperidine ring stereochemistry related | BRA | Torres et al., 2002 |
| Caminoside A (7)/sponge | Lipopolysaccharidef | Antibiotic-resistant S. aureus and enterococci | Inhibition of bacterial type III secretory system | CAN, NETH | Linington et al., 2002 | |
| Bogorol A (8)/bacterium | Peptidee | Antibiotic-resistant S. aureus and enterococci | Undetermined | CAN | Barsby et al., 2001 | |
| Chalcomycin B (9)/bacterium | Macrolided | S. aureus inhibition | Undetermined | GER | Asolkar et al., 2002 | |
| Dicynthaurin (10)/tunicate | Peptidee | Gram-negative and Gram-positive inhibition | Undetermined | S. KOR, USA | Lee et al., 2001 | |
| Halocidin (11)/tunicate | Peptidee | Antibiotic resistant S. aureus and MDR-resistant P. Aeruginosa | Undetermined | S. KOR | Jang et al., 2002 | |
| Iyengaroside-A (12)/alga | Steroidal glycosideg | Gram-negative and Gram-positive inhibition | Undetermined | CAN, PAK | Ali et al., 2002 | |
| Lembyne A (13)/alga | Halogenated acetogenins | Inhibition of marine bacteria | Undetermined | JAPN | Vairappan et al., 2001 | |
| Pannosanol and pannosane (14 and 15)/alga | Sesquiterpeneg | Inhibition of marine bacteria | Undetermined | JAPN | Suzuki et al., 2001 | |
| Pestalone (16)/fungus | Halogenated benzophenone | Antibiotic-resistant S. aureus and enterococci inhibition | Undetermined | USA | Cueto et al., 2001 | |
| Sumiki’s acid (17)/fungus | Macrolided | B. subtilis and S. aureus inhibition | Undetermined | GER | Jadulco et al., 2001 | |
| Zamamistatin (18)/sponge | Bromotyrosinee | Rhodospirillum salexigens inhibition | Undetermined | JAPN | Takada et al., 2001 | |
| Zopfiellamides A and B (19 and 20)/fungus | Polyketided | Gram-negative and Gram-positive inhibition | Undetermined | GER, SWE | Daferner et al., 2002 | |
| Anticoagulant | Galactan and fucans (21 and 22)/sea urchin | Sulfated galactans and fucansf | Coagulation inhibition | Enhancement of thrombin or factor Xa inhibition | BRA | Pereira et al., 2002 |
| Antifungal | Basiliskamides A and B (23 and 24)/bacterium | Polyketided | C. albicans and A. fumigatus inhibition | Undetermined | CAN | Barsby et al., 2002 |
| Corticatic acids A and E (25 and 26)/sponge | Polyacetylenic acid | C. albicans and A. fumigatus inhibition | Selective GGTase I inhibition | JAPN | Nishimura et al., 2002 | |
| Swinhoeiamide A (27)/sponge | Polyketided | C. albicans and A. fumigatus inhibition | Undetermined | GER, INDO, AUS, NETH | Edrada et al., 2002a | |
| Patagonicoside A (28)/sea cucumber | Triterpene glycosideg | Cladosporium cucumerinum inhibition | Sulfate groups in oligosaccharide related to activity | ARG | Murray et al., 2001 | |
| Oxybis methyl phenol (29)/fungus | Polyketided | C. albican, T. rubrum and A. niger inhibition | Undetermined | CHI | Liu et al., 2002a | |
| Polyester 15G256β (30)/fungus | Macrolided | Cell wall biosynthesis inhibition | Undetermined | USA | Schlingmann et al., 2002 | |
| Xestodecalactones B (31)/fungus | Macrolided | C. albicans inhibition | Undetermined | GER, INDO | Edrada et al., 2002b | |
| Antimalarial | Aigialomycin D (32)/fungus | Macrolided | P. falciparum inhibition | Undetermined | THAI | Isaka et al., 2002 |
| Halorosellinic acid (33)/fungus | Sesterterpeneg | P. falciparum inhibition | Undetermined | THAI | Chinworrungsee et al., 2001 | |
| Heptyl prodigiosin (34)/bacterium | Pyrrole alkaloide | P. falciparum and P. berghei inhibition in vitro and in vivo | Undetermined | FRA, PHIL | Lazaro et al., 2002 | |
| ent-8-hydroxymanzamine A, manzamine F, and neo-kauluamine (35, 36, and 37)/sponge | Alkaloide | P. berghei inhibition in vivo | Undetermined | N. ZEL, SING, USA | El Sayed et al., 2001 | |
| (S)-(+)-15-hydroxycurcuphenol (38)/sponge | Sesquiterpenee | P. falciparum inhibition | Undetermined | N. ZEL, USA | El Sayed et al., 2002 | |
| Jasplakinolide (39)/sponge | Cyclic peptidee | P. falciparum inhibition | Apical protrusion in merozoites, F-actin increase | JAPN | Mizuno et al., 2002 | |
| Lepadins E-F (40 and 41)/tunicate | Alkaloide | P. falciparum inhibition | Tyrosine kinase p56lck inhibition | GER, SWI | Wright et al., 2002 | |
| Plakortide F (42)/sponge | Polyketided | P. falciparum inhibition | Undetermined | USA | Gochfeld and Hamann, 2001 | |
| Antiprotozoal | Plakortolide G (43)/sponge | Polyketided | Toxomplasma gondii | Undetermined | N. ZEL, USA | Perry et al., 2001 |
| Antituberculosis | Cyanthiwigin C (44)/sponge | Diterpeneg | M. tuberculosis inhibition | Undetermined | N. ZEL, USA | Peng et al., 2002 |
| Erogorgiaene and 7-hydroxyerogorgiaene (45 and 46)/sea whip | Diterpeneg | M. tuberculosis inhibition | Undetermined | USA | Rodriguez and Ramirez, 2001 | |
| Antiplatelet | Alkylpyridinium (47)/sponge | Pyridinee | In vivo and in vitro platelet aggregation | Undetermined | SLO | Bunc et al., 2002 |
| Antiviral | Clathsterol (48)/sponge | Sulfated sterolg | HIV reverse transcriptase inhibition | Undetermined | ISRA, S. AFR | Rudi et al., 2001 |
| Microspinosamide (49)/sponge | Depsipeptidee | HIV-growth inhibition | Undetermined | USA | Rashid et al., 2001 | |
| Polyacetylenetriol (50)/sponge | Fatty acidd | RNA- and DNA-directed DNA polymerase inhibition | Reversible non-competitive inhibition, with hydrophobic interactions | ISRA | Loya et al., 2002 | |
| Thalassiolins A–C (51, 52, and 53)/sea grass | Sulfated flavonesd,f | HIV-1 integrase inhibition and HIV growth in vitro | Binding to catalytic domain of HIV-1 integrase | USA | Rowley et al., 2002 | |
| Calyceramides A–C (54, 55, and 56)/sponge | Fatty acidd | Neuraminidase inhibition | Undetermined | JAPN | Nakao et al., 2001 |
Organism, Kingdom Animalia: sea urchin and cucumber (Phylum Echinodermata), sponge (Phylum Porifera), tunicate (Phylum Chordata), and sea whips (Phylum Cnidaria); Kingdom Fungi: fungus; Kingdom Plantae: alga and sea grass; and Kingdom Monera: bacterium (Phylum Cyanobacteria).
MMOA: molecular mechanism of action.
Country: ARG: Argentina; AUS: Australia; BRA: Brazil; CAN: Canada; CHI: China; FRA: France; GER: Germany; INDO: Indonesia; ISRA: Israel; ITA: Italy; JAPN: Japan; NETH: The Netherlands; N. ZEL: New Zealand; PAK: Pakistan; PHIL: The Philippines; SING: Singapore; S. AFR.: South Africa; S. KOR: South Korea; SLO: Slovenia; SWE: Sweden; SWZ: Switzerland; and THAI: Thailand.
Polyketides.
Nitrogen-containing compound.
Polysaccharide.
Terpene.
Fig. 1.
Marine pharmacology in 2001–2002: marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities.
Table 2.
Marine pharmacology in 2001–2002: marine compounds with anti-inflammatory and antidiabetic activities and affecting the cardiovascular, immune and nervous systems
| Drug class | Compound/organisma | Chemistry | Pharmacological activity | MMOAb | Countryc | References |
|---|---|---|---|---|---|---|
| Anti-inflammatory | Halipeptins A and B (57 and 58)/sponge | Depsipeptidesd | Inhibition of carrageenan-induced edema | Undetermined | ITA, FRA | Randazzo et al., 2001 |
| Hymenamide C (59)/sponge | Cyclopeptided | Neutrophil and macrophage mediator modulation | Elastase, PGE2 and NO inhibition | ITA, SPA | Napolitano et al., 2001 | |
| Petrosaspongiolide (60)/sponge | Sesterterpenee | Phospholipase A2 inhibition | Hydroxybutenolide required for PLA2 inhibition | ITA | Dal Piaz et al., 2002 | |
| Scytonemin (61)/bacterium | Amino acidd | Inhibition of PMA-induced mouse ear edema | Inhibition of polo-like kinase 1 and PKCβ1 | USA | Stevenson et al., 2002b,a | |
| Antidiabetic | Insulin (62)/shark | Peptided | Glucose metabolism in sharks | High affinity binding to human insulin receptor | UK, USA, SWE, DEN | Anderson et al., 2002 |
| Cardiovascular | Gramine analogue (TBG) (63)/bryozoa | Alkaloidd | Vasorelaxation of isolated rat aorta | Ca2+ inhibition and increase cyclic AMP | JAPN | Iwata et al., 2001 |
| Lepadiformine (64)/tunicate | Alkaloidd | Inhibition of cardiocirculatory system in vivo and in vitro | Reduction of inward K+ current | FRA | Juge et al., 2001 | |
| Immune system | Domoic acid (65)/diatom | Amino acidd | Limited TNF-α and matrix metalloproteinase-9 release from brain microglia | Undetermined | USA | Mayer et al., 2001 |
| Nervous system | Antillatoxin B (66)/bacterium | Lipopeptidee | Activator of voltage sensitive-sodium channel | Undetermined | USA | Nogle et al., 2001 |
| Dysiherbaine (67)/sponge | Amino acidd | Induction of convulsant action in mice | Inhibition of kainic acid and mGluR5 glutamate receptors | JAPN, USA | Sakai et al., 2001b | |
| N-3′-ethylaplysinopsin (68)/sponge | Alkaloidd | Undetermined | Binding to human serotonin 5-HT2C receptor | N. ZEL, SING, USA | Hu et al., 2002 | |
| Gangliosides HLG-1, HLG-2, HLG-3 (69–71)/sea cucumber | Glycosphingolipidf | In vitro neuritogenic assay | Undetermined | JAPN | Yamada et al., 2001 | |
| Manoalide (72)/sponge | Sesterterpenee | Inhibition of seizures and epileptogenic properties of crotoxin | Dissociation of crotoxin complex | FRA | Dorandeu et al., 2002 | |
| Neodysiherbaine A (73)/sponge | Amino acidd | Induction of convulsant action in mice | Inhibition of kainic acid glutamate receptors | JAPN | Sakai et al., 2001a | |
| Conantokin-G (74)/snail | Peptided | In vitro NMDA receptor-transfected, oocyte electrophysiology | Interaction with NMDA glutamate- binding pocket | GER, USA | Wittekindt et al., 2001 | |
| Conantokin-L (75)/snail | Peptided | Anticonvulsant in mouse epilepsy model. Neuroprotective. | NMDA receptor antagonist | PHIL, USA | Jimenez et al., 2002 |
Organism, Kingdom Animalia: bryozoa (Phylum Ectoprocta), sea anemones (Phylum Cnidaria), shark and tunicate (Phylum Chordata), sea cucumber (Phylum Echinodermata), snail (Phylum Mollusca), and sponge (Phylum Porifera); Kingdom Plantae: dinoflagellate and alga; and Kingdom Monera: bacterium (Phylum Cyanobacteria).
MMOA: molecular mechanism of action.
Country: DEN: Denmark; FRA: France; GER: Germany; ITA: Italy; JAPN: Japan; N. ZEL: New Zealand; PHIL: Philippines; SING: Singapore; SLO: Slovenia; SPA: Spain; SWE: Sweden; and UK: United Kingdom.
Nitrogen-containing compounds.
Terpenes.
Polyketides.
Fig. 2.
Marine pharmacology in 2001–2002: marine compounds with anti-inflammatory and antidiabetic activities and affecting the cardiovascular, immune and nervous systems.
Table 3.
Marine pharmacology in 2001 – 2002: marine compounds with miscellaneous mechanisms of action
| Compound/organisma | Chemistry | Pharmacological activity | MMOAb | Countryc | References |
|---|---|---|---|---|---|
| Aeroplysinin-1 (76)/sponge | Amino acid derivedd | Antiangiogenic | Undetermined | SPA | Rodriguez-Nieto et al., 2002 |
| Antillatoxin (77)/bacterium | Lipopeptided | Voltage-dependent Na+ channel activation | Sodium channel α subunit binding | JAPN, USA | Li et al., 2001 |
| Aplysiallene (78)/sea hare | Polyketidee | Na+, K+ – ATPase inhibition | Undetermined | JAPN | Okamoto et al., 2001a |
| Azaspiracid-1 (79)/alga | Polyketidee | Toxicity to lymphocytes and neuroblastoma cells | Decrease in F-actin pools and increased [Ca2+]i | SPA, JAPN | Roman et al., 2002 |
| Bistratene A (80)/ascidian | Polyketidee | Induction of cell-cycle arrest in Go/G1 and G2/M | Protein kinase C δ activation | AUS, USA | Frey et al., 2001 |
| Bryoanthrathiophene (81)/bryozoa | Polyketidee | Angiogenesis inhibition | Undetermined | JAPN | Jeong et al., 2002 |
| Bryostatin-1 (82)/bryozoa | Polyketidee | IgE synthesis inhibition | Iε germline transcription modulation | USA | Rabah et al., 2001 |
| Coscinosulfate (83)/sponge | Sesquiterpenef | Cell cycle regulation | Dual specificity phosphatase CDC25 inhibition | FRA | Loukaci et al., 2001 |
| Debromohymenialdisine (84)/sponge | Alkaloidd | G2 checkpoint inhibition | Protein kinase Chk1 and Chk2 inhibition | CAN, UK, USA | Curman et al., 2001 |
| Chlorogentisylquinone (85)/fungus | Polyketidee | Neutral sphingomyelinase inhibition | Undetermined | JAPN | Uchida et al., 2001 |
| Discodermin A (86)/sponge | Peptided | Permeabilization of plasma membrane | Undetermined | JAPN | Sato et al., 2001 |
| Farnesylhydroquinone (87)/fungus | Terpenef | Radical scavenging | Undetermined | S. KOR | Son et al., 2002 |
| Halenaquinol (88)/sponge | Polyketide | Na+, K+ – ATPase inhibition | Oxidation of sulfhydryl groups | RUS | Gorshkova et al., 2001 |
| Halenaquinone (89)/sponge | Polyketidee | Induction of apoptosis | Inhibition of phosphatidyl inositol 3-kinase | JAPN | Fujiwara et al., 2001 |
| Hectochlorin (90)/bacterium | Peptided | Inhibition of cell growth | Induction of actin polymerization | USA | Marquez et al., 2002 |
| Iantherans A and B (91 and 92)/sponge | Peptided | Na+, K+ – ATPase and plasmin inhibition | Undetermined | JAPN | Okamoto et al., 2001b |
| Jaspaquinol (93)/sponge | Terpenef | Human 15-lipoxygenase inhibition | Undetermined | USA | Carroll et al., 2001 |
| Jasplankinolide (39)/sponge | Peptide/Polyketidee | Increased outflow facility in monkey eye | Undetermined | USA | Tian et al., 2001 |
| Linckosides A and B (94 and 95)/starfish | Sterol glycosidef | Induction of neuritogenesis | Undetermined | JAPN | Qi et al., 2002 |
| Maitotoxin (96)/alga | Complex polyketidee | Modulation of calcium and sodium influx | Undetermined | MEX | Morales-Tlalpan and Vaca, 2002 |
| Complex polyketidee | Regulation of excocytosis in Xenopus laevis oocytes | Activation of cation conductance | GER, RUS, UK, FRA | Diakov et al., 2001 | |
| Micropeptins (97)/bacterium | Depsipeptidesd | Inhibition of trypsin and chymotrypsin | Undetermined | ISRA | Reshef and Carmeli, 2001 |
| Pectenotoxin-6 (98)/alga | Macrolidee | Disruption of F-actin cytoskeletal | Induction of F-actin depolymerization | SPA, JAPN, ITA | Leira et al., 2002 |
| Sculezonone-A and B (99 and 100)/fungus | Polyketidee | Inhibition of DNA polymerase α, β and γ | Differential electrostatic charges elicit different inhibition spectra | JAPN | Perpelescu et al., 2002 |
| Scytonemin (61)/bacterium | Amino acidd | Inhibition of active cell proliferation | Inhibition of polo-like and cell-cycle kinases | USA | Stevenson et al., 2002a |
| Stolonoxides (101)/tunicate | Fatty acide | Mitochondrial respiratory chain inhibition | Effect on mitochondrial complex II and III | ITA, SPA | Fontana et al., 2001 |
| Swinholide A (102)/sponge | Complex Polyketidee | Increased outflow facility in monkey eye | Undetermined | USA | Tian et al., 2001 |
| Wondonins A and B (103 and 104)/sponge | Alkaloidd | Modulation of angiogenesis in vitro | Undetermined | S. KOR | Shin et al., 2001 |
| Xetospongin-C (105)/sponge | Alkaloidd | Inhibition of smooth muscle contraction | Voltage-dependent K+ and L-type Ca2+ channel inhibition | JAPN | Ozaki et al., 2002 |
| Yessotoxin (106)/alga | Polyketidee | Lymphocyte [Ca2+]i homeostasis modulation | Inhibition of calcium channels | SPA | De la Rosa et al., 2001 |
Organism, Kingdom Animalia: ascidians and tunicates (Phylum Chordata), anemones, corals and hydroids (Phylum Cnidaria), sea cucumber and starfish (Phylum Echinodermata), bryozoa (Phylum Ectoprocta), sea hares (Phylum Mollusca), and sponge (Phylum Porifera); Kingdom Fungi: fungus; Kingdom Plantae: alga; and Kingdom Monera: bacterium (Phylum Cyanobacteria).
MMOA: molecular mechanism of action.
Country: AUS: Australia; CAN: Canada; CHI: China; FRA: France; GER: Germany; ISRA: Israel; ITA: Italy; JAPN: Japan; MEX: Mexico; RUS: Russia; S. KOR: South Korea; SPA: Spain; and UK: United Kingdom.
Nitrogen-containing compounds.
Polyketides.
Terpenes.
Polysaccharides.
Fig. 3.
Marine pharmacology in 2001–2002: marine compounds with miscellaneous mechanisms of action.
2. Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities
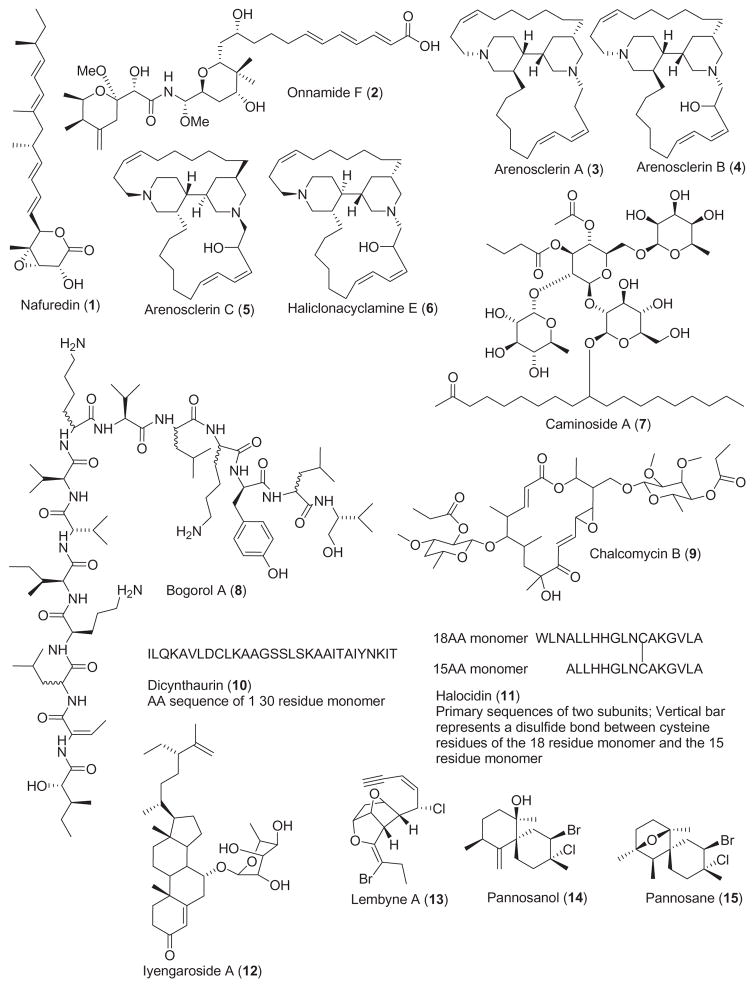
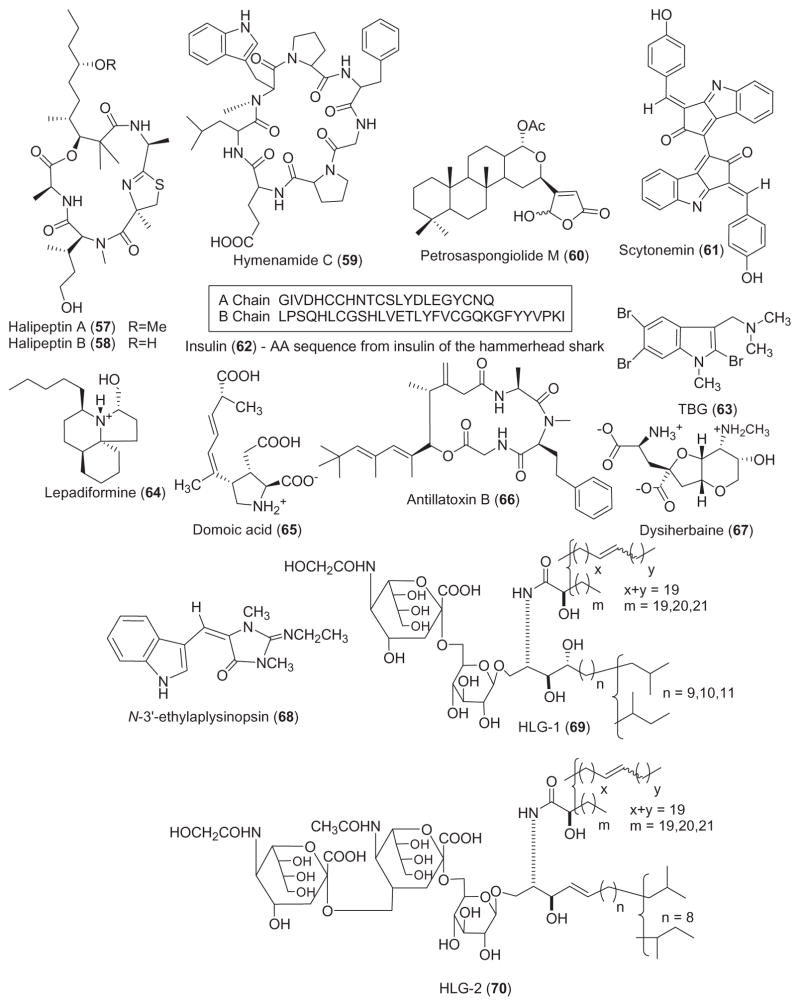
Table 1 summarizes new pharmacological findings reported during 2001–2002 on the preclinical anthelmintic, antibacterial, anticoagulant, antifungal, antimalarial, anti-platelet, antiprotozoal, antituberculosis, and antiviral pharmacology of the 56 marine natural products shown in Fig. 1.
2.1. Anthelmintic and antibacterial compounds
Two studies contributed to the search of novel anthelmintic marine natural products during 2001–2002. Nafuredin (1), an epoxy-δ-lactone with a methylated olefinic side chain isolated from the culture broth of Aspergillus niger, exerted anthelmintic activity against the ruminant parasite worm Haemonchus contortus and the dwarf tapeworm Hymenolepsis nana in mice (Omura et al., 2001). The mechanism of action involved inhibition at the nanomolar level of NADH-fumarate reductase activity, a “unique anaerobic electron transport system in helminth mitochondria”. The marine natural product onnamide F (2), isolated from the Australian marine sponge Trachycladus laevispirulifer, showed potent inhibition of larval development of the parasitic nematode Haemonchus contortus with an in vitro LD99 value of 2.6 μg/mL (Vuong et al., 2001).
Reflecting the fact that the development of resistance toward current antibiotics continues to be a significant problem in the treatment of infectious diseases, during 2001–2002 thirteen studies contributed to the antibacterial pharmacology of marine natural products, a marked increase from 1998–2000 (Mayer and Lehmann, 2000; Mayer and Hamann, 2002, 2004). Two studies reported on the mechanism of action of two novel marine antibiotics. Torres et al. (2002) investigated the arenosclerins A–C (3–5) and haliclonacyclamine E (6), novel tetracyclic alkylpiperidine alkaloids isolated from the marine sponge Arenosclera brasiliensis. The investigators reported that differences in the stereochemistry at the bis-piperidine ring system played a significant role in the potent antibiotic activity of these compounds against antibiotic-resistant Staphylococcus aureus strains, observations that lead the authors to suggest that “these compounds may be regarded as potentially useful new drug leads”. Linington et al. (2002) developed a high throughput assay to screen marine compounds for their ability to inhibit a type III secretory system which is an essential component of the pathogenicity of enteropathogenic and enterohermorragic E. coli. Their efforts resulted in the isolation of a novel antimicrobial glycolipid caminoside A (7) from the marine sponge Caminus spaeroconia. Caminoside A was “reasonably potent” against methicillin-resistant S. aureus (MIC=12 μg/mL) and vancomycin-resistant enterococcal strains (MIC=12 μg/mL).
Although additional novel marine antibiotics were reported in 2001–2002, no mechanism of action studies were reported for marine compounds (8–20). Nevertheless, the reports highlight the observations that potentially novel antibiotics are present in marine bacteria, tunicates, fungi, algae and sponges. Two papers reported on antibiotic activity in compounds isolated from marine bacteria: Barsby et al. (2001) reported the isolation of the peptide bogorol A (8) from a culture of a marine Bacillus sp., which was active against methicillin-resistant S. aureus (MIC=2 μg/mL) and vancomycin-resistant enterococcal strains (MIC =10 μg/mL). Because bogorol A represents a new cationic peptide antibiotic template the authors proposed that it might become “an attractive lead structure for SAR optimization”. Asolkar et al. (2002) reported on the isolation of a novel macrolide antibiotic chalcomycin B (9) from the culture broth of a marine Streptomycete isolate which was particularly potent against S. aureus (MIC=0.39 μg/mL). Two papers reported on new antimicrobial peptides isolated from marine tunicates: Lee et al. (2001) discovered an unusual peptide, dicynthaurin (10), from hemocytes of the marine tunicate Halocynthia aurantium. Dicynthaurin (10), a peptide that contains an unpaired cysteine and forms covalent homodimers, was active against Gram-negative and Gram-positive bacteria. Jang et al. (2002) reported a new antimicrobial heterodimeric peptide halocidin (11) from the hemocytes of the solitary marine tunicate, H. aurantium, the same source of the peptide dicynthaurin. Although the investigators hypothesized that the main target of halocidin might be the bacterial cell membrane, this heterodimeric peptide demonstrated significant potency against methicillin-resistant S. aureus and multidrug-resistant Pseudomonas aeruginosa. Three papers reported on the presence of antibacterial compounds in marine algae: a novel steroidal glycoside, iyengaroside-A (12), was isolated from the marine green alga Codium iyengarii (Ali et al., 2002) and was shown to be slightly less potent than tetracycline against a battery of Gram-negative and Gram-positive bacteria. Vairappan et al. (2001) reported a new halogenated G15 acetogenin, lembyne-A (13), from the marine red alga Laurencia sp. that had antibacterial activity against a panel of 13 species of marine bacteria isolated from algal habitats. Suzuki et al. (2001) contributed two novel halogenated sesquiterpenoids, pannosanol (14) and pannosane (15), from the red alga Laurencia pannosa, which demonstrated antibacterial activity against 13 species of marine bacteria. Three antibiotic compounds were isolated from marine fungi: a novel chlorinated benzophenone antibiotic, pestalone (16), was isolated from a member of the marine fungus genus Pestalotia, in response to a bacterial challenge (Cueto et al., 2001). Pestalone showed very potent antibiotic activity against methicillin-resistant S. aureus (MIC=37 ng/mL) and vancomycin-resistant Enterococus faecium (MIC=78 ng/mL), suggesting that pestalone should be evaluated in advanced models of infection. Jadulco et al. (2001) reported the isolation of a new furan carboxylic acid, an acetyl derivative of sumiki’s acid (17) from the marine fungus Cladosporium herbarum that was found to be active against Bacillus subtilis and S. aureus. Zopfiellamides A and B (19 and 20), antimicrobial pyrrolidinone derivatives, were isolated from the marine fungus Zopfiella latipes (Daferner et al., 2002). Although the zopfiellamides appeared to be moderately antibacterial to Gram-positive and Gram-negative bacteria (MIC = 2–10 μg/mL) they exhibited no cytotoxicity up to 100 μg/mL. Finally, the bromotyrosine antibiotic, zamastatin (18), was derived from the Okinawan sponge Pseudoceratina purpurea (Takada et al., 2001) and while zamastatin affected the growth of the marine biofouling bacteria Rhodospirillum salexigens, it remains to be demonstrated that this compound will affect antibiotic-resistant bacteria.
2.2. Anticoagulant compounds
One paper was published during 2001–2002 on the anticoagulant properties of two marine polysaccharides, a decrease from our previous reviews (Mayer and Lehmann, 2000; Mayer and Hamann, 2002, 2004). Pereira et al. (2002) reported a novel sulfated α-L-galactan (21) and a sulfated α-L-fucan (22) isolated from the crude egg jelly of the sea urchins Echinometra lucunter and Strongylocentrotus franciscanus collected in Brazil and the USA. Interestingly, only the sulfated galactan had potent anticoagulant activity as shown by the enhancement of thrombin or factor Xa inhibition by antithrombin or heparin cofactor II, similar to heparin.
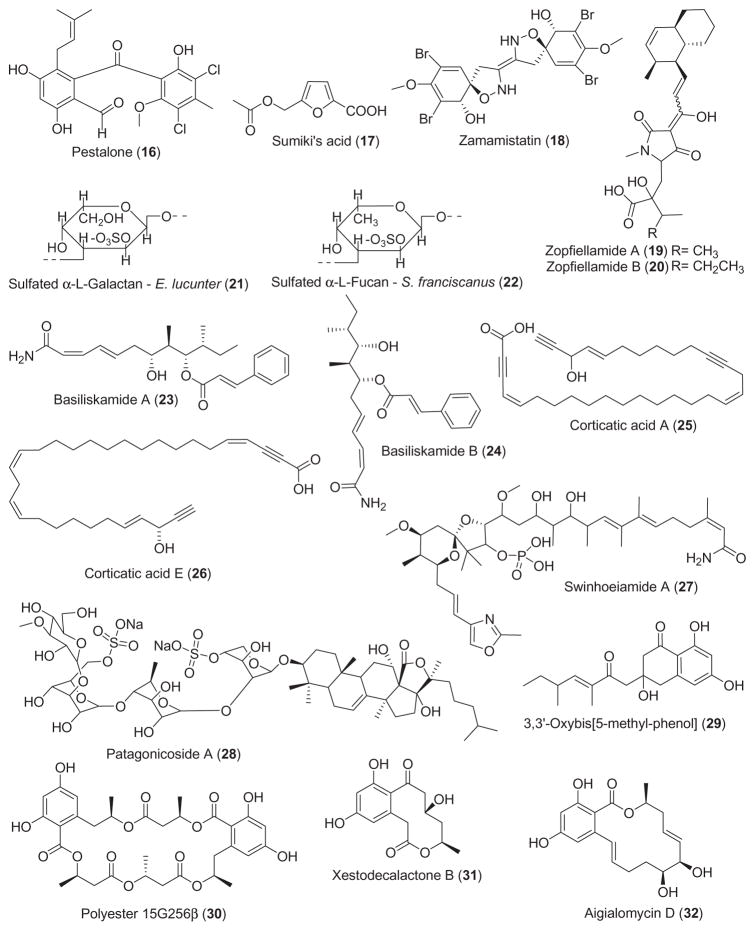
2.3. Antifungal compounds
Seven studies during 2001–2002 reported on the antifungal properties of 9 novel marine natural products, a slight increase from 1998–2000 (Mayer and Lehmann, 2000; Mayer and Hamann, 2002, 2004). Antifungal activity was noted in novel compounds isolated from marine bacteria, sponges, sea cucumbers and fungi.
Two novel antifungal amides resulted from screening marine bacterial cultures against common fungal pathogens. Laboratory cultures of the marine bacterium Bacillus laterosporus produced the novel polyketides basiliskamides A and B (23 and 24) (Barsby et al., 2002). Both compounds showed potent activity against Candida albicans (MIC=1.0 and 3.1 μg/mL, respectively) and Aspergillus fumigatus (MIC = 2.5 and 5.0 μg/mL, respectively), which was comparable to amphotericin B, an agent currently used for the treatment of systemic fungal infections. Interestingly, basiliskamide A was 4-fold less cytotoxic to normal human fibroblasts than amphotericin B.
Two novel polyacetylenic acids, corticatic acids D and E (25 and 26) isolated from the marine sponge Petrosia corticata (Nishimura et al., 2002) were shown to inhibit geranylgeranyltransferase type I (GGTase I), an enzyme involved in fungal cell wall biosythesis. Interestingly, while corticatic acids D and E inhibited C. albicans with IC50 values of 3.3 and 7.3 μM, the fact that there is little sequence identity between human and Candida GGTase I suggested that these marine compounds may become leads for novel and “selective antifungal agents”. Swinhoeiamide A (27), a novel antifungal calyculin derivative was isolated from the marine sponge Theonella swinhoei (Edrada et al., 2002a). Swinhoeiamide A showed strong antifungal activity towards C. albicans and A. fumigatus (MIC=1.2 and 1.0 μg/mL, respectively). Murray et al. (2001) reported the novel disulfated triterpene glycoside patagonicoside A (28) from the sea cucumber Psolus patagonicus that was active against the pathogenic fungus C. cucumerinum (apparent MIC=8 μg/spot), which may be the result of sulfate groups in the oligosaccharide chain.
Three novel marine antifungals resulted from screening marine fungal cultures against common fungal pathogens. An antifungal metabolite, 3,3′-oxybis[5-methyl-phenol] (29) was isolated from the fermentation broth of the filamentous marine fungus Keissleriella sp. (Liu et al., 2002a,b). This novel metabolite moderately inhibited growth of the human pathogens C. albicans, Tricophyton rubrum and A. niger (MIC=10–80 μg/ml). A new antifungal polyester 15G256β (30) was isolated from the fermentation broth of the marine fungus Hypoxylon oceanicum (Schlingmann et al., 2002). Although the exact molecular mechanism responsible for the reported antifungal activity has not been established, the polylactone was observed to potently inhibit a variety of phytopathogenic fungi (MIC=2 μg/mL). Culture filtrates of Penicillium cf. montanense obtained from the marine sponge Xestospongia exigua yielded a novel decalactone xestodecalactone B (31) (Edrada et al., 2002b) which was found to be active against the yeast C. albicans.
2.4. Antimalarial, antiplatelet, antiprotozoal and antituberculosis compounds
During 2001–2002 and as shown in Table 1, 11 studies were reported in the area of antimalarial, antiprotozoal and antituberculosis pharmacology of structurally characterized marine natural products.
Eleven compounds depicted in Fig. 1 were shown to possess antimalarial activity. Moderate antimalarial activity (IC50 =6.6 μg/mL) against the multidrug resistant Plasmodium falciparum (K1 strain) was reported for aigialomycins D (32), a new 14-membered resorcyclic macrolide isolated from the marine mangrove fungus Aigialus parvus BCC 5311 (Isaka et al., 2002). As part of an ongoing program on biologically active substances from bioresources in Thailand, Chinworrungsee et al. (2001) reported the ophiobolane sesterterpene halorosellinic acid (33) from the marine fungus Halorosellinia oceanica BCC 5149. Halorosellinic acid demonstrated moderate antimalarial activity (IC50 =13 μg/mL) against the parasite P. falciparum (K1, multidrug resistant strain). In vitro and in vivo antimalarial studies were conducted with the trypyrrole bacterial pigment heptyl prodigiosin (34), purified from the culture of a proteobacteria from a marine tunicate in the Philippines (Lazaro et al., 2002). The investigators reported that the in vitro activity of heptyl prodigiosin against P. falciparum 3D7 was similar to chloroquine (IC50 =0.07 vs. 0.015 μM, respectively). Interestingly, a single subcutaneous administration of 5–20 mg/kg heptyl prodigiosin significantly extended survival of P. berghei ANKA strain-infected mice, suggesting that “the molecule might be used as a lead compound”. New enantiomers of ent-8-hydroxymanzamine A (35), manzamine F (36) and neo-kauluamine (37) were isolated from an undescribed Indo-Pacific sponge (El Sayed et al., 2001). When assayed in vivo, ent-8-hydroxymanzamine A and neo-kauluamine reduced parasitemia in P. berghei-infected mice, with a concomitant increase in survival; additionally ent-8-hydroxymanzamine A and manzamine F inhibited Mycobacterium tuberculosis (MIC < 12.5 μg/mL). In vitro antimalarial activity (MIC=3.8–2.9 μg/mL) against P. falciparum (D6 and W2 clones) was reported for (S)-(+)-15-hydroxycurcuphenol (38), a microbial transformation product of the sesquiterpene (S)-(+)-curcuphenol isolated from the Jamaican sponge Didiscus oxeata and transformed using Kluyveromyces marxianus var. lactis (El Sayed et al., 2002). Mizuno et al. (2002) extended the pharmacology of jasplakinolide (39), a cyclic peptide isolated from the marine sponge Jaspis sp. These investigators observed that jasplakinolide markedly decreased parasitemia of P. falciparum by virtue of “an apical protrusion that appears to interfere with the erythrocyte invasion by the merozoites” and whose mechanism of formation is possibly related to an increase in F-actin content of the merozoites treated with this marine agent. Wright et al. (2002) reported an extensive study on two novel alkaloids, lepadins E (40) and F (41) isolated from a tropical marine tunicate Didemnum sp., which showed significant antiplasmodial activity. Interestingly, the bioactivity of the two molecules against two P. falciparum strains (strain K1: IC50 =0.4 and 0.2 μg/mL, respectively; strain NF5: IC50 =0.9 and 0.3 μg/mL, respectively) appeared to be dependent on the configuration at C-2 and the nature of the functionality at C-3 in the decahydroquinoline, as well as the moderate inhibition of the p56lck tyrosine kinase. A peroxide-containing metabolite, plakortide F (42) was isolated from the Jamaican sponge Plakortis sp. (Gochfeld and Hamann, 2001). The fact that plakortide F had significant in vitro activity against P. falciparum (D6 and W2 clones: IC50 =0.48 and 0.39 μg/mL, respectively) suggested that the peroxide is necessary for antimalarial activity of this compound.
Perry et al. (2001) contributed to antiprotozoal pharmacology by reporting the isolation of plakortolide G (43), a new peroxylactone from the Jamaican sponge Plakinastrella onkodes. Plakortolide G at 10 μM exhibited 100% inhibition of infection by the obligate intracellular parasite Toxoplasma gondii, the cause of toxoplasmosis and concomitant serious pathology, including hepatitis, pneumonia, blindness, and severe neurological disorders that is especially true in individuals whose immune systems are compromised (e.g., AIDS patients).
Three novel compounds were contributed to the search for novel antituberculosis agents. A new bioactive diterpene, cyanthiwigin C (44), was isolated from the Jamaican sponge Myrmekioderma styx (Peng et al., 2002). At 6.25 μg/mL, cyanthiwigin C inhibited the growth of M. tuberculosis by 50%. Two new serrulatane diterpenes, erogorgiaene (45) and 7-hydroxyerogorgiaene (46), inhibited M. tuberculosis growth by 96% and 77% at a concentration of 12.5 and 6.25 μg/mL, respectively (Rodriguez and Ramirez, 2001).
Bunc et al. (2002) contributed to the antiplatelet pharmacology of the water soluble polymeric 3-alkylpyridinium salts (47) isolated from the marine sponge Raniera sarai. These salts, previously shown to be cholinesterase inhibitors, appeared to aggregate in vivo inducing formation of “non-covalently bound supra-molecular structures”, ultimately inducing blood coagulation, platelet aggregation and cytotoxicity in rats.
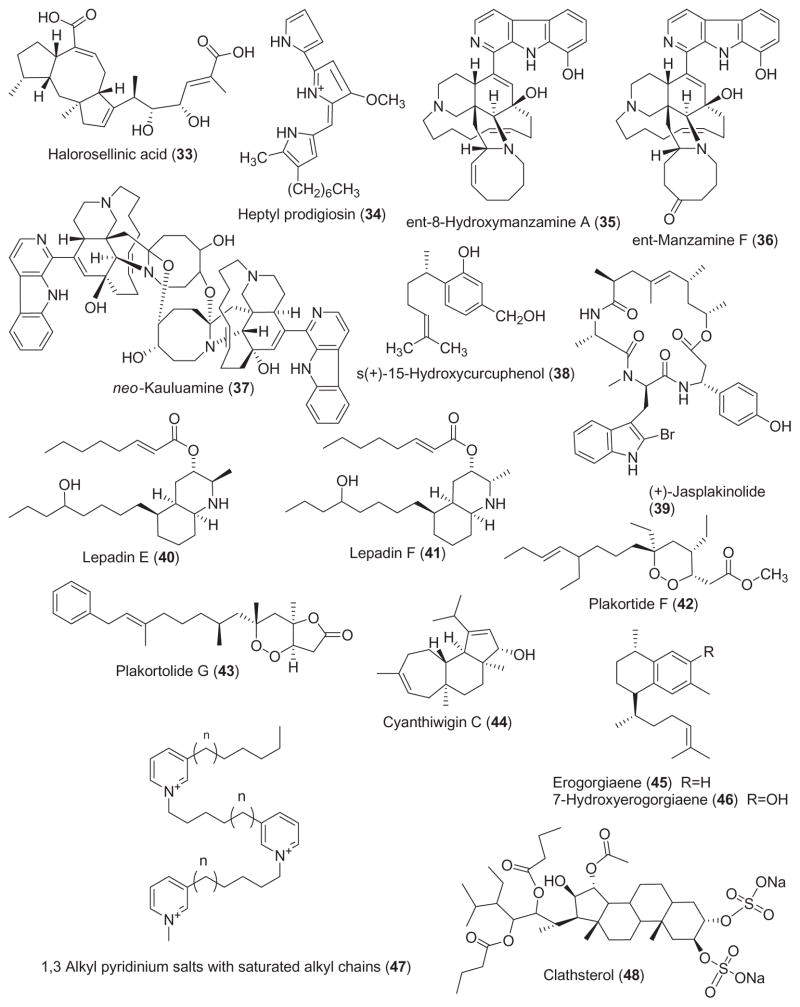
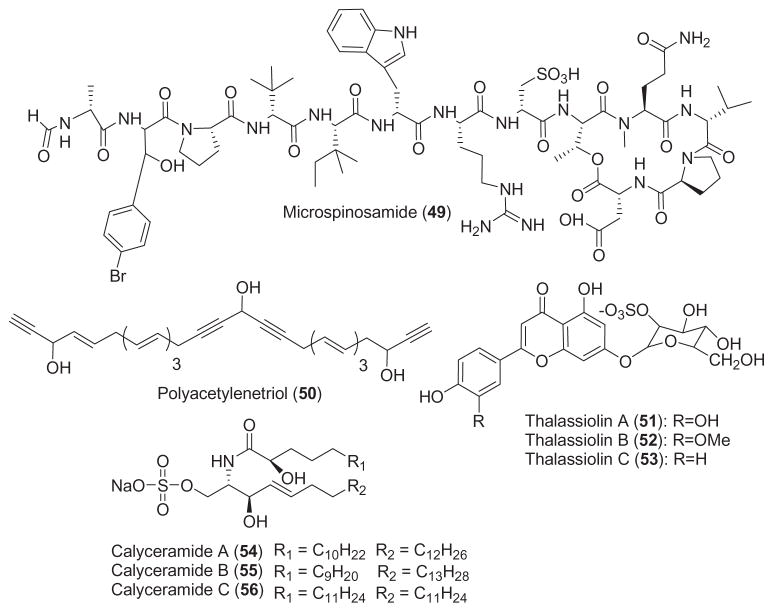
2.5. Antiviral compounds
As shown in Table 1, interest in the antiviral pharmacology of marine natural products remained high during 2001–2002. During this two-year period, six novel marine compounds (Fig. 1) were reported to possess antiviral properties against human immunodeficiency (HIV), herpes simplex (HSV) and influenza viruses. Rudi et al. (2001) reported the isolation of clathsterol (48), a novel and active sulfated sterol from the Red Sea sponge Clathria sp., which was active against HIV-1 reverse transcriptase at 10 μM. An HIV-inhibitory cyclic depsipeptide, microspinosamide (49) was isolated from the marine sponge Sidonops microspinosa (Rashid et al., 2001). Microspinosamide inhibited the cytopathic effect of HIV-1 infection in a cell-based in vitro assay with an EC50 of 0.2 μg/mL. Loya et al. (2002) reported an extensive study on the mechanism of action of poly-acetylenetriol (50), isolated from the marine sponge Petrosia sp. Polyacetylenetriol evidenced selective inhibition of the RNA- and DNA-dependent DNA polymerase activities of retroviral reverse transcriptases (IC50 =0.95 μM), as compared to cellular DNA polymerases (IC50 =2.6 μM). Furthermore, a reversible non-competitive mechanism involving a putative hydrophobic interaction was shown to play a critical role in the inhibition of the HIV-1 reverse transcriptase enzyme. Although polyacetylenetriol lacked sufficient specificity and thus could probably not be used as an anti-HIV agent, the authors concluded that “… structural modification of the side chains of the lead polyacetylenic molecule may produce new potent and selective anti-AIDS drugs”. As a result of an ongoing program focused on the discovery of new “small molecule” inhibitors of HIV-1 integrase, Rowley et al. (2002) reported on the discovery of the sulfated-flavone glycosides, thalassiolins A–C (51, 52, and 53), isolated from the Caribbean sea grass Thalassia testudinum. Thalassiolin A, the most active compound of this series, inhibited HIV integrase catalyzed strand transfer (IC50 =0.4 μM) as well as HIV replication in cell culture (IC50 =30 μM). Interestingly, the presence of sulfated β-D-glucose functionality increased potency against the HIV integrase, while molecular modeling studies indicated that the probable binding site of the molecule was the catalytic core domain of the HIV-1 integrase.
As part of their search for novel influenza virus neuraminidase inhibitors, Nakao et al. (2001) reported three active sulfated calyceramides A–C (54, 55, and 56) isolated from the marine sponge Discodermia calyx. Interestingly, calyceramides A–C inhibited neuraminidase from bacterium Clostridium perfringens with IC50 values of 0.4, 0.2 and 0.8 μg/mL, respectively, which was slightly more potent than 4-acetyl neuraminic acid (IC50 =1.5 mg/mL). It remains to be determined if these compounds will also inhibit influenza virus neuraminidase with similar potency.
3. Marine compounds with anti-inflammatory and antidiabetic effects and affecting the cardiovascular, immune and nervous systems
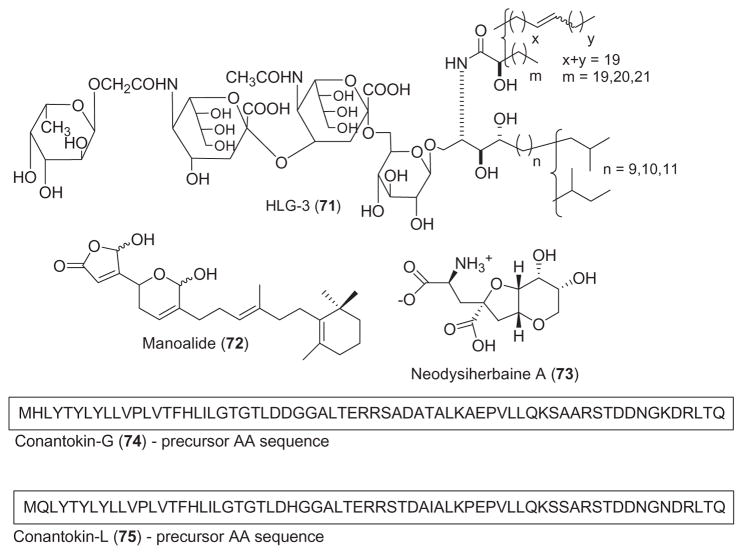
Table 2 summarizes preclinical pharmacological research completed during 2001–2002 with the 19 marine chemicals shown in Fig. 2.
3.1. Anti-inflammatory compounds
The anti-inflammatory pharmacology of the marine compounds halipeptins A and B, hymenamide C, petrosas-pongiolide and scytonemin was reported during 2001–2002, a slight decrease from our previous report (Mayer and Hamann, 2004).
Two anti-inflammatory 17-membered cyclic depsipeptides, halipeptins A and B (57 and 58) were isolated from the marine sponge Haliclona sp. (Randazzo et al., 2001). Halipeptin A at 300 μg/kg potently and dose-dependently inhibited carrageenan-induced paw edema in a mouse model of inflammation. Although no mechanism of action study was reported, interestingly, indomethacin and naproxen showed an ED50 of 12 and 40 mg/kg, respectively, in the same in vivo assay, thus suggesting that halipeptin A was “40 and 130 times more potent” than these clinically used non-steroidal anti-inflammatory agents. The immunomodulating activity of the marine cyclopeptide hymenamide C (59), isolated from the marine sponge Axinella carteri, was explored investigating its effects on neutrophils and macrophages (Napolitano et al., 2001). Although hymenamide C inhibition of human neutrophil degranulation (IC50 =18 μM) was not particularly impressive, the authors noted that cyclosporine, a clinically used immunosuppressive cyclopeptide, exerted “weaker inhibitory effect on elastase release”. A structure–activity relationship study completed with this cyclopeptide concluded a “non-receptorial mode of action” for hymenamide C and its synthetic analogs (Napolitano et al., 2002). Dal Piaz et al. (2002) reported an extensive investigation of the mechanism of phospholipase A2 (PLA2) inactivation by the novel marine sesterterpene petrosaspongiolide M (60), a bioactive sesterterpene isolated from the marine sponge Petrosaspongia nigra. The reported results suggest that the PLA2 α-amino terminal group of the IIe-1 residue is the only covalent binding site for petrosaspongiolide M, a compound that inhibits bee venom PLA2 with an IC50 =0.6 μM. Stevenson et al. (2002a,b) extended the pharmacology of scytonemin (61), a yellow-pigment isolated from marine cyanobacteria which demonstrated interesting anti-inflammatory and anti-proliferative activities. In vitro, scytonemin inhibited both polo-like kinase 1 (IC50 =2.3 μM) and protein kinase C β1 (IC50 =5.4 μM), while in vivo, topical application of this novel pharmacophore reduced phorbol-ester induced mouse ear edema (IC50 =10.9 μg/ear).
3.2. Antidiabetic and cardiovascular compounds
Only 3 reports during 2001–2002 contributed to the antidiabetic and cardiovascular pharmacology of marine natural products.
Anderson et al. (2002) reported the purification, characterization and biological activity of insulins (62) from the European spotted dogfish, Scyliorhinus canalicula, and the hammerhead shark, Sphyrna lewini. Although the elasmobranch insulins were noted to be markedly different from human insulin, with 17 amino acid substitutions identified, all residues that are required for binding to the recombinant human insulin receptor were shown to be conserved. The bolus arterial injection of dogfish insulin caused a significant drop in blood glucose only after 12 h but it persisted for 48 h, indicating metabolic actions similar to those “described for mammalian insulin”.
Contributions to the cardiovascular pharmacology of the marine natural products 2,5,6-tribromo-1-methylgramine (TBG) and lepadiformine were reported during 2001–2002. Iwata et al. (2001) extended the pharmacology of 2,5,6-tribromo-1-methylgramine (TBG) (63), a compound isolated from the marine bryozoan Zoobotryon pellucidum, by examining its effect on the contraction of the rat aorta. Interestingly, while at concentrations up to 10 μM the halogen-containing gramine analogue inhibited muscle contraction by affecting Ca2+ entry, at 30 μM the inhibitory mechanism involved an increase in intracellular cyclic AMP. Juge et al. (2001) investigated the cardiovascular effects of lepadiformine (64), an alkaloid isolated from the marine ascidians Clavelina lepadiformis and C. moluccensis. Using in vivo arterial blood pressure recordings and electrocardiograms in anaesthetised rats and in situ peripheral vascular pressure recordings in perfused rabbit ear, they observed that lepadiformine had marked effects on the cardiocirculatory system, inducing bradycardia, prolonging electrocardiogram parameters, producing a transient fall of blood pressure in the rat and decreasing blood flow in the rabbit ear. The authors concluded that the pharmacological effects of lepadiformine might result from a reduction of the inward rectifying K+ current, suggesting that this marine compound has “antiar-rhythmic properties” that warrant further investigation.
3.3. Compounds affecting the immune system
Mayer et al. (2001) extended the pharmacology of the marine excitatory amino acid domoic acid (65), a glutamate and kainic acid analog which can cause amnesic shellfish poisoning in humans and is produced by the widely distributed marine diatom genus Pseudo-nitzschia. Domoic acid at in vitro concentrations that were toxic to neuronal cells (1 mM) was shown to trigger a limited activation of rat brain microglia, an immune cell type that contributes to circa 10% of the total glial population in the central nervous system, and the concomitant release of two potentially neurotoxic mediators, namely TNF-α and matrix metalloproteinase-9.
3.4. Compounds affecting the nervous system
Reports on both central and autonomic nervous system pharmacology of marine natural products during 2001–2002 studies involved antillatoxin B, dysiherbaine, N-3′-ethylaplysinopsin, neodysiherbaine A, gangliosides of Holothuria leucospilota, the peptides conantokins-G and L and manoalide.
Bioassay-guided fractionation of organic extracts from the marine cyanobacterium Lyngbya majuscula led to the isolation of the neurotoxic lipopeptide antillatoxin B (66), an analogue of the potent neurotoxin antillatoxin (Nogle et al., 2001). Although antillatoxin B was a potent activator of voltage-sensitive sodium channel in a mouse neuro-2a neuroblastoma cell line (EC50 =1.77 μM), its biological activity was 10-fold less than that of antillatoxin, probably as a result of a substitution of a larger N-methyl homo-phenylalanine residue for an N-methyl valine residue in the peptide-derived metabolite. An extensive characterization of the pharmacological properties of the potent epileptogenic amino acid dysiherbaine (67), isolated from the marine sponge Dysidea herbacea was reported by Sakai et al. (2001b). Dysiherbaine, which demonstrated “potent convulsant activity”, caused seizures upon injection into mice (ED50 =13 pmol/mouse i.c.v) and was shown to be a non-NMDA-type glutamate receptor agonist with high selectivity for kainic acid receptors (IC50 =210 nM) as well as mGluR5 receptors. The authors concluded that this novel marine amino acid might be useful for the evaluation of “physiological and pathological roles of non-NMDA receptors, especially kainic acid receptors, in the central nervous system”. A new indole alkaloid, N-3′-ethylaplysinopsin (68), isolated from the Jamaican sponge Smenospongia aurea, was shown to potently bind to the human serotonin 5-HT2C receptor subtype expressed in a mammalian cell line (Hu et al., 2002). The authors suggest that the R2 functional groups at position 2′ may play an important role in regulating subtype selective binding to the 5-HT2C receptor, a receptor found in high density in the choroid plexus, the site of cerebrospinal fluid production.
Yamada et al. (2001) reported new gangliosides (HLG-1, HLG-2, and HLG-3) (69–71) from the sea cucumber Holothuria leucospilota which induced neurite growth outgrowth. Although the molecular mechanism remains unexplored the ganglioside species displayed neuritogenic activity at 10 μM in the presence of nerve growth factor. Dorandeu et al. (2002) presented novel information on potential anticonvulsant pharmacology of the nonsteroidal sesterterpene manoalide (72), a well-known phospholipase A2 inhibitor isolated from the marine sponge Luffariella variabilis. Manoalide was reported to fully and irreversibly inhibit the catalytic activity of crotoxin, the heterodimeric β-neurotoxin from the venom of the South American rattlesnake Crotalus durissus terrificus, preventing central neurotoxicity after intracerebroventricular injection and peripheral toxicity after intravenous injection. Sakai et al. (2001a) reported the isolation of a neodysiherbaine A (73), a new excitatory amino acid from the sponge D. herbacea. Neodysiherbaine A was observed to be a potent epileptogenic amino acid (ED50 =15 pmol/mouse i.c.v), comparable to dysiherbaine, and a potent and novel non-NMDA-type glutamate receptor agonist with high selectivity for kainic acid receptors (IC50 =66 nM). Two studies extended the pharmacology of the marine conantokins, the only natural biochemically characterized peptides known to be N-methyl-D-asparate (NMDA) antagonists and potent anticonvulsants. Wittekindt et al. (2001) investigated the binding of conantokin-G (74), a peptide derived from the venom of the marine cone snail Conus geographus, on recombinant NMDA receptors carrying point mutations within the glycine and glutamate binding pockets of the NR1 and NR2B subunits. Because mutations located in the glutamate binding site of the NR2B subunit were found to significantly affect conantokin-G binding, the investigators concluded that this peptide inhibited the NMDA receptor currents at the “glutamate binding site via a competitive mechanism”. A new member of the conantokin peptide family, conantokin-L (75), was isolated and characterized from a heretofore unexamined species, the marine fish-hunting cone snail Conus lynceus (Jimenez et al., 2002). Conantokin-L had extensive sequence identities with conantokin-R and was a potent NMDA receptor antagonist in mammalian CNS neurons in culture. However, conantokin-L was a far less potent anticonvulsant in the audiogenic mouse model of epilepsy, probably as a result of the lack of C-terminal acids. The authors note that the discovery of conantokin-L will definitely contribute to the development of a “clinically effective and well-tolerated NMDA antagonist that possesses both anticonvulsant and neuroprotective properties”.
4. Marine compounds with miscellaneous mechanisms of action
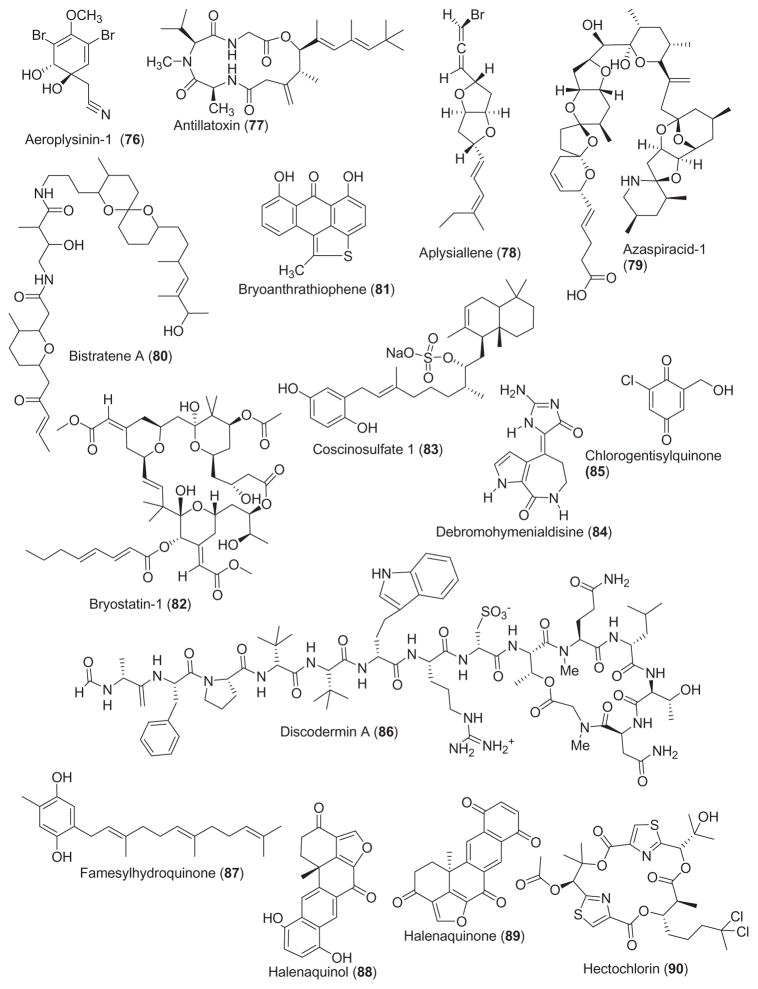
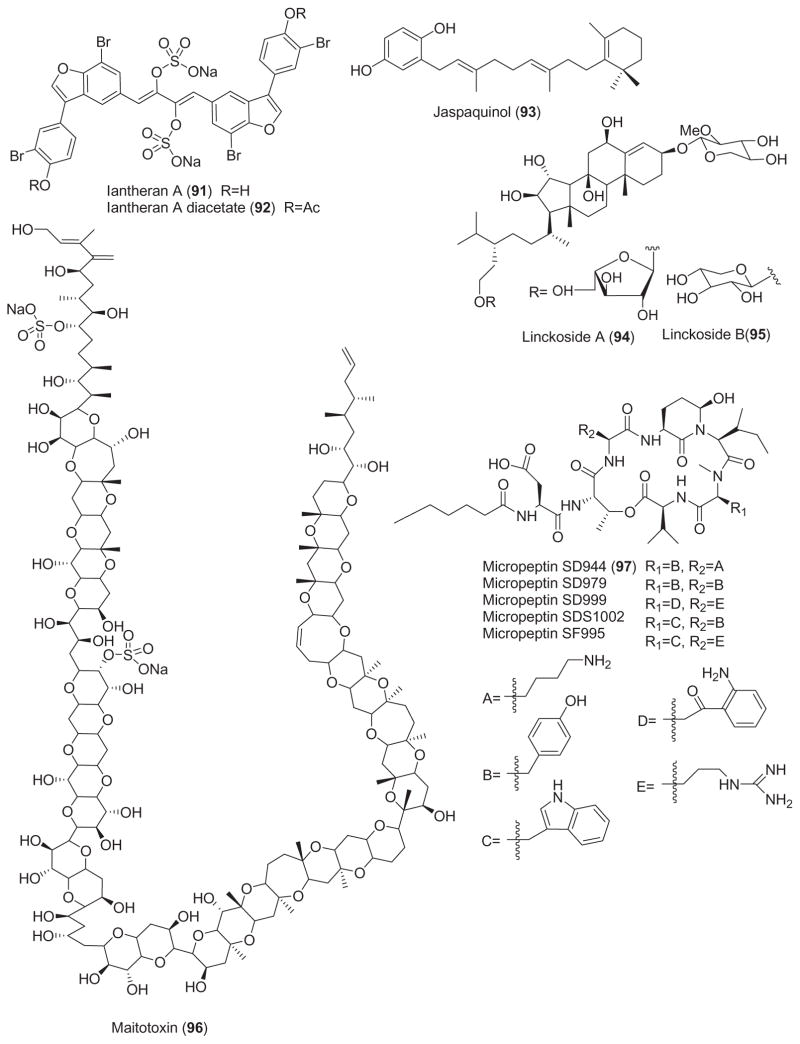
The structures of marine compounds with miscellaneous mechanisms of action are presented in Fig. 3. Interestingly and in contrast with the 75 chemicals included in Figs. 1 and 2, this third group of 31 marine compounds includes not only nitrogen-containing compounds (i.e. proteins, peptides), terpenes and polyketides but also a few polysaccharides.
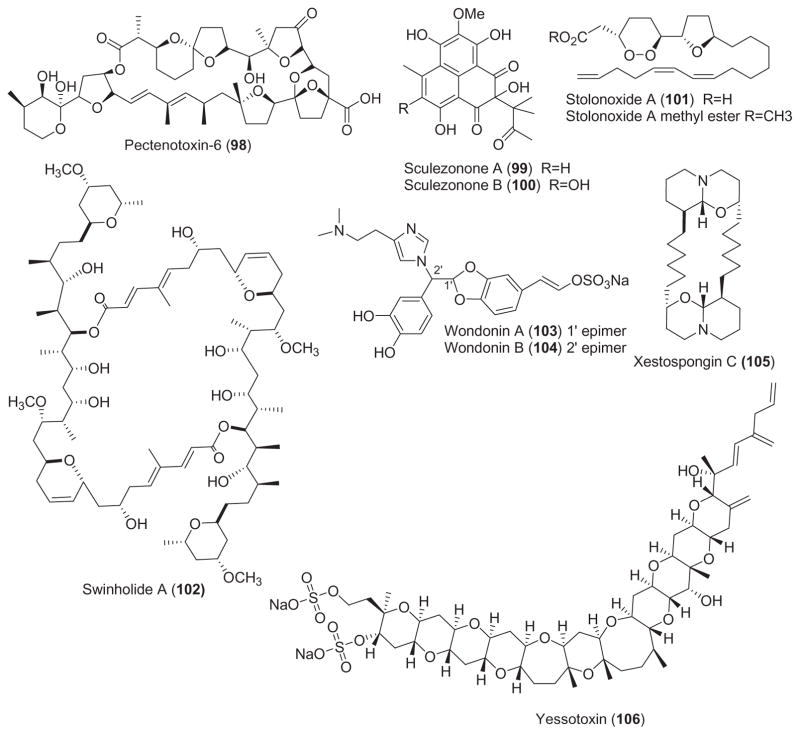
As shown in Table 3, for some of these marine natural products, namely antillatoxin (77), azaspiracid-1 (79), bistratene A (80), bryostatin-1 (82), coscinosulfate (83), debromohymenialdisine (84), halenaquinol (88), halenaquinone (89), hectochlorin (90), maitotoxin (96), pectenotoxin-6 (98), sculezonone A and B (99 and 100), scytonemin (61), stolonoxides (101), xetospongin-C (105) and yessotoxin (106), both the pharmacological activity and molecular mechanism of action were reported.
In contrast, for the marine compounds aeroplysinin-1 (76), aplysiallene (78), bryoanthrathiophene (81), chlorogentisylquinone (85), discodermin A (86), farnesylhydroquinone (87), iantherans A and B (91 and 92), jaspaquinol (93), jasplankinolide (39), linckosides A and B (94 and 95), micropeptins (97), swinholide A (102) and wondonins A and B (103 and 104), while a pharmacological activity was investigated, no additional information was reported on the molecular mechanism of action.
5. Reviews on marine pharmacology
Several reviews covering selected aspects of marine pharmacology and toxicology were published during 2001–2002: the chemistry and biological function of natural marine toxins (Yasumoto, 2001); a retrospective on the conotoxins (Olivera and Cruz, 2001); α-conotoxins as pharmacological tools and potential drug leads (Dutton and Craik, 2001); ciguatera fish poisoning (Hokama and Yoshikawa-Ebesu, 2001); toxic marine microalgae (Daranas et al., 2001); advances in chemical and biological research with marine indoles and carbazole alkaloids (Pindur and Lemster, 2001); biologically active marine proteins (O’Keefe, 2001); bioactive marine compounds from coral reef invertebrates (Higa et al., 2001); glycolipids with immunomodulating activity from marine sponges (Costantino et al., 2001); marine lipids and coronary heart disease (Colquhoun, 2001); anticoagulant properties of sulfated glycosaminoglycans (Pavao, 2002); sea-anemone pore-forming proteins and their pharmacological and medical use (Anderluh and Menestrina, 2001); cytolytic peptide and protein toxins from sea anemones (Anderluh and Macek, 2002); okadaic acid as the archetypal serine/threonine protein phosphatase inhibitor (Dounay and Forsyth, 2002) and useful tool for studying cellular processes (Fernandez et al., 2002); marine microorganisms for the production of bioactive metabolites (Wagner-Dobler et al., 2002); cyanobacterial toxins and their implications for human health (Rao et al., 2002); the pharmacological activity of fish venoms (Church and Hodgson, 2002); and the chemistry of marine natural products (Faulkner, 2001; Faulkner, 2002).
6. Conclusion
Although during 2001–2002 no new marine natural product was approved for patient care by the U.S. Food and Drug Administration, the present review documents the fact that during 2001–2002 preclinical pharmacological research with marine chemicals continued to proceed at a very active pace, involving both natural product chemists and pharmacologists from 29 countries including the United States. Although this review has mainly focused on recent developments in the preclinical pharmacology of 106 marine natural products, the reader should be aware that concomitant to the mechanistic characterization of marine natural products, the issues of supply, formulation, and manufacturing represent important challenges that need to be met for the successful development of novel pharmaceutical agents. These issues were approached in 2002 by three companies involved in the development of novel pharmaceuticals from marine sources (Fenical et al., 2002; Garcia-Fernandez et al., 2002; Mayer, 2002; Sennett et al., 2002).
Acknowledgments
This publication was made possible by grant number 1R15 ES012654, from the National Institute of Environmental Health Sciences, NIH (to AMSM); 1R01A136596, from the National Institute of Allergy and Infectious Diseases, NIH and the Medicines for Malaria Venture (MMV) to MTH; and Midwestern University. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS, NIH. Jennifer Allman is gratefully acknowledged for her assistance with the preparation of figures (MTH). Excellent support for literature searches and article retrieval by library staff members as well as medical and pharmacy students from Midwestern University is most gratefully acknowledged. The authors wish to specially thank Mrs. Victoria Sears and Ms. Mary Hall for their assistance in the preparation of this manuscript.
References
- Ali MS, Saleem M, Yamdagni R, Ali MA. Steroid and antibacterial steroidal glycosides from marine green alga Codium iyengarii Borgesen. Nat Prod Lett. 2002;16:407–413. doi: 10.1080/10575630290034249. [DOI] [PubMed] [Google Scholar]
- Anderluh G, Macek P. Cytolytic peptide and protein toxins from sea anemones (Anthozoa: Actiniaria) Toxicon. 2002;40:111–124. doi: 10.1016/s0041-0101(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Anderluh G, Menestrina G. Pore forming proteins from sea anemones and the construction of immunotoxins for selective killing of harmful cells. In: Fingerman M, Rachakonda N, editors. Bio-organic Compounds: Chemistry and Biomedical Applications. Science Publishers; Enfield: 2001. pp. 131–148. [Google Scholar]
- Anderson WG, Ali MF, Einarsdottir IE, Schaffer L, Hazon N, Conlon JM. Purification, characterization, and biological activity of insulins from the spotted dogfish, Scyliorhinus canicula, and the hammerhead shark, Sphyrna lewini. Gen Comp Endocrinol. 2002;126:113–122. doi: 10.1006/gcen.2002.7787. [DOI] [PubMed] [Google Scholar]
- Asolkar RN, Maskey RP, Helmke E, Laatsch H. Chalcomycin B, a new macrolide antibiotic from the marine isolate Streptomyces sp B7064. J Antibiot (Tokyo) 2002;55:893–898. doi: 10.7164/antibiotics.55.893. [DOI] [PubMed] [Google Scholar]
- Barsby T, Kelly MT, Gagne SM, Andersen RJ. Bogorol A produced in culture by a marine Bacillus sp reveals a novel template for cationic peptide antibiotics. Org Lett. 2001;3:437–440. doi: 10.1021/ol006942q. [DOI] [PubMed] [Google Scholar]
- Barsby T, Kelly MT, Andersen RJ. Tupuseleiamides and basiliskamides, new acyldipeptides and antifungal polyketides produced in culture by a Bacillus laterosporus isolate obtained from a tropical marine habitat. J Nat Prod. 2002;65:1447–1451. doi: 10.1021/np0201321. [DOI] [PubMed] [Google Scholar]
- Bunc M, Strupi-Suput J, Vodovnik A, Suput D. Toxic effects of head-to-tail 3-alkylpyridinium polymers isolated from the marine sponge Reniera sarai in rat. Toxicon. 2002;40:843–849. doi: 10.1016/s0041-0101(01)00147-7. [DOI] [PubMed] [Google Scholar]
- Carroll J, Jonsson EN, Ebel R, Hartman MS, Holman TR, Crews P. Probing sponge-derived terpenoids for human 15-lipoxygenase inhibitors. J Org Chem. 2001;66:6847–6851. doi: 10.1021/jo015784t. [DOI] [PubMed] [Google Scholar]
- Chinworrungsee M, Kittakoop P, Isaka M, Rungrod A, Tanticharoen M, Thebtaranonth Y. Antimalarial halorosellinic acid from the marine fungus Halorosellinia oceanica. Bioorg Med Chem Lett. 2001;11:1965–1969. doi: 10.1016/s0960-894x(01)00327-4. [DOI] [PubMed] [Google Scholar]
- Church JE, Hodgson WC. The pharmacological activity of fish venoms. Toxicon. 2002;40:1083–1093. doi: 10.1016/s0041-0101(02)00126-5. [DOI] [PubMed] [Google Scholar]
- Colquhoun DM. Nutraceuticals: vitamins and other nutrients in coronary heart disease. Curr Opin Lipidol. 2001;12:639–646. doi: 10.1097/00041433-200112000-00007. [DOI] [PubMed] [Google Scholar]
- Costantino V, Fattorusso E, Mangoni A. Glycolipids with immunomodulating activity from marine sponges. In: Tringali C, editor. Bioactive Compounds from Natural Sources: Isolation, Characterization and Biological Properties. Taylor and Francis; London: 2001. pp. 555–575. [Google Scholar]
- Cueto M, Jensen PR, Kauffman C, Fenical W, Lobkovsky E, Clardy J. Pestalone, a new antibiotic produced by a marine fungus in response to bacterial challenge. J Nat Prod. 2001;64:1444–1446. doi: 10.1021/np0102713. [DOI] [PubMed] [Google Scholar]
- Curman D, Cinel B, Williams DE, Rundle N, Block WD, Goodarzi AA, Hutchins JR, Clarke PR, Zhou BB, Lees-Miller SP, Andersen RJ, Roberge M. Inhibition of the G2 DNA damage checkpoint and of protein kinases Chk1 and Chk2 by the marine sponge alkaloid debromohymenialdisine. J Biol Chem. 2001;276:17914–17919. doi: 10.1074/jbc.M100728200. [DOI] [PubMed] [Google Scholar]
- Daferner M, Anke T, Sterner O. Zopfiellamides A and B, antimicrobial pyrrolidinone derivatives from the marine fungus Zopfiella latipes. Tetrahedron. 2002;58:7781–7784. [Google Scholar]
- Dal Piaz F, Casapullo A, Randazzo A, Riccio R, Pucci P, Marino G, Gomez-Paloma L. Molecular basis of phospholipase A2 inhibition by petrosaspongiolide M. Chembiochem. 2002;3:664–671. doi: 10.1002/1439-7633(20020703)3:7<664::AID-CBIC664>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Daranas AH, Norte M, Fernandez JJ. Toxic marine microalgae. Toxicon. 2001;39:1101–1132. doi: 10.1016/s0041-0101(00)00255-5. [DOI] [PubMed] [Google Scholar]
- de la Rosa LA, Alfonso A, Vilarino N, Vieytes MR, Botana LM. Modulation of cytosolic calcium levels of human lymphocytes by yessotoxin, a novel marine phycotoxin. Biochem Pharmacol. 2001;61:827–833. doi: 10.1016/s0006-2952(01)00549-4. [DOI] [PubMed] [Google Scholar]
- Diakov A, Koch JP, Ducoudret O, Muller-Berger S, Fromter E. The disulfonic stilbene DIDS and the marine poison maitotoxin activate the same two types of endogenous cation conductance in the cell membrane of Xenopus laevis oocytes. Pflugers Arch. 2001;442:700–708. doi: 10.1007/s004240100593. [DOI] [PubMed] [Google Scholar]
- Dorandeu F, Hesters R, Girard F, Four E, Foquin A, Bon C, Lallement G, Faure G. Inhibition of crotoxin phospholipase A(2) activity by manoalide associated with inactivation of crotoxin toxicity and dissociation of the heterodimeric neurotoxic complex. Biochem Pharmacol. 2002;63:755–761. doi: 10.1016/s0006-2952(01)00896-6. [DOI] [PubMed] [Google Scholar]
- Dounay AB, Forsyth CJ. Okadaic acid: the archetypal serine/threonine protein phosphatase inhibitor. Curr Med Chem. 2002;9:1939–1980. doi: 10.2174/0929867023368791. [DOI] [PubMed] [Google Scholar]
- Duarte ME, Noseda DG, Noseda MD, Tulio S, Pujol CA, Damonte EB. Inhibitory effect of sulfated galactans from the marine alga Bostrychia montagnei on herpes simplex virus replication in vitro. Phytomedicine. 2001;8:53–58. doi: 10.1078/0944-7113-00007. [DOI] [PubMed] [Google Scholar]
- Dutton JL, Craik DJ. Alpha-conotoxins: nicotinic acetylcholine receptor antagonists as pharmacological tools and potential drug leads. Curr Med Chem. 2001;8:327–344. doi: 10.2174/0929867013373453. [DOI] [PubMed] [Google Scholar]
- Edrada RA, Ebel R, Supriyono A, Wray V, Schupp P, Steube K, Van Soest R, Proksch P. Swinhoeiamide A, a new highly active calyculin derivative from the marine sponge Theonella swinhoei. J Nat Prod. 2002a;65:1168–1172. doi: 10.1021/np020049d. [DOI] [PubMed] [Google Scholar]
- Edrada RA, Heubes M, Brauers G, Wray V, Berg A, Grafe U, Wohlfarth M, Muhlbacher J, Schaumann K, Sudarsono S, Bringmann G, Proksch P. Online analysis of xestodecalactones AC, novel bioactive metabolites from the fungus Penicillium cfmontanense and their subsequent isolation from the sponge Xestospongia exigua. J Nat Prod. 2002b;65:1598–1604. doi: 10.1021/np020085c. [DOI] [PubMed] [Google Scholar]
- El Sayed KA, Kelly M, Kara UA, Ang KK, Katsuyama I, Dunbar DC, Khan AA, Hamann MT. New manzamine alkaloids with potent activity against infectious diseases. J Am Chem Soc. 2001;123:1804–1808. doi: 10.1021/ja002073o. [DOI] [PubMed] [Google Scholar]
- El Sayed KA, Yousaf M, Hamann MT, Avery MA, Kelly M, Wipf P. Microbial and chemical transformation studies of the bioactive marine sesquiterpenes (S)-(+)-curcuphenol and -curcudiol isolated from a deep reef collection of the Jamaican sponge Didiscus oxeata. J Nat Prod. 2002;65:1547–1553. doi: 10.1021/np020213x. [DOI] [PubMed] [Google Scholar]
- Ermakova SP, Burtseva YV, Sova VV, Kratchun VV, Zvyagintseva TN. Proteins of brown seaweeds as inhibitors of endo-1-3-beta-D-glucanases of marine invertebrates. Biochemistry (Mosc) 2001;66:188–194. doi: 10.1023/a:1002895632026. [DOI] [PubMed] [Google Scholar]
- Faulkner DJ. Marine natural products. Nat Prod Rep. 2001;18:1–49. doi: 10.1039/b006897g. [DOI] [PubMed] [Google Scholar]
- Faulkner DJ. Marine natural products. Nat Prod Rep. 2002;19:1–48. doi: 10.1039/b009029h. [DOI] [PubMed] [Google Scholar]
- Fenical W, Sethna KM, Lloyd GK. Marine microorganisms as a developing resource for drug discovery. Pharm News. 2002;9:489–494. [Google Scholar]
- Fernandez JJ, Candenas ML, Souto ML, Trujillo MM, Norte M. Okadaic acid, useful tool for studying cellular processes. Curr Med Chem. 2002;9:229–262. doi: 10.2174/0929867023371247. [DOI] [PubMed] [Google Scholar]
- Fontana A, Cimino G, Gavagnin M, Gonzalez MC, Estornell E. Novel inhibitors of mitochondrial respiratory chain: endoperoxides from the marine tunicate Stolonica socialis. J Med Chem. 2001;44:2362–2365. doi: 10.1021/jm0011373. [DOI] [PubMed] [Google Scholar]
- Frey MR, Leontieva O, Watters DJ, Black JD. Stimulation of protein kinase C-dependent and -independent signaling pathways by bistratene A in intestinal epithelial cells. Biochem Pharmacol. 2001;61:1093–1100. doi: 10.1016/s0006-2952(01)00596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Matsunaga K, Saito M, Hagiya S, Furukawa K, Nakamura H, Ohizumi Y. Halenaquinone, a novel phosphatidylinositol 3-kinase inhibitor from a marine sponge, induces apoptosis in PC12 cells. Eur J Pharmacol. 2001;413:37–45. doi: 10.1016/s0014-2999(00)00944-4. [DOI] [PubMed] [Google Scholar]
- Garcia-Fernandez LF, Reyes F, Sanchez-Puelles JM. The marine pharmacy: new antitumoral compounds from the sea. Pharm News. 2002;9:495–501. [Google Scholar]
- Gochfeld DJ, Hamann MT. Isolation and biological evaluation of filiformin, plakortide F, and plakortone G from the Caribbean sponge Plakortis sp. J Nat Prod. 2001;64:1477–1479. doi: 10.1021/np010216u. [DOI] [PubMed] [Google Scholar]
- Gorshkova IA, Gorshkov BA, Fedoreev SA, Stonik VA. Halenaquinol, a natural cardioactive pentacyclic hydroquinone, interacts with sulfhydryls on rat brain Na(+), K(+)–ATPase. Comp Biochem Physiol C, Comp Pharmacol Toxicol. 2001;128:531–540. doi: 10.1016/s1532-0456(01)00175-2. [DOI] [PubMed] [Google Scholar]
- Higa T, Tanaka J, Ohtani II, Musman M, Roy MC, Kuroda I. Bioactive compounds from coral reef invertebrates. Pure Appl Chem. 2001;73:589–593. [Google Scholar]
- Hokama Y, Yoshikawa-Ebesu JSM. Ciguatera fish poisoning: a foodborne disease [Review] J Toxicol, Toxin Rev. 2001;20:85–139. [Google Scholar]
- Hu JF, Schetz JA, Kelly M, Peng JN, Ang KK, Flotow H, Leong CY, Ng SB, Buss AD, Wilkins SP, Hamann MT. New antiinfective and human 5-HT2 receptor binding natural and semi-synthetic compounds from the Jamaican sponge Smenospongia aurea. J Nat Prod. 2002;65:476–480. doi: 10.1021/np010471e. [DOI] [PubMed] [Google Scholar]
- Isaka M, Suyarnsestakorn C, Tanticharoen M, Kongsaeree P, Thebtaranonth Y. Aigialomycins A–E, new resorcylic macrolides from the marine mangrove fungus Aigialus parvus. J Org Chem. 2002;67:1561–1566. doi: 10.1021/jo010930g. [DOI] [PubMed] [Google Scholar]
- Iwata S, Saito S, Kon-ya K, Shizuri Y, Ohizumi Y. Novel marine-derived halogen-containing gramine analogues induce vaso-relaxation in isolated rat aorta. Eur J Pharmacol. 2001;432:63–70. doi: 10.1016/s0014-2999(01)01476-5. [DOI] [PubMed] [Google Scholar]
- Jadulco R, Proksch P, Wray V, Sudarsono Berg A, Grafe U. New macrolides and furan carboxylic acid derivative from the sponge-derived fungus Cladosporium herbarum. J Nat Prod. 2001;64:527–530. doi: 10.1021/np000401s. [DOI] [PubMed] [Google Scholar]
- Jang WS, Kim KN, Lee YS, Nam MH, Lee IH. Halocidin: a new antimicrobial peptide from hemocytes of the solitary tunicate, Halocynthia aurantium. FEBS Lett. 2002;521:81–86. doi: 10.1016/s0014-5793(02)02827-2. [DOI] [PubMed] [Google Scholar]
- Jeong SJ, Higuchi R, Miyamoto T, Ono M, Kuwano M, Mawatari SF. Bryoanthrathiophene, a new antiangiogenic constituent from the bryozoan Watersipora subtorquata (d’Orbigny, 1852) J Nat Prod. 2002;65:1344–1345. doi: 10.1021/np010577+. [DOI] [PubMed] [Google Scholar]
- Jimenez EC, Donevan S, Walker C, Zhou LM, Nielsen J, Cruz LJ, Armstrong H, White HS, Olivera BM. Conantokin-L, a new NMDA receptor antagonist: determinants for anticonvulsant potency. Epilepsy Res. 2002;51:73–80. doi: 10.1016/s0920-1211(02)00101-8. [DOI] [PubMed] [Google Scholar]
- Juge M, Grimaud N, Biard JF, Sauviat MP, Nabil M, Verbist JF, Petit JY. Cardiovascular effects of lepadiformine, an alkaloid isolated from the ascidians Clavelina lepadiformis (Muller) and C. moluccensis (Sluiter) Toxicon. 2001;39:1231–1237. doi: 10.1016/s0041-0101(01)00079-4. [DOI] [PubMed] [Google Scholar]
- Kaji T, Fujiwara Y, Inomata Y, Hamada C, Yamamoto C, Shimada S, Lee JB, Hayashi T. Repair of wounded monolayers of cultured bovine aortic endothelial cells is inhibited by calcium spirulan, a novel sulfated polysaccharide isolated from Spirulina platensis. Life Sci. 2002;70:1841–1848. doi: 10.1016/s0024-3205(01)01555-7. [DOI] [PubMed] [Google Scholar]
- Lazaro JE, Nitcheu J, Predicala RZ, Mangalindan GC, Nesslany F, Marzin D, Concepcion GP, Diquet B. Heptyl prodigiosin, a bacterial metabolite, is antimalarial in vivo and non-mutagenic in vitro. J Nat Toxins. 2002;11:367–377. [PubMed] [Google Scholar]
- Lee IH, Lee YS, Kim CH, Kim CR, Hong T, Menzel L, Boo LM, Pohl J, Sherman MA, Waring A, Lehrer RI. Dicynthaurin: an antimicrobial peptide from hemocytes of the solitary tunicate, Halocynthia aurantium. Biochim Biophys Acta. 2001;1527:141–148. doi: 10.1016/s0304-4165(01)00156-8. [DOI] [PubMed] [Google Scholar]
- Leira F, Cabado AG, Vieytes MR, Roman Y, Alfonso A, Botana LM, Yasumoto T, Malaguti C, Rossini GP. Characterization of F-actin depolymerization as a major toxic event induced by pectenotoxin-6 in neuroblastoma cells. Biochem Pharmacol. 2002;63:1979–1988. doi: 10.1016/s0006-2952(02)00993-0. [DOI] [PubMed] [Google Scholar]
- Li WI, Berman FW, Okino T, Yokokawa F, Shioiri T, Gerwick WH, Murray TF. Antillatoxin is a marine cyanobacterial toxin that potently activates voltage-gated sodium channels. Proc Natl Acad Sci U S A. 2001;98:7599–7604. doi: 10.1073/pnas.121085898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linington RG, Robertson M, Gauthier A, Finlay BB, Van Soest R, Andersen RJ. Caminoside A, an antimicrobial glycolipid isolated from the marine sponge Caminus sphaeroconia. Org Lett. 2002;4:4089–4092. doi: 10.1021/ol0268337. [DOI] [PubMed] [Google Scholar]
- Liu CH, Meng JC, Zou WX, Huang LL, Tang HQ, Tan RX. Antifungal metabolite with a new carbon skeleton from Keissleriella sp Y54108, a marine filamentous fungus. Planta Med. 2002a;68:363–365. doi: 10.1055/s-2002-26756. [DOI] [PubMed] [Google Scholar]
- Liu RS, Yang JH, Liu WY. Isolation and enzymatic characterization of lamjapin, the first ribosome-inactivating protein from cryptogamic algal plant (Laminaria japonica A) Eur J Biochem. 2002b;269:4746–4752. doi: 10.1046/j.1432-1033.2002.03165.x. [DOI] [PubMed] [Google Scholar]
- Loukaci A, Le SI, Samadi M, Leclerc S, Damiens E, Meijer L, Debitus C, Guyot M. Coscinosulfate, a CDC25 phosphatase inhibitor from the sponge Coscinoderma mathewsi. Bioorg Med Chem. 2001;9:3049–3054. doi: 10.1016/s0968-0896(01)00208-5. [DOI] [PubMed] [Google Scholar]
- Loya S, Rudi A, Kashman Y, Hizi A. Mode of inhibition of HIV-1 reverse transcriptase by polyacetylenetriol, a novel inhibitor of RNA- and DNA-directed DNA polymerases. Biochem J. 2002;362:685–692. doi: 10.1042/0264-6021:3620685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez BL, Watts KS, Yokochi A, Roberts MA, Verdier-Pinard P, Jimenez JI, Hamel E, Scheuer PJ, Gerwick WH. Structure and absolute stereochemistry of hectochlorin, a potent stimulator of actin assembly. J Nat Prod. 2002;65:866–871. doi: 10.1021/np0106283. [DOI] [PubMed] [Google Scholar]
- Matou S, Helley D, Chabut D, Bros A, Fischer AM. Effect of fucoidan on fibroblast growth factor-2-induced angiogenesis in vitro. Thromb Res. 2002;106:213–221. doi: 10.1016/s0049-3848(02)00136-6. [DOI] [PubMed] [Google Scholar]
- Mayer AMS. Current marine pharmacology contributions to new drug development in the biopharmaceutical industry. Pharm News. 2002;9:479–482. [Google Scholar]
- Mayer AMS, Hamann MT. Marine pharmacology in 1999: compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities; affecting the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Comp Biochem Physiol C, Comp Pharmacol Toxicol. 2002;132:315–339. doi: 10.1016/s1532-0456(02)00094-7. [DOI] [PubMed] [Google Scholar]
- Mayer AMS, Hamann MT. Marine pharmacology in 2000: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar Biotechnol (NY) 2004;6:37–52. doi: 10.1007/s10126-003-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AMS, Lehmann VKB. Marine pharmacology in 1998: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, antiplatelet, antiprotozoal, and antiviral activities; with actions on the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Pharmacologist. 2000;42:62–69. [Google Scholar]
- Mayer AM, Hall M, Fay MJ, Lamar P, Pearson C, Prozialeck WC, Lehmann VK, Jacobson PB, Romanic AM, Uz T, Manev H. Effect of a short-term in vitro exposure to the marine toxin domoic acid on viability, tumor necrosis factor-alpha, matrix metal-loproteinase-9 and superoxide anion release by rat neonatal microglia. BMC Pharmacol. 2001;1:7. doi: 10.1186/1471-2210-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Makioka A, Kawazu S, Kano S, Kawai S, Akaki M, Aikawa M, Ohtomo H. Effect of jasplakinolide on the growth, invasion, and actin cytoskeleton of Plasmodium falciparum. Parasitol Res. 2002;88:844–848. doi: 10.1007/s00436-002-0666-8. [DOI] [PubMed] [Google Scholar]
- Mohapatra BR, Bapuji M, Sree A. Antifungal efficacy of bacteria isolated from marine sedentary organisms. Folia Microbiol (Praha) 2002;47:51–55. doi: 10.1007/BF02818565. [DOI] [PubMed] [Google Scholar]
- Morales-Tlalpan V, Vaca L. Modulation of the maitotoxin response by intracellular and extracellular cations. Toxicon. 2002;40:493–500. doi: 10.1016/s0041-0101(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Murray AP, Muniain C, Seldes AM, Maier MS. Patagonicoside A: a novel antifungal disulfated triterpene glycoside from the sea cucumber Psolus patagonicus. Tetrahedron. 2001;57:9563–9568. [Google Scholar]
- Nakao Y, Takada K, Matsunaga S, Fusetani N. Calyceramides A–C: neuraminidase inhibitory sulfated ceramides from the marine sponge Discodermia calyx. Tetrahedron. 2001;57:3013–3017. [Google Scholar]
- Napolitano A, Bruno I, Rovero P, Lucas R, Peris MP, Gomez-Paloma L, Riccio R. Synthesis, structural aspects and bioactivity of the marine cyclopeptide hymenamide C. Tetrahedron. 2001;57:6249–6255. doi: 10.1002/psc.396. [DOI] [PubMed] [Google Scholar]
- Napolitano A, Bruno I, Rovero P, Lucas R, Peris MP, Gomez-Paloma L, Riccio R. Synthesis and biological properties of the seven alanine-modified analogues of the marine cyclopeptide hymenamide C. J Pept Sci. 2002;8:407–417. doi: 10.1002/psc.396. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Matsunaga S, Shibazaki M, Suzuki K, Harada N, Naoki H, Fusetani N. Corticatic acids D and E, polyacetylenic geranylgeranyltransferase type I inhibitors, from the marine sponge Petrosia corticata. J Nat Prod. 2002;65:1353–1356. doi: 10.1021/np020080f. [DOI] [PubMed] [Google Scholar]
- Nogle LM, Okino T, Gerwick WH. Antillatoxin B, a neurotoxic lipopeptide from the marine cyanobacterium Lyngbya majuscula. J Nat Prod. 2001;64:983–985. doi: 10.1021/np010107f. [DOI] [PubMed] [Google Scholar]
- O’Keefe BR. Biologically active proteins from natural product extracts. J Nat Prod. 2001;64:1373–1381. doi: 10.1021/np0103362. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Nitanda N, Ojika M, Sakagami Y. Aplysiallene, a new bromoallene as an Na, K–ATPase inhibitor from the sea hare, Aplysia kurodai. Biosci Biotechnol Biochem. 2001a;65:474–476. doi: 10.1271/bbb.65.474. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Ojika M, Suzuki S, Murakami M, Sakagami Y. Iantherans A and B, unique dimeric polybrominated benzofurans as Na, K–ATPase inhibitors from a marine sponge, Ianthella sp. Bioorg Med Chem. 2001b;9:179–183. doi: 10.1016/s0968-0896(00)00234-0. [DOI] [PubMed] [Google Scholar]
- Olivera BM, Cruz LJ. Conotoxins, in retrospect [Review] Toxicon. 2001;39:7–14. doi: 10.1016/s0041-0101(00)00157-4. [DOI] [PubMed] [Google Scholar]
- Omura S, Miyadera H, Ui H, Shiomi K, Yamaguchi Y, Masuma R, Nagamitsu T, Takano D, Sunazuka T, Harder A, Kolbl H, Namikoshi M, Miyoshi H, Sakamoto K, Kita K. An anthelmintic compound, nafuredin, shows selective inhibition of complex I in helminth mitochondria. Proc Natl Acad Sci U S A. 2001;98:60–62. doi: 10.1073/pnas.011524698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Hori M, Kim YS, Kwon SC, Ahn DS, Nakazawa H, Kobayashi M, Karaki H. Inhibitory mechanism of xestospongin-C on contraction and ion channels in the intestinal smooth muscle. Br J Pharmacol. 2002;137:1207–1212. doi: 10.1038/sj.bjp.0704988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavao MS. Structure and anticoagulant properties of sulfated glycosaminoglycans from primitive Chordates. An Acad Bras Cienc. 2002;74:105–112. doi: 10.1590/s0001-37652002000100007. [DOI] [PubMed] [Google Scholar]
- Peng JN, Walsh K, Weedman V, Bergthold JD, Lynch J, Lieu KL, Braude IA, Kelly M, Hamann MT. The new bioactive diterpenes cyanthiwigins E–AA from the Jamaican sponge Myrmekioderma styx. Tetrahedron. 2002;58:7809–7819. [Google Scholar]
- Pereira MS, Vilela-Silva AC, Valente AP, Mourao PA. A 2-sulfated, 3-linked alpha-L-galactan is an anticoagulant polysaccharide. Carbohydr Res. 2002;337:2231–2238. doi: 10.1016/s0008-6215(02)00215-x. [DOI] [PubMed] [Google Scholar]
- Perpelescu M, Kobayashi J, Furuta M, Ito Y, Izuta S, Takemura M, Suzuki M, Yoshida S. Novel phenalenone derivatives from a marine-derived fungus exhibit distinct inhibition spectra against eukaryotic DNA polymerases. Biochemistry. 2002;41:7610–7616. doi: 10.1021/bi020115a. [DOI] [PubMed] [Google Scholar]
- Perry TL, Dickerson A, Khan AA, Kondru RK, Beratan DN, Wipf P, Kelly M, Hamann MT. New peroxylactones from the Jamaican sponge Plakinastrella onkodes, with inhibitory activity against the AIDS opportunistic parasitic infection Toxoplasma gondii. Tetrahedron. 2001;57:1483–1487. [Google Scholar]
- Pindur U, Lemster T. Advances in marine natural products of the indole and annelated indole series: chemical and biological aspects. Curr Med Chem. 2001;8:1681–1698. doi: 10.2174/0929867013371941. [DOI] [PubMed] [Google Scholar]
- Preeprame S, Hayashi K, Lee JB, Sankawa U, Hayashi T. A novel antivirally active fucan sulfate derived from an edible brown alga, Sargassum horneri. Chem Pharm Bull (Tokyo) 2001;49:484–485. doi: 10.1248/cpb.49.484. [DOI] [PubMed] [Google Scholar]
- Qi J, Ojika M, Sakagami Y. Linckosides A and B, two new neuritogenic steroid glycosides from the Okinawan starfish Linckia laevigata. Bioorg Med Chem. 2002;10:1961–1966. doi: 10.1016/s0968-0896(02)00006-8. [DOI] [PubMed] [Google Scholar]
- Rabah D, Grant S, Ma C, Conrad DH. Bryostatin-1 specifically inhibits in vitro IgE synthesis. J Immunol. 2001;167:4910–4918. doi: 10.4049/jimmunol.167.9.4910. [DOI] [PubMed] [Google Scholar]
- Randazzo A, Bifulco G, Giannini C, Bucci M, Debitus C, Cirino G, Gomez-Paloma L. Halipeptins A and B: two novel potent anti-inflammatory cyclic depsipeptides from the Vanuatu marine sponge Haliclona species. J Am Chem Soc. 2001;123:10870–10876. doi: 10.1021/ja010015c. [DOI] [PubMed] [Google Scholar]
- Rao PV, Gupta N, Bhaskar AS, Jayaraj R. Toxins and bioactive compounds from cyanobacteria and their implications on human health. J Environ Biol. 2002;23:215–224. [PubMed] [Google Scholar]
- Rashid MA, Gustafson KR, Cartner LK, Shigematsu N, Pannell LK, Boyd MR. Microspinosamide, a new HIV-inhibitory cyclic depsipeptide from the marine sponge Sidonops microspinosa. J Nat Prod. 2001;64:117–121. doi: 10.1021/np0002379. [DOI] [PubMed] [Google Scholar]
- Reshef V, Carmeli S. Protease inhibitors from a water bloom of the cyanobacterium Microcystis aeruginosa. Tetrahedron. 2001;57:2885–2894. doi: 10.1021/np900340t. [DOI] [PubMed] [Google Scholar]
- Rodriguez AD, Ramirez C. Serrulatane diterpenes with anti-mycobacterial activity isolated from the West Indian sea whip Pseudopterogorgia elisabethae. J Nat Prod. 2001;64:100–102. doi: 10.1021/np000196g. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Nieto S, Gonzalez-Iriarte M, Carmona R, Munoz-Chapuli R, Medina MA, Quesada AR. Antiangiogenic activity of aeroplysinin-1, a brominated compound isolated from a marine sponge. FASEB J. 2002;16:261–263. doi: 10.1096/fj.01-0427fje. [DOI] [PubMed] [Google Scholar]
- Roman Y, Alfonso A, Louzao MC, de la Rosa LA, Leira F, Vieites JM, Vieytes MR, Ofuji K, Satake M, Yasumoto T, Botana LM. Azaspiracid-1, a potent, nonapoptotic new phycotoxin with several cell targets. Cell Signal. 2002;14:703–716. doi: 10.1016/s0898-6568(02)00015-3. [DOI] [PubMed] [Google Scholar]
- Rowley DC, Hansen MS, Rhodes D, Sotriffer CA, Ni H, McCammon JA, Bushman FD, Fenical W. Thalassiolins A–C: new marine-derived inhibitors of HIV cDNA integrase. Bioorg Med Chem. 2002;10:3619–3625. doi: 10.1016/s0968-0896(02)00241-9. [DOI] [PubMed] [Google Scholar]
- Rudi A, Yosief T, Loya S, Hizi A, Schleyer M, Kashman Y. Clathsterol, a novel anti-HIV-1 RT sulfated sterol from the sponge Clathria species. J Nat Prod. 2001;64:1451–1453. doi: 10.1021/np010121s. [DOI] [PubMed] [Google Scholar]
- Sakai R, Koike T, Sasaki M, Shimamoto K, Oiwa C, Yano A, Suzuki K, Tachibana K, Kamiya H. Isolation, structure determination, and synthesis of neodysiherbaine a, a new excitatory amino acid from a marine sponge. Org Lett. 2001a;3:1479–1482. doi: 10.1021/ol015798l. [DOI] [PubMed] [Google Scholar]
- Sakai R, Swanson GT, Shimamoto K, Green T, Contractor A, Ghetti A, Tamura-Horikawa Y, Oiwa C, Kamiya H. Pharmacological properties of the potent epileptogenic amino acid dysiherbaine, a novel glutamate receptor agonist isolated from the marine sponge Dysidea herbacea. J Pharmacol Exp Ther. 2001b;296:650–658. [PubMed] [Google Scholar]
- Sato K, Horibe K, Amano K, Mitusi-Saito M, Hori M, Matsunaga S, Fusetani N, Ozaki H, Karaki H. Membrane permeabilization induced by discodermin A, a novel marine bioactive peptide. Toxicon. 2001;39:259–264. doi: 10.1016/s0041-0101(00)00123-9. [DOI] [PubMed] [Google Scholar]
- Schlingmann G, Milne L, Carter GT. Isolation and identification of antifungal polyesters from the marine fungus Hypoxylon oceanicum LL-15G256. Tetrahedron. 2002;58:6825–6835. [Google Scholar]
- Schmitz FJ, Bowden BF, Toth SI. Antitumor and cytotoxic compounds from marine organisms. In: Attaway DH, Zaborsky OR, editors. Marine Biotechnology, Pharmaceutical and Bioactive Natural Products. Vol. 1. Plenum Press; New York: 2003. pp. 197–308. [Google Scholar]
- Sennett SH, McCarthy PJ, Wright AE, Pomponi SA. Natural products from marine invertebrates: the harbor branch oceanographic institution experience. Pharm News. 2002;9:483–488. [Google Scholar]
- Shin J, Rho JR, Seo Y, Lee HS, Cho KW, Kwon HJ, Sim CJ. Wondonins A and B, new bis(dihydroxystyryl)imidazoles from a two-sponge association. Tetrahedron Lett. 2001;42:1965–1968. [Google Scholar]
- Son BW, Kim JC, Choi HD, Kang JS. A radical scavenging farnesylhydroquinone from a marine-derived fungus Penicillium sp. Arch Pharm Res. 2002;25:77–79. doi: 10.1007/BF02975266. [DOI] [PubMed] [Google Scholar]
- Stevenson CS, Capper EA, Roshak AK, Marquez B, Eichman C, Jackson JR, Mattern M, Gerwick WH, Jacobs RS, Marshall LA. The identification and characterization of the marine natural product scytonemin as a novel antiproliferative pharmacophore. J Pharmacol Exp Ther. 2002a;303:858–866. doi: 10.1124/jpet.102.036350. [DOI] [PubMed] [Google Scholar]
- Stevenson CS, Capper EA, Roshak AK, Marquez B, Grace K, Gerwick WH, Jacobs RS, Marshall LA. Scytonemin—a marine natural product inhibitor of kinases key in hyperproliferative inflammatory diseases. Inflamm Res. 2002b;51:112–114. doi: 10.1007/BF02684014. [DOI] [PubMed] [Google Scholar]
- Suput D, Frangez R, Bunc M. Cardiovascular effects of equinatoxin III from the sea anemone Actinia equina (L.) Toxicon. 2001;39:1421–1427. doi: 10.1016/s0041-0101(01)00102-7. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Daitoh M, Vairappan CS, Abe T, Masuda M. Novel halogenated metabolites from the Malaysian Laurencia pannosa. J Nat Prod. 2001;64:597–602. doi: 10.1021/np0006317. [DOI] [PubMed] [Google Scholar]
- Takada N, Watanabe R, Suenaga K, Yamada K, Ueda K, Kita M, Uemura D. Zamamistatin, a significant antibacterial bromotyrosine derivative, from the Okinawan sponge Pseudoceratina purpurea. Tetrahedron Lett. 2001;42:5265–5267. [Google Scholar]
- Tian B, Kiland JA, Kaufman PL. Effects of the marine macrolides swinholide A and jasplakinolide on outflow facility in monkeys. Invest Ophthalmol Vis Sci. 2001;42:3187–3192. [PubMed] [Google Scholar]
- Torres YR, Berlinck RG, Nascimento GG, Fortier SC, Pessoa C, de Moraes MO. Antibacterial activity against resistant bacteria and cytotoxicity of four alkaloid toxins isolated from the marine sponge Arenosclera brasiliensis. Toxicon. 2002;40:885–891. doi: 10.1016/s0041-0101(01)00286-0. [DOI] [PubMed] [Google Scholar]
- Trento F, Cattaneo F, Pescador R, Porta R, Ferro L. Antithrombin activity of an algal polysaccharide. Thromb Res. 2001;102:457–465. doi: 10.1016/s0049-3848(01)00264-x. [DOI] [PubMed] [Google Scholar]
- Uchida R, Tomoda H, Arai M, Omura S. Chlorogentisylquinone, a new neutral sphingomyelinase inhibitor, produced by a marine fungus. J Antibiot (Tokyo) 2001;54:882–889. doi: 10.7164/antibiotics.54.882. [DOI] [PubMed] [Google Scholar]
- Vairappan CS, Daitoh M, Suzuki M, Abe T, Masuda M. Antibacterial halogenated metabolites from the Malaysian Laurencia species. Phytochemistry. 2001;58:291–297. doi: 10.1016/s0031-9422(01)00243-6. [DOI] [PubMed] [Google Scholar]
- Vuong D, Capon RJ, Lacey E, Gill JH, Heiland K, Friedel T. Onnamide F: a new nematocide from a southern Australian marine sponge, Trachycladus laevispirulifer. J Nat Prod. 2001;64:640–642. doi: 10.1021/np000474b. [DOI] [PubMed] [Google Scholar]
- Wagner-Dobler I, Beil W, Lang S, Meiners M, Laatsch H. Integrated approach to explore the potential of marine microorganisms for the production of bioactive metabolites. Adv Biochem Eng Biotechnol. 2002;74:207–238. doi: 10.1007/3-540-45736-4_10. [DOI] [PubMed] [Google Scholar]
- Wittekindt B, Malany S, Schemm R, Otvos L, Maccecchini ML, Laube B, Betz H. Point mutations identify the glutamate binding pocket of the N-methyl-D-aspartate receptor as major site of conantokin-G inhibition. Neuropharmacology. 2001;41:753–761. doi: 10.1016/s0028-3908(01)00112-5. [DOI] [PubMed] [Google Scholar]
- Wright AD, Goclik E, Konig GM, Kaminsky R. Lepadins D–F: antiplasmodial and antitrypanosomal decahydroquinoline derivatives from the tropical marine tunicate Didemnum sp. J Med Chem. 2002;45:3067–3072. doi: 10.1021/jm0110892. [DOI] [PubMed] [Google Scholar]
- Yamada K, Matsubara R, Kaneko M, Miyamoto T, Higuchi R. Constituents of holothuroidea: 10. Isolation and structure of a biologically active ganglioside molecular species from the sea cucumber Holothuria leucospilota. Chem Pharm Bull. 2001;49:447–452. doi: 10.1248/cpb.49.447. [DOI] [PubMed] [Google Scholar]
- Yasumoto T. The chemistry and biological function of natural marine toxins. Chem Rec-Age. 2001;1:228–242. doi: 10.1002/tcr.1010. [DOI] [PubMed] [Google Scholar]