Abstract
Purpose
We sought to evaluate the effectiveness of bone substitutes in circumferential peri-implant defects created in the rabbit tibia.
Methods
Thirty rabbits received 45 implants in their left and right tibia. A circumferential bone defect (6.1 mm in diameter/4 mm depth) was created in each rabbit tibia using a trephine bur. A dental implant (4.1 mm × 8.5 mm) was installed after the creation of the defect, providing a 2-mm gap. The bone defect gaps between the implant and the bone were randomly filled according to the following groups: blood clot (CO), particulate Bio-Oss® (BI), and Bio-Oss® Collagen (BC). Ten animals were euthanized after periods of 15, 30, and 60 days. Biomechanical analysis by means of the removal torque of the implants, as well as histologic and immunohistochemical analyses for protein expression of osteocalcin (OC), Runx2, OPG, RANKL, and TRAP were evaluated.
Results
For biomechanics, BC showed a better biological response (61.00±15.28 Ncm) than CO (31.60±14.38 Ncm) at 30 days. Immunohistochemical analysis showed significantly different OC expression in CO and BC at 15 days, and also between the CO and BI groups, and between the CO and BC groups at 60 days. After 15 days, Runx2 expression was significantly different in the BI group compared to the CO and BC groups. RANKL expression was significantly different in the BI and CO groups and between the BI and BC groups at 15 days, and also between the BI and CO groups at 60 days. OPG expression was significantly higher at 60 days postoperatively in the BI group than the CO group.
Conclusions
Collectively, our data indicate that, compared to CO and BI, BC offered better bone healing, which was characterized by greater RUNX2, OC, and OPG immunolabeling, and required greater reversal torque for implant removal. Indeed, along with BI, BC presents promising biomechanical and biological properties supporting its possible use in osteoconductive grafts for filling peri-implant gaps.
Keywords: Bone substitutes, Bone transplantation, Dental implantation, Osseointegration, Rabbits
Graphical Abstract
INTRODUCTION
Tooth extraction results in significant resorption of the alveolar ridge [1]. Some studies [2,3] have revealed that the alveolar ridge reduces by approximately 0.34 mm to 7.7 mm horizontally and 0.22 mm to 3.25 mm vertically, 6-12 months after tooth extraction. Moreover, this resorption is more severe in the first 3 months.
In areas of bone resorption, satisfactory esthetic and functional results are challenging for delayed loading of dental implants [3]. To overcome this limitation, Schulte and Heimke [4] described the insertion of implants in fresh extraction sockets and classified this procedure as immediate dental implant placement. However, considering that after tooth extraction the alveolar socket is usually larger than the implant diameter, a marginal gap between bone crest wall and the implant surface is commonly observed, which could result in marked dimensional changes in the alveolar walls with respect to thickness and height [5,6,7]. Depending on the gap size, osseointegration can be achieved with or without the use of bone substitutes. The peri-implant marginal defect is usually widest in its marginal part and demands osseous healing from the defect walls to the implant surface. A previous study [8] evaluated a smooth surface implant placed in peri-implant bone defects in dogs. The results indicate that bone regeneration was found in a 0.5-mm wide defect gap. On the other hand, Botticelli et al. [9] demonstrated that spontaneous bone fill could occur after 4 months in circumferential gap defects up to 1.25 mm in combination with a rough surface implant (SLA) and guided tissue regeneration (barrier membrane). However, a wider circumferential peri-implant bone defect (above 1.25 mm) could not be histologically restored without the use of various bone-regenerative techniques and materials to encourage bone growth within these defects [10].
Several materials have been described in the literature to fill peri-implant gaps surrounding dental implants in an attempt to increase the reliability of the treatment. In this context, a marginal gap, extraction socket, and peri-implant gap can be filled with bone substitutes including autogenous, homogenous, heterogeneous, or alloplastic bone. In recent years, Bio-Oss® Collagen (Geistlich Pharma AB, Wolhusen, Switzerland), a mineralized bovine bone matrix with the addition of 10% porcine collagen, has been highlighted as a bone substitute option in which the addition of collagen provides pro-angiogenic qualities, accelerated growth, and maturation and proliferation of the endothelial cells, which promotes physiological regeneration [11]. Several studies [12,13,14] in the scientific literature have been performed evaluating Bio-Oss Collagen to fill extraction sockets and peri-implant gaps in pre-clinical and clinical studies. Currently, studies using this biomaterial for marginal gap filling have not yet provided much information regarding bone healing, especially in short periods of time [12,14]. Moreover, no report has addressed the dynamics of tissue repair and biomechanics of Bio-Oss Collagen measured over different periods of evaluation.
Therefore, considering the need for better understanding of the dynamics of tissue repair using Bio-Oss® Collagen, we aimed to evaluate peri-implant circumferential bone gap defects created in the rabbit tibia that were filled with a blood clot (CO), particulate Bio-Oss® (BI) (Geistlich Pharma AB, Wolhusen, Switzerland), or Bio-Oss® Collagen (BC). The rationale for this study is that particulate Bio-Oss® plays major role in bone regeneration, and thus we want to compare it with Bio-Oss® Collagen to evaluate the actual capability of this material to improve peri-implant bone formation for implants placed after osteotomies that resulted in circumferential gap defects. In this regard, our hypothesis was that Bio-Oss® Collagen would favor the healing process in circumferential gap defects of 2 mm around osseointegrated implants when compared to blood clot and particulate Bio-Oss®. To achieve this goal, biomechanical, histological, and immunohistochemical analyses for detection of osteocalcin (OC), runt-related transcription factor 2 (RUNX 2), osteoprotegerin (OPG), receptor activator of nuclear factor kappa B-ligand (RANKL) and tartrate-resistant acid phosphatase (TRAP) proteins were evaluated in this study after 15, 30, and 60 days postoperatively.
MATERIAL AND METHODS
Animals
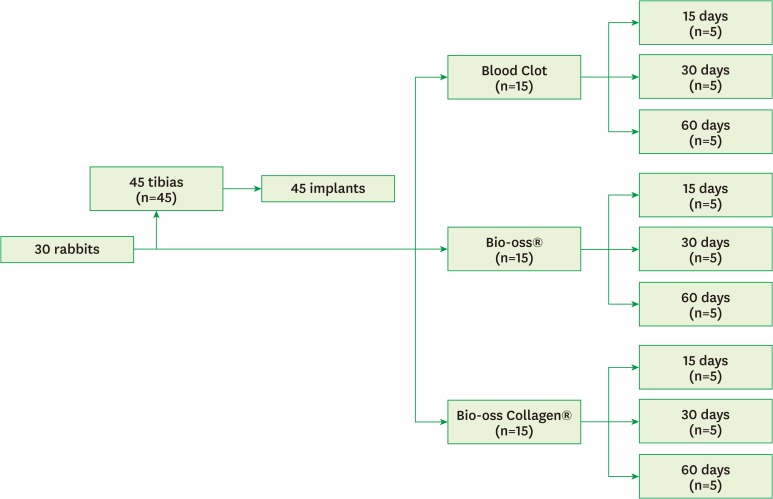
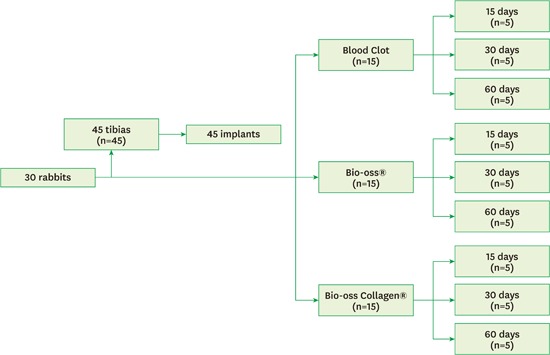
For our animal experiment, the sample size calculation was based on previously published papers [15,16,17,18,19,20]. We followed a randomized, prospective, controlled, animal model design following all the recommendations of the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines for the execution and submission of studies in animals [21]. All rabbits were housed in animal facilities at 25°C, in 12-hour light:dark cycles. Throughout the experimental period, the rabbits were housed in individual plastic cages, and a normal chow diet and water were provided ad libitum. A total of 30 5-month-old male Albinus rabbits (New Zealand) with an average body weight between 3 kg and 4 kg were enrolled in this study. The animals were divided into three experimental periods: 15, 30, and 60 postoperative days (10 animals per period). For each period, 5 animals received 1 implant in each tibia while 5 animals received only 1 implant in either the right or left tibia. The right or left tibias were randomly selected. Then, the rabbits were randomly divided into three experimental groups of 5 animals each: control (blood clot - Group CO), particulate Bio-Oss® (Group BI) and Bio-Oss Collagen® (Group BC) (Figure 1).
Figure 1.
Flowchart of the classification of the study groups.
Surgical procedure
Experimental surgery for implant placement was performed as previously described [17]. Briefly, the animals were anesthetized by intramuscular injection of ketamine hydrochloride at a concentration of 50 mg/kg (Francotar; Vibrac do Brasil Ltda., SP, Brazil) and xylazine hydrochloride at 5 mg/kg (Rompun, Bayer AS, Saúde Animal, SP, Brazil). Then, trichotomy and antisepsis were performed in each tibia with iodine solution (PVPI 10%, Riodeine Degermante, Rioquímica, SP, Brazil) and topical PVPI before surgical incision. Local anesthesia by infiltrative injection with mepivacaine hydrochloride (0.3 mL/kg, Scandicaine 2% with adrenalin 1:100,000, Septodont, France) was performed to assist hemostasis of the wound healing.
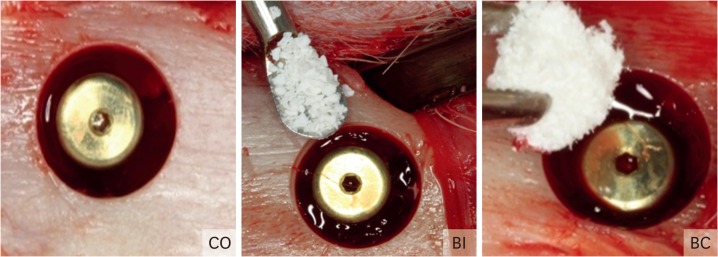
An incision 3.0 cm in length was made on the proximal tibia just below the knee to the depth of the bone tissue. The soft tissue was carefully dissected and lifted with the aid of a periosteal elevator, exposing the bone tissue for implant insertion. Subsequently, the receptor beds were prepared using a progressive sequence of drills (Sistemas de Implantes [SIN], SP, Brazil). Initially, a trephine bur was used for determination of implant positioning and for removal of the cortical bone (6.1 mm diameter/4 mm depth). Then, a sequence of lance drill, 2.0-mm drill, 2.0/3.0-mm pilot drill, and 3.0-mm drill was used to enlarge the osteotomy at the center of the 6.1-mm perforation under constant irrigation with 0.9% sodium chloride at 1,200 RPM (Darrow, Rio de Janeiro, RJ, Brazil). One 4.1-mm diameter and 8.5-mm length conical implant, with a machined surface and external hexagon (Sistemas de Implantes [SIN], SP, Brazil) was inserted in the proximal region of the tibia under 40 N of torque using an electric motor with a contra angle handpiece (BLM 600, Driller Electric Equipment Ltda, Sao Paulo, SP, Brazil), totaling 45 implants. Later, the cover screws were placed. The bone defects were filled with: CO, particulate BI (Geistlich Pharma AB, Wolhusen, Switzerland), and BC (Geistlich Pharma AB, Wolhusen, Switzerland) (Figure 2). For the BI group, the amount of particulate Bio-Oss® was based upon completely filling the circumferential gap defect using of the form of Bio-Oss® with small particles (0.25- to 1-mm particle size).
Figure 2.
Bone defects were filled with: I - blood clot (CO), II - Bio-Oss® (BI), and III - Bio-Oss® Collagen (BC).
The soft tissue was carefully repositioned and sutured in different layers for primary wound closure using an absorbable suture (Poligalactina 910 – Vycril 4.0, Ethicon, Johnson Prod., SP, Brazil). Monofilament suture (Nylon 5.0, Ethicon, Johnson, São José dos Campos, SP, Brazil) was used for interrupted skin suturing. Additional antisepsis with PVPI was conducted after the suturing. Intramuscular pentabiotic (0.1 mL/kg, Fort Dodge Saúde Animal Ltda, SP, Brazil) was injected immediately and at 5 days postoperatively. Sodic dipyrone (1 mg/kg/day, Ariston Indústrias Químicas e Farmacêuticas Ltda, São Paulo, SP, Brazil) was also administered. Neither food or movement restriction was applied to the animals that remained in individual cages during the experimental period. Ten rabbits were euthanized by a lethal dose of pentobarbital (200 mg/kg) at 15 days, at 30 days, and at 60 postoperatively days.
Analysis of removal torque of implants
After the sacrifice of the animals in all the three periods evaluated, the tibial metaphyses of the rabbits were reopened for exposure and removal of the implants using reverse torque. An analogic torque gauge (ATG24CN, Tohnichi, Tokyo, Japan) with a scale range of 3-24 Ncm and divisions of 0.05 Ncm was attached to the torque driver for implant removal. Counterclockwise rotation was conducted to apply reverse torque and rotate the implant inside the bone tissue. The maximum torque value (N.cm) necessary for rupture of the bone/implant interface was measured, as described [22].
Histological processing
After implant removal using reverse torque, samples of the bone/implant interface were removed and immersed in 10% paraformaldehyde for 72 hours. Then, the samples were decalcified in 5% ethylenediaminetetraacetic acid (EDTA) for 3 months. Subsequently, the samples were washed in running water for 24 hours, dehydrated, diaphonized and paraffin embedded, and 5-µm sections were obtained. Hematoxylin and eosin (H&E)-stained slices were obtained for morphological analysis of bone repair in the different groups and periods. For histological analysis, images were captured at 125× using a digital camera (Leica DFC 300FX, Leica microsystems, Heerbrugg, Switzerland) attached to a light microscope (Leica Aristoplan Microsystems- Leitz, Benshein, Germany).
Immunohistochemistry
For immunohistochemistry, the primary antibodies were used as following: osteoprotegerin (OPG, goat anti-OPG; Santa Cruz Biotechnology, SC21038), receptor activator of nuclear factor kappa-B ligand (RANKL, goat anti-RANKL; Santa Cruz Biotechnology, SC7627), osteocalcin (OC, goat anti-OC; Santa Cruz Biotechnology, SC18319), tartrate-resistant acid phosphatase (TRAP, goat anti-TRAP; Santa Cruz Biotechnology), and runt-related transcription factor 2 (RUNX2, goat anti-RUNX2; Santa Cruz Biotechnology).
Immunohistochemical analysis was performed by the immunoperoxidase method using the biotinylated donkey anti-goat secondary antibody (Jackson Immunoresearch). The avidin/biotin complex (Vector Laboratories) was used for signal amplification with diaminobenzidine (DAB, Dako) as chromogen according to the manufacturer's instructions. The sections were counterstained with Harris hematoxylin. As a negative control, primary antibody was omitted and the sections were incubated with 1% PBS to assess background staining while the lower cortex of the tibia was used as a positive control to check the effectiveness of the antibodies. A calibrated examiner, blinded to the experimental groups, analyzed the digital images in an optical microscopy (Leica microsystem GmbH, Wetzlar, Germany) at 40× magnification. Quantification of protein expression was evaluated in the region of the implant threads by an ordinal quantitative analysis based on scores (hyper-positive +++, super-positive ++, positive +, and negative -) according to previous published studies [23,24,25]. The RANKL:OPG ratio was also used to evaluate the tendency toward osteoclast formation or inhibition in the different groups [26].
Statistics
The GraphPad Prism 6.0 software package (Graph-Pad Software, Inc., La Jolla, CA, USA) was used for statistical analysis and visualization of the data. All data were expressed as mean±the standard error of the mean (SEM). After testing of the normal distribution (Kolmogorov-Smirnov test), differences were analyzed by the Kruskal-Wallis test or one-way ANOVA followed by post hoc Dunn's and Tukey's test, respectively, for multiple comparisons among groups. In addition, correlations between the reverse torque values and immunohistochemical staining's were investigated for all groups and periods using Spearman's rank correlation coefficient. The following correlation values (r) and categories were considered positive: r=0 to 0.3, weak correlation; r=0.3 to 0.7, moderate correlation; and r=greater than or equal to 0.7, strong correlation. Differences were considered significant at P<0.05.
RESULTS
Biomechanical analysis
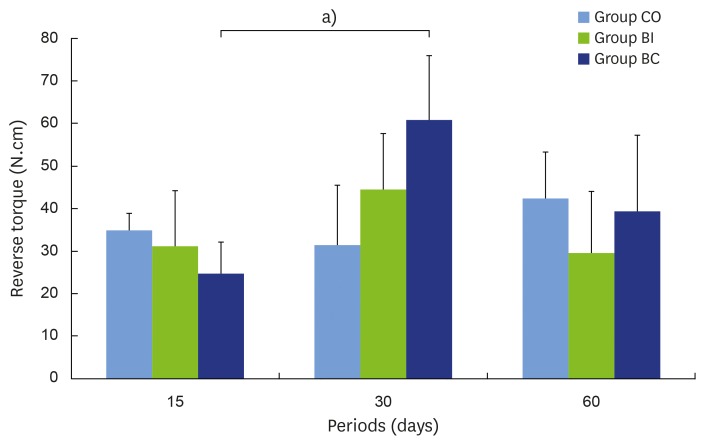
Analysis of the implant removal was performed using revere torque after the animals were sacrificed. No statistical difference was found among periods in the CO and BI groups. However, in the BC group, lower reverse torque was found at 15 days (mean±SD = 24.80±7.22 Ncm) in comparison with the 30-days point (61.00±15.28 Ncm) (P=0.005), but no difference was found between 15 and 60 (39.40±17.84 Ncm) days (P=0.273). For comparison among the groups, the BC group exhibited higher torque (61.00±15.28 Ncm) than the CO group (31.60±14.38 Ncm) at 30 days (P=0.005). According to Figure 3, the mean reverse torque values of the BI (31.20±13.29 Ncm and 44.60±13.12 Ncm for the 15- and 30-days periods, respectively) and BC (24.80±7.22 Ncm and 61.00±15.28 Ncm for 15- and 30-day periods, respectively) groups increased from 15 to 30 days but fell from 30 to 60 days (BI = 29.60±14.41 Ncm and BC = 39.40±17.84 Ncm in the 60-day period). However, for the CO group, the mean reverse torque values remained constant at the 15- and 30-day points in time (35.00±3.94 Ncm and 31.60±14.38 Ncm, respectively), but increased by 60 days (42.40±10.90 Ncm) (Figure 3).
Figure 3.
Removal torque analysis in all groups and periods.
a)Statistically significant between indicated groups, P<0.05.
Morphological analysis
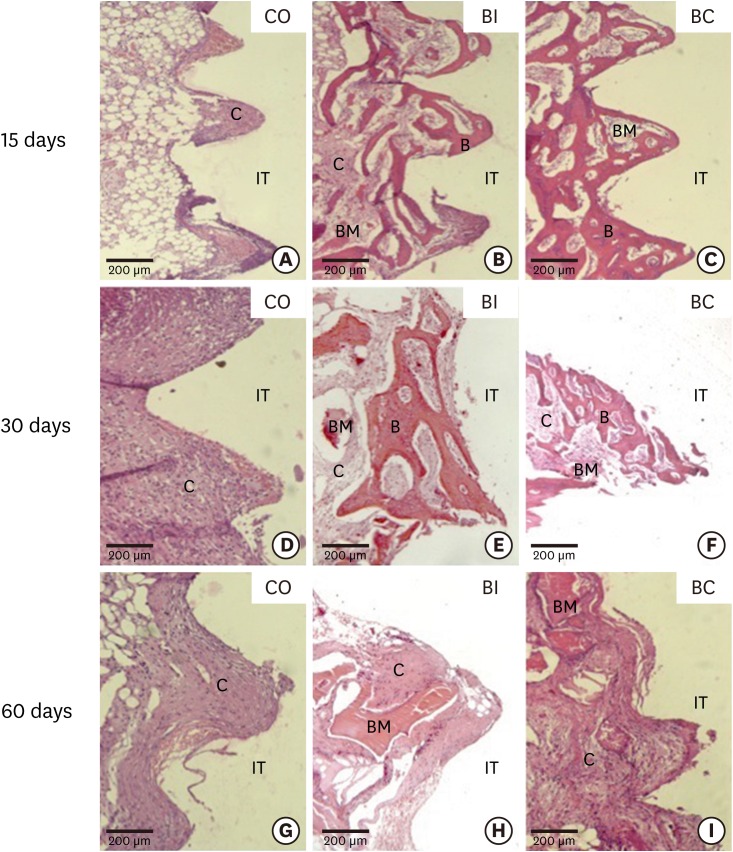
At 15 days, in the CO group, the region of the implant threads exhibited initial bone tissue repair with intense proliferation of connective tissue (CT) and the presence of blood clotting. Direct contact between the implant and CT was also observed (Figure 4A). In the upper cortical region, the cement line and bone trabeculae (BT) were surrounded by cellularized CT (Figure 5A). In the BI and BC groups, the region of the implant threads presented bone neoformation with osteoblasts at the implant/bone interface and CT between the threads (Figure 4B and C). In the upper cortical region, a cement line and bone neoformation surrounding the biomaterial and CT were observed (Figure 5B and C).
Figure 4.
Light micrographs of bone portions from implant threads in the CO (4A, 4D, and 4G), BI (4B, 4E, and 4H), and BC (4C, 4F, and 4I) groups at 15 (4A-4C), 30 (4D-4F), and 60 days (4G-4H). Hematoxylin and eosin staining.
B, bone trabeculae; C, connective tissue; IT, implant thread; BM, biomaterial.
Figure 5.
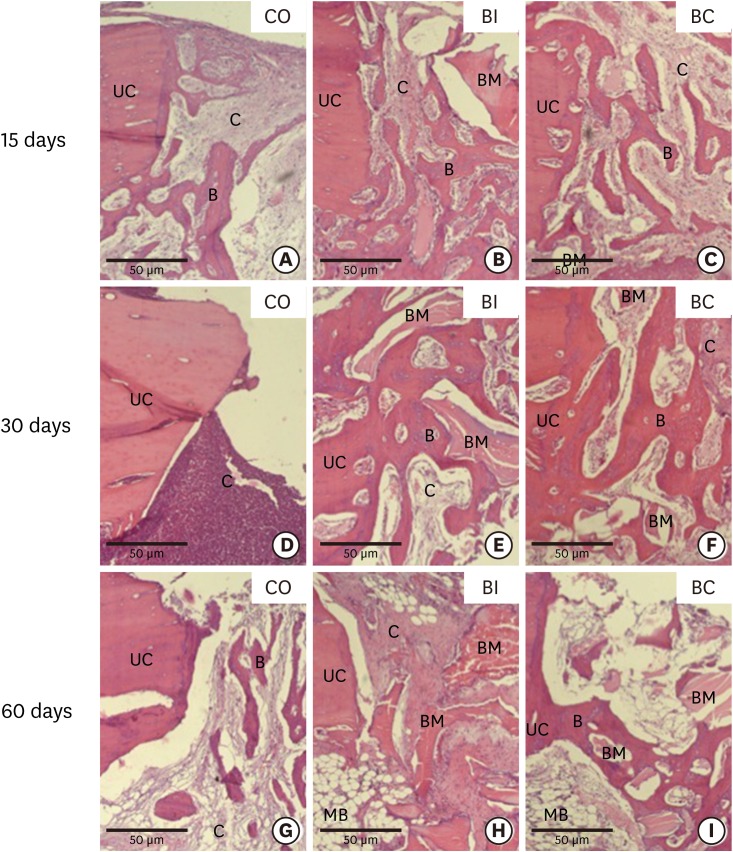
Light micrographs from upper cortical region and bone defects in the CO (5A, 5D and 5G), BI (5B, 5E and 5H), and BC (5C, 5F and 5I) groups at 15 (5A-5C), 30 (5D-5F), and 60 days (5G-5I). Hematoxylin and eosin staining.
B, bone trabeculae; C, connective tissue; UC, upper cortical region; BM, biomaterial; MB, medullary bone.
At 30 days, in the CO group, CT was found at the implant threads and upper cortical region without signs of osseointegration (Figure 4D and 5D). However, in the BI and BC groups, the region of the implant threads exhibited bone neoformation with osteoblasts at the bone/implant interface and smaller areas with CT (Figure 4E and F). In the upper cortical region, bone neoformation surrounding the biomaterial and minor areas with CT were observed (Figure 5E and F).
At 60 days, the results of the CO group were similar to the previous 15 and 30-day periods, with fibrous CT in the region of the implant threads and upper cortical region without signs of osseointegration (Figure 4G and 5G). In the BI and BC groups, similar results were found at the region of the implant threads with CT surrounding the biomaterial (Figure 4H and I). In the upper cortical region of the BI group, fibrous CT was found surrounding the biomaterial (Figure 5H). However, in the upper cortical region of the BC group, bone trabeculae were found adjacent to the biomaterial. A reduced number and volume of bone trabeculae were also observed in this period (Figure 5I).
Immunohistochemical analysis (IHC)
IHC reactions were conducted using primary antibodies anti-OC, -RUNX2, -OPG, -RANKL, and -TRAP. The reaction was analyzed during the healing period at the bone/implant interface.
Osteocalcin (OC)
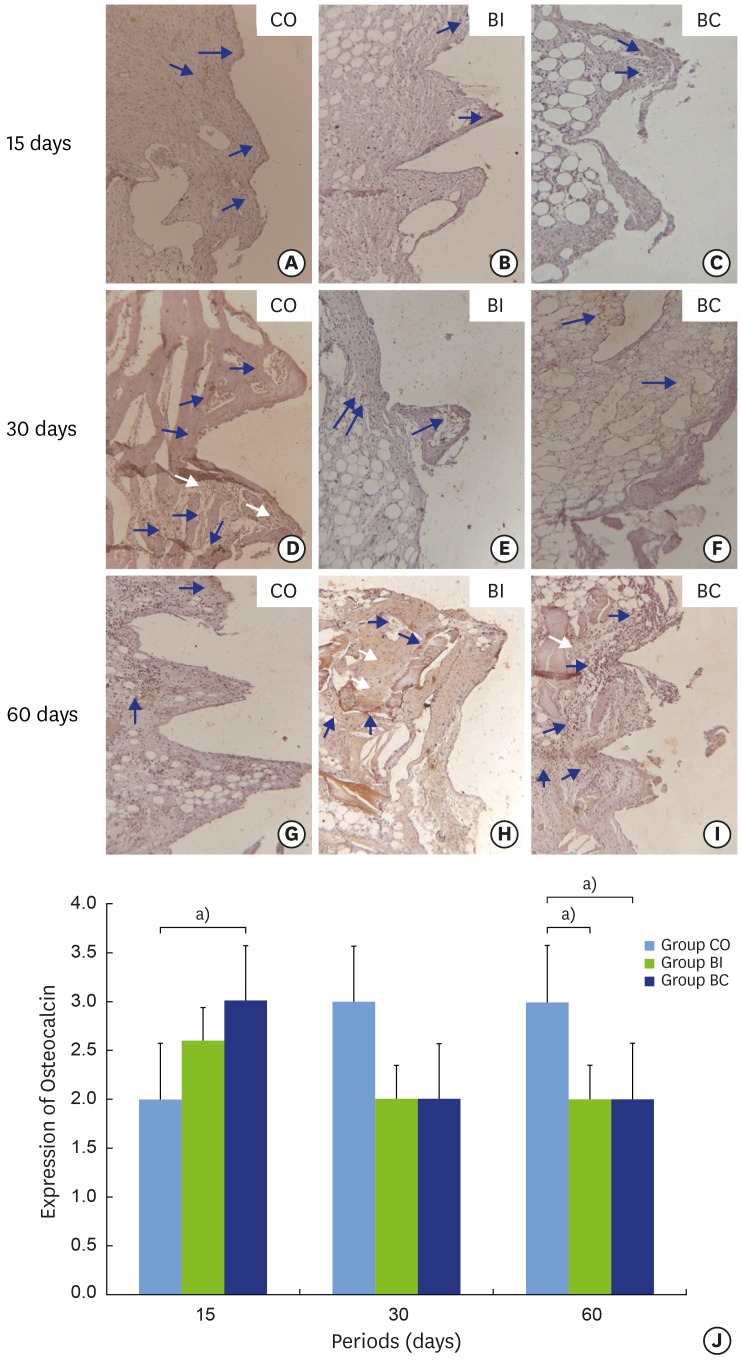
The labeling of OC was observed in the region of the implant threads in the CO group in all periods, which confirmed cell viability. In this group, a significant statistical difference (P=0.001) was found for labeling at 15 days (2.00±0.00) (positive) and the other periods (3.00±0.00) (super-positive) (Figure 6A, D, and G). In the BI group, positive labeling of OC was found, and the value remained stable over the postoperative periods (Figure 6B, E, and H). However, Figure 6J shows reduced expression between 15 (2.60±0.54) and 30 (2.00±1.00) days, and equilibrium between 30 and 60 (3.00±0.00) days. In the BC group, OC labeling ranged from super-positive to positive in the initial and final periods, respectively (Figure 6C, F, and H). There was a statistically significant difference (P=0.001) between 15 days (3.00±0.00) and the other periods evaluated (2.00±0.00) (Figure 6J). In comparing OC labeling among the different groups, at 15 days, a statistically significant difference (P=0.004) was found between CO and BC, with higher labeling in the BC group (Figure 6J). Although no statistically significant difference was found between groups at 30 days, higher OC labeling was seen for the CO group. At 60 days, the CO group showed higher OC labeling than the other groups (P=0.001).
Figure 6.
Light micrographs of sections submitted to immunohistochemical analysis for detection of osteocalcin (OC). Samples from implant thread tissues in CO (6A, 6D and 6G), BI (6B, 6E and 6H), and BC (6C, 6F and 6I) groups at 15 (6A-6C), 30 (6D-6F) and 60 days (6G-6H). Graphic representation of balanced OC labeling over time(6J). Blue arrows: labeling in cytoplasm; White arrows: background labeling.
a)Statistically significant between indicated groups, P<0.05.
Runt-related transcription factor 2 (RUNX2)
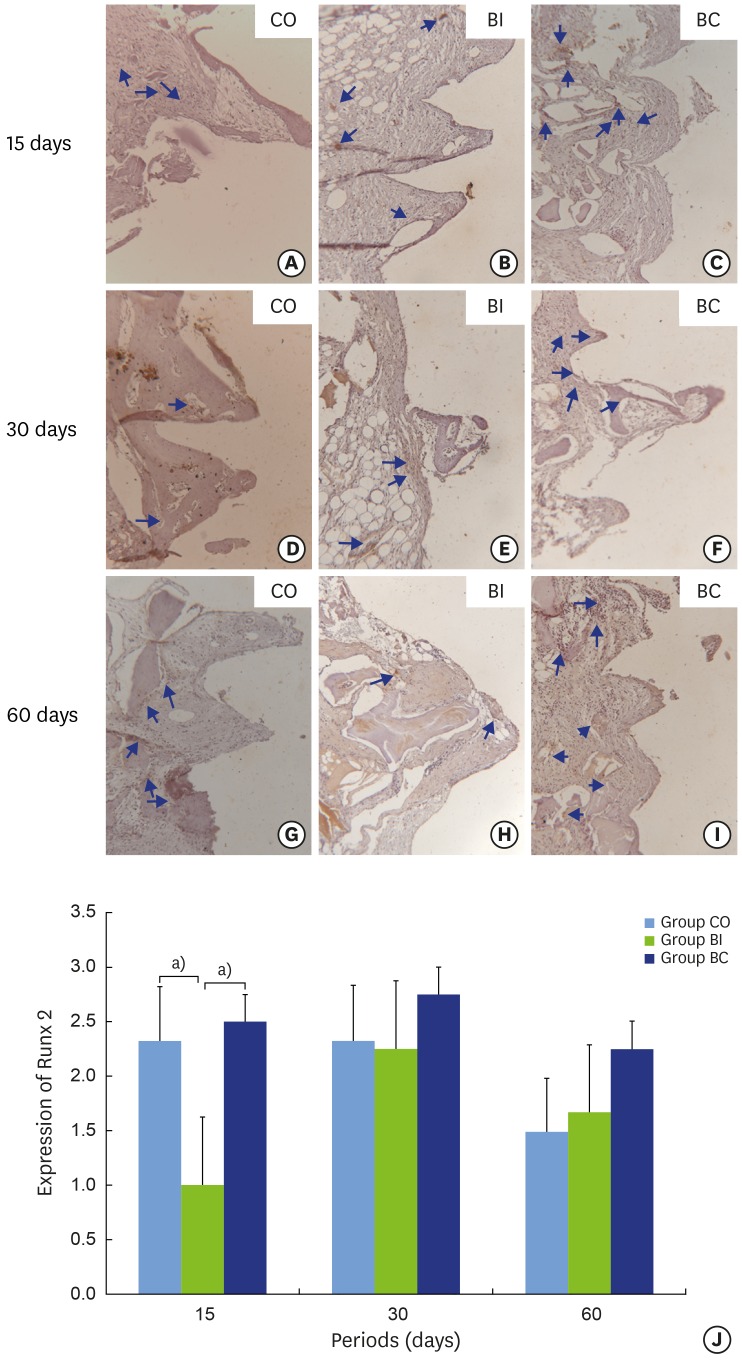
No statistically significant difference was found for RUNX2 labeling between the CO and BC groups, and stable labeling was exhibited over the periods (Figure 7A and C). However, the CO group presented reduced labeling from positive to negative at 60 days (Figure 7G). In the BI group, RUNX2 labeling ranged from negative to positive. There was a statistically significant difference at 15 (1.00±0.00) and 30 (2.25±0.95) days, with reduced labeling at the initial period (Figure 7B, E, H, and J). Comparing the groups, BI (1.00±0.00) showed lower RUNX2 labeling than CO (2.35±0.57) and BC (2.50±0.57) at 15 days with a statistically significant difference (P=0.006). Nevertheless, stable labeling was found in all groups at 30 and 60 days (Figure 7J).
Figure 7.
Light micrographs of immunohistochemical sections representing RUNX2 labeling in samples from implant threads in the CO (7A, 7D, and 7G), BI (7B, 7E, and 7H), and BC (7C, 7F, and 7I) groups at 15 (7A-7C), 30 (7D-7F), and 60 (7G-7I) days. Graphic representation of balanced RUNX2 labeling over time(7J). Blue arrows: labeling in cytoplasm.
a)Statistically significant between indicated groups, P<0.05.
Osteoprotegerin (OPG)
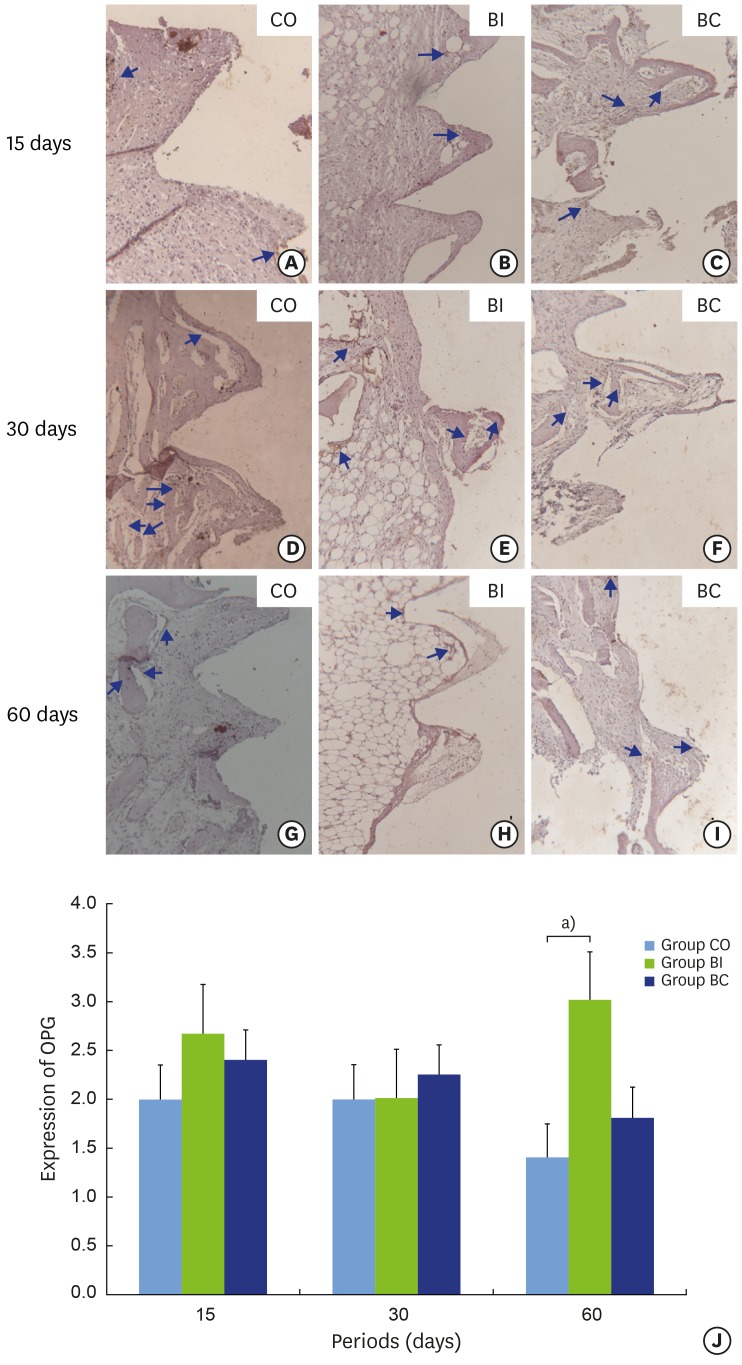
The OPG labeling ranged between negative and positive in the CO and BC groups. No qualitative alteration was found over the periods (Figure 8A, C, D, F, G, and I). In the BI group, OPG labeling ranged from positive to super-positive. A statistically significant difference was identified (P=0.004) at 30 (2.00±0.00) and 60 (3.00±0.00) days with increased labeling at the final period (Figure 8B, E, H, and J). No statistically significant difference in OPG labeling was observed among the three groups at 15 and 30 days. However, higher OPG labeling was found in the BI (3.00±0.00) than in the CO group (1.40±0.54) at 60 days (P=0.004) (Figure 8J).
Figure 8.
Light micrographs of immunohistochemical sections representing OPG labeling in implant thread tissue in CO (8A, 8D and 8G), BI (8B, 8E and 8H), and BC (8C, 8F and 8I) groups at 15 (8A-8C), 30 (8D-8F), and 60 days (8G-8I). Graphic representation of balanced OPG labeling over time (8J). Blue arrows: labeling in cytoplasm.
a)Statistically significant between indicated groups, P<0.05.
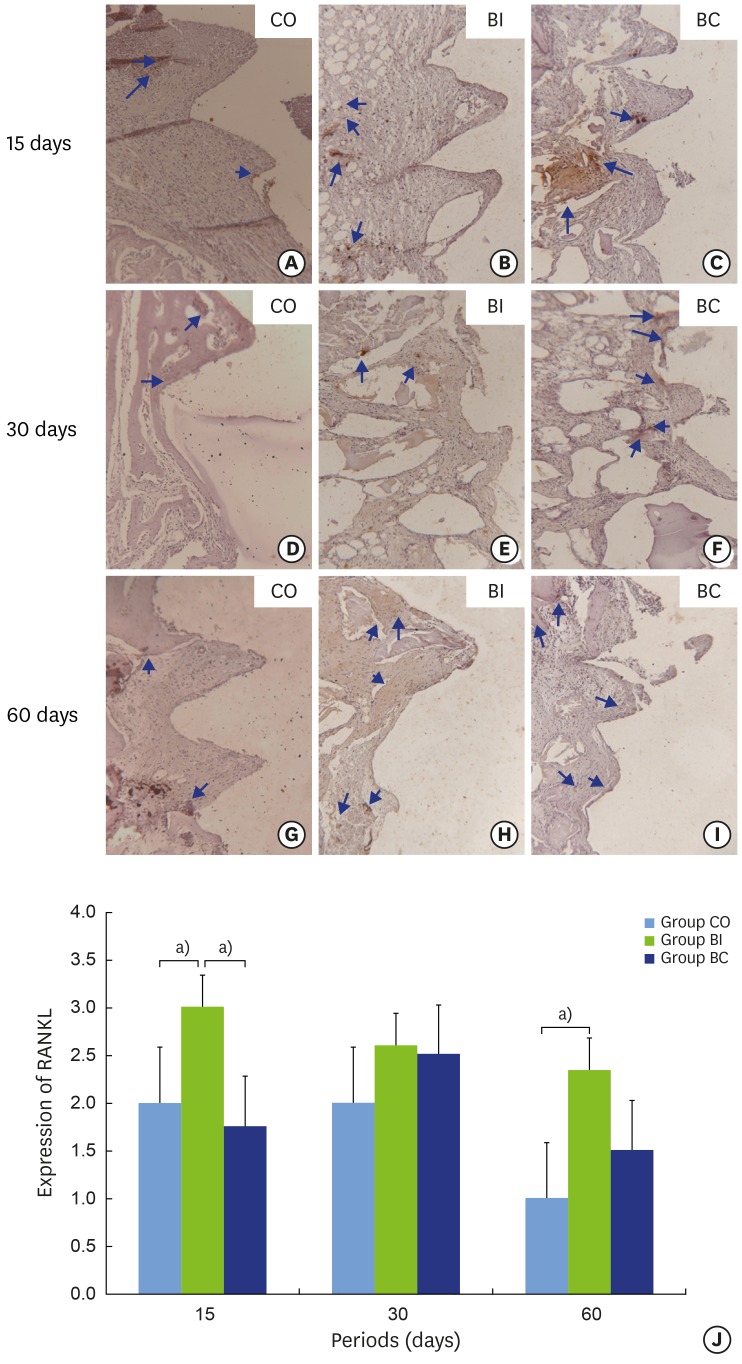
Receptor activator of nuclear factor kappa-b ligand (RANKL)
In the CO group, RANKL labeling ranged from negative to positive. A statistically significant difference (P=0.001) was found between 60 days (1.00±0.00) (negative) and the other periods (2.00±0.00) (positive) (Figure 9A, D, and G). In the BI group, there was no statistically significant difference in RANKL labeling over the periods (Figure 9B, E, and H). However, labeling reduction was observed over time, ranging from positive to super-positive (Figure 9J). In the BC group, RANKL labeling ranged from negative to positive. A statistically significant difference (P=0.028) was found at 30 (2.50±0.57) and 60 (1.50±0.57) days with reduced labeling at the final period (Figure 9C, F, I, and J). Comparing the groups, BI (3.00±0.00) exhibited higher RANKL labeling than CO (2.00±0.00) and BC (1.75±0.50) at 15 days (P=0.002), but no difference was found at 30 days. At 60 days, BI (2.33±0.57) showed higher RANKL labeling than did CO (1.00±0.00) (P=0.005) (Figure 9J). Although no statistically significant difference was found between the groups for the RANKL/OPG ratio, the BC group showed lower values than the other groups in all periods of evaluation (data not shown).
Figure 9.
Light micrographs of immunohistochemical slices representing RANKL labeling in implant thread tissue in CO (9A, 9D and 9G), BI (9B, 9E and 9H), and BC (9C, 9F and 9I) groups at 15 (9A-9C), 30 (9D-9F), and 60 days (9G-9I). Graphic representation of balanced RANKL labeling over time (9J). Blue arrows: labeling in cytoplasm.
a)Statistically significant between indicated groups, P<0.05.
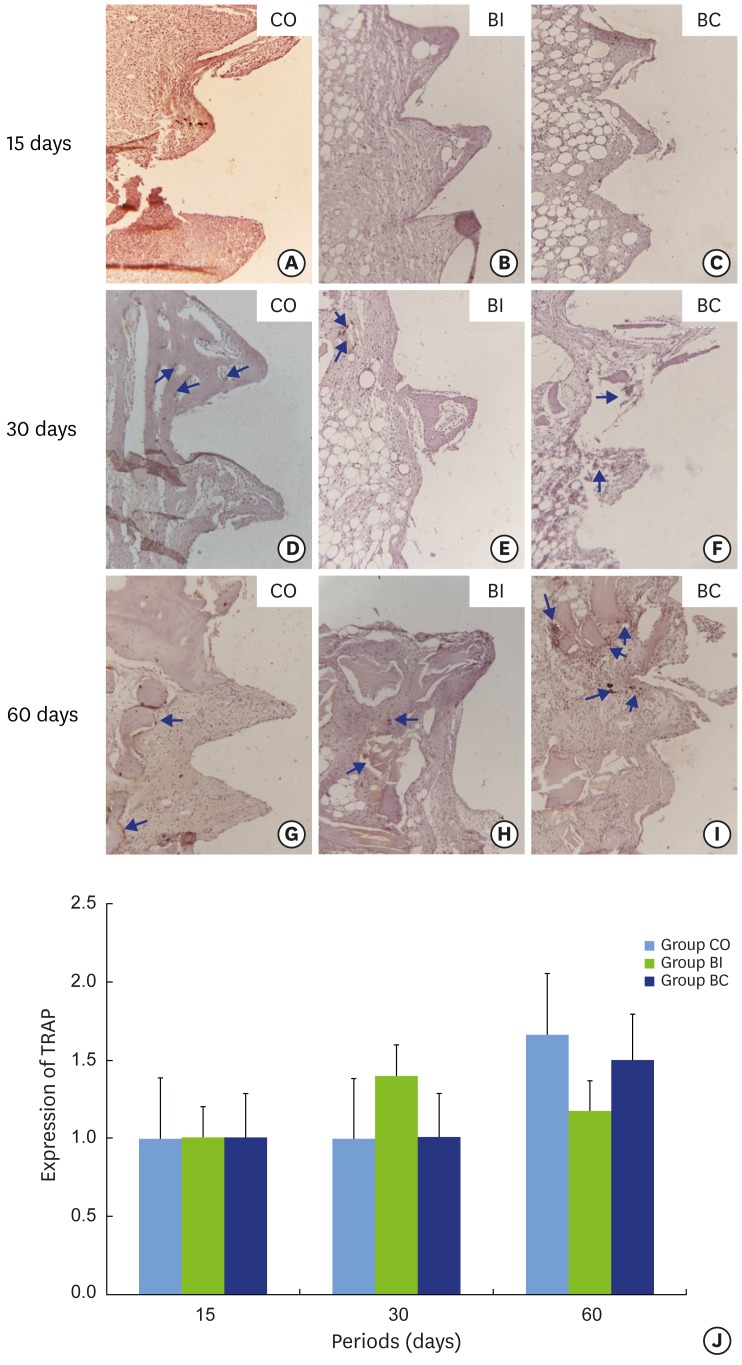
Tartrate-resistant acid phosphatase (TRAP)
The CO group presented increasing TRAP labeling from negative to positive at 60 days, which indicates a higher tendency toward bone resorption than neoformation. However, no statistically significant difference was observed since labeling remained stable (Figure 10A, D, and G). In the BI group, no statistically significant difference in TRAP labeling was found over the different periods. However, a slight increase in TRAP labeling at 30 days in comparison to the other periods was observed (Figure 10B, E, H, and J). Although no statistically significant difference in TRAP labeling was found in the BC group, increased expression was noted at 60 days (Figure 10C, F, and I).
Figure 10.
Light micrograph immunohistochemical sections representing TRAP labeling containing samples from implant threads in CO (10A, 10D and 10G), BI (10B, 10E and 10H), and BC (10C, 10F and 10I) groups at 15 (10A-10C), 30 (10D-10F), and 60 (10G-10I) days. Graphic representation of balanced TRAP labeling over time(10J). Blue arrows: labeling in cytoplasm.
Correlations between the reverse torque values and immunohistochemical stainings
Some relevant correlations between reverse torque values and immunohistochemical stainings were observed. In the CO group, moderate correlation between the torque value and RUNX2 expression was found at 30 days. Strong correlations were observed between torque values at 30 days and RUNX2 expression at 60 days. In the BI group, 15-day torque values were moderately correlated with 15- and 60-day OC, 30- and 60-day OPG, and 60-day RANKL expression. There were strong correlations between 30-day torque and 15-day OC expression as well as between 60-day torque and OC expression at 15 and 30 days. In the BC group, a moderate correlation was found between 15-day torque data and 30-day RANKL expression. Furthermore, moderate correlation was observed between the 30-day torque and the expression of OPG at 30 days, RANKL at 30 and 60 days, and RUNX2 at 15 and 60 days. We observed strong correlations between the torque of 30 days and the expressions of OPG and TRAP at 15 and 30 days, RUNX2 at 30 days, and OC at 60 days.
DISCUSSION
The healing of a fresh alveolar defect (i.e. the 4-wall defect) is conducted by maintenance of blood clotting in the region. To preserve the dimensions of the alveolar ridge and promote healing, filling the alveolus with biomaterial is indicated [1,27,28]. In addition, the insertion of biomaterial simultaneously with implant placement may enhance functional and esthetic restoration. Thus, the aim of this study was to evaluate the bone repair surrounding dental implants in circumferential gap defects filled with CO, BI, and BC. To the best of our knowledge, this is the first investigation regarding the use Bio-Oss® Collagen in peri-implant defects for short periods of time (15, 30, and 60 days), using biomechanical, morphological, and immunohistochemical analysis. Our results indicate that BC offered improved bone healing compared to the CO and BI groups, which was characterized by increased RUNX2, OC, and OPG immunolabeling, and increased reversal torque for implant removal especially in the 30-day period. Indeed, both BC and BI have promising biomechanical and biological properties supporting their possible use as osteoconductive grafts for filling peri-implant gaps.
Considering the biomechanical and immunohistochemical results, bone formation was observed in the BC group, as evidenced by increased RUNX2, OC, and OPG immunolabeling, and increased reversal torque for implant removal, especially in the 30-day period. It should be emphasized that in the BC group in the 15-day period, the OC expression was significantly higher than in the CO group. Accordingly, the removal torque of the BC group was statistically significantly different compared to the CO group in the 30-day period. However, it is important to highlight that bone resorption characterized by RANKL and TRAP staining revealed osteoclast activity that continued consistently over time. This fact, associated with reduction in OC expression in the BI and BC groups during the 60-day period, resulted in decreased bone formation, and consequently, the presence of higher amounts of connective tissue. These findings reflect the values obtained in the removal torque analysis, which showed lower values for the BI and BC groups at day 60.
Primary implant stability is determined by the physical contact between bone and implant and is due to the resistance produced by elevated insertion torque [17], and it is essential for osseointegration [29], which is produced by the biologic contact between implant and bone. Unstable implants may lead to formation of peri-implant fibrous connective tissue [30]. The biomechanical analysis of osseointegration in animals is based on removal torque that is proportional to the percentage of bone/implant contact [31]. Here, the biomechanical test revealed a statistically significant difference in the BC group at 15 and 30 days. The BC group showed increased removal torque values (61.00±15.28) compared to the CO group (31.60±14.38) at 30 days. This finding could probably be attributed to the collagen that is rapidly resorbed, and the particles of Bio-Oss® act as a framework for mineralization, improving the osseointegration of the implants and favoring bone-to-implant contact [32]. In the 60-day period, the torque removal analysis showed no statistically significant difference between the CO (42.4±10.90), BI (29.6±14.41), and BC (39.4±17.84) groups. The BI and BC groups showed a decrease in the reverse torque at 60 days without a statistically significant difference. Similar results were observed in histological analysis since connective tissue was found surrounding the biomaterial. In this context, Antunes et al. [33] showed that implants associated with Bio-Oss® present poor stability after 2 months in bone defects created in the dog mandibles. Another fact that might influence the biomechanical analysis is the lack of mechanical loading on the implant since loading can be a positive effect on bone-to-implant contact in peri-implant defects filled or not filled with bone grafts [34].
Several studies in the literature have evaluated bone repair in peri-implant defects filled with biomaterials using animal [8,12,14,29,35] and human [1,5] models. The peri-implant defect reconstruction can be evaluated through clinical, histological, radiographic, and immunohistochemical analyses. In the present study, rabbits were selected as the experimental model because they are easy to handle, and due to the size of their tibia, allowing the creation of a large peri-implant defect and the installation of conventional implants in the proximal region of the tibia, far from the growth plate (tibial metaphysis).
The conical shape of the alveolus is usually wider than the implant dimensions, which results in a gap between the threads of the implant and the bone tissue. Some studies [5,12,29] evaluated this marginal gap with respect to the insertion of biomaterials. Akimoto et al. [8] demonstrated that the diameter of the bone defect influences the percentage of bone/implant contact, which hints to the potential usefulness of inserting biomaterial for filling of those defects. Accordingly, Carlsson et al. [36] concluded that residual gaps remained in defects of 0.85 mm. In the present study, a peri-implant defect with cervical diameter of 1 mm wider than the implant diameter was reproduced to simulate the insertion of a dental implant into a fresh extraction socket. The lack of bone neoformation in the cervical and medial (medullary) areas in the CO group confirmed the maintenance of residual gaps.
In the evaluations at 15 and 30 days, the BI and BC groups demonstrated significant bone neoformation at the bone-to-implant interface with the presence of biomaterial. However, after 60 days, the biomaterial was surrounded by connective tissue in the BI group. Comparably, Carmagnola et al. [11] evaluated peri-implant tissue reactions using Bio-Oss® and concluded that the demineralized bovine bone did not integrate into the host tissue after 4 months, which suggests that the bone defect was wide and deep. Here, the rabbit tibia was the receptor site, which represented a medullary region without bone trabeculae to support the biomaterial. Carmagnola et al. [37] also conducted a study in humans and reported that Bio-Oss® shows slow resorption and healing with fibrous encapsulation, which delayed bone remodeling. These findings parallel observations made by Artzi et al. [38] that showed the presence of non-resorbable particles of bovine bone mineral surrounded by connective tissue in human extraction sockets. However, Araújo et al. [12] conducted a study in dogs to evaluate the bone repair in peri-implant defects filled with Bio-Oss® Collagen. The biopsies were collected after 6 months for histological analysis. The authors concluded that the Bio-Oss® Collagen changed the healing of the hard tissue, improving the bone-to-implant contact. These results closely resemble observations made by Han et al. [14] that showed in a study in dogs that the particles of Bio-Oss® and Bio-Oss® Collagen were completely integrated into the bone tissue after 8 weeks.
The immunohistochemical analyses revealed important information regarding cell behavior based on the expression of proteins for bone metabolism during osseointegration. RUNX2 is a transcription factor from Runt genes found in pre-osteoblasts and osteoblasts. This protein regulates osteoblast differentiation, formation of extracellular matrix, and bone mineralization being essential for bone tissue formation and development. Its action might occur by either binding to specific regions of DNA or by activation of a cascade reaction recruiting enzymes that will catalyze the transcription of osteoblastic genes [39]. In this study, the RUNX2 staining in the BI group was reduced below the CO and BC groups at 15 days. These findings could be explained because the Bio-Oss® is an osteoconductive material with a greater volume of non-resorbable particles, which results in reduced space between the particles for bone neoformation. In the BC group, the collagen was rapidly absorbed, leaving more space for bone neoformation and higher RUNX2 labeling.
Osteocalcin was also studied since it is expressed by osteoblasts and binds to the hydroxyapatite of bone matrix. It is important for maturation of bone mineral content, and it is only found after the end of osteoblast proliferative activity, representing a differentiated and mature stage of those cells. Its activity occurs during the initial steps of bone repair for regulation of osteoblast activity [40]. The results of this study showed that the BC group exhibited higher OC labeling than the CO group at 15 days, which is associated with the higher RUNX2 labeling in the BC group. The CO group showed higher OC labeling than the BI and BC groups at 60 days. This result is probably associated with bone turnover, which means that bone mineralization occurred before the last period of evaluation in this study (i.e. 60-day period) [29].
Osteoprotegerin is considered a regulatory protein for osteoclast formation and activity. Its presence is related to inhibition of bone resorption. Although all groups have exhibited similar OPG labeling at 15 and 30 days, the BI group showed higher labeling than the CO group at 60 days. On the other hand, RANKL labeling is related to activation of bone resorption. The results of this study showed that the BI group demonstrated more RANKL labeling than the CO group at 15 and 60 days. Considering that OPG and RANKL have opposite functions, the analysis of their ratio reveals their relative influence on bone remodeling [26]. In this regard, the RANKL/OPG ratio was calculated for all groups. Considering that the BC group presented lower values than the other groups in all periods, Bio-Oss® Collagen represents a greater tendency toward bone resorption inhibition. Tartrate-resistant acid phosphatase (TRAP) is a protein related to bone resorption and an important marker of osteoclastic activity [26]. The results showed equilibrium of TRAP labeling between the groups, indicating similar resorption activation.
Regarding the correlations between torque values and bone formation markers, in the CO group, a strong correlation was found between the torque at 30 days and RUNX2 expression at 60 days. In the BI group, 60-day torque analysis was strongly correlated with OC expression at 15 and 30 days. The BC group showed a strong correlation between 30-day torque and 60-day OC expression. When bone resorption proteins were considered, there was strong correlation between 30-day torque and the expression of OPG and TRAP at 15 days in the BC group. All these findings indicate that there was a relationship between torque values and proteins associated with bone formation or resorption. The bone remodeling in the early period (15 days) apparently resulted in high torque values in the next period (30 days). The early expression of RUNX2 (related to osteoblast differentiation and then bone formation) may be associated, at least in part, with the torque at 30 days. In the latter period (60 days), the expression of OC (related to bone mineralization) may be responsible for torque values.
In summary, using a peri-implant bone defect in the rabbit tibia, we demonstrated through biomechanical, histological, and immunohistochemical analyses that, compared to the CO and BI groups, BC enhanced bone healing, which was characterized by increased RUNX2, OC, and OPG immunolabeling, and higher reversal torque for implant removal, especially at 30 days. Accordingly, the BI group showed increased expression of OPG at 15 and 60 days. Indeed, based on our findings, it can be concluded that BC, as well as BI, presents promising biomechanical and biological properties supporting its possible use in osteoconductive grafts for filling peri-implant gaps and may be considered a safe and predictable biomaterial for pre-clinical and clinical use.
ACKNOWLEDGEMENTS
We are extremely grateful to Geistlich biomaterials (Geistlich Pharma AB, Wolhusen, Switzerland) for the free donation of the Bio-Oss and Bio-Oss collagen used in this study.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Mardas N, Chadha V, Donos N. Alveolar ridge preservation with guided bone regeneration and a synthetic bone substitute or a bovine-derived xenograft: a randomized, controlled clinical trial. Clin Oral Implants Res. 2010;21:688–698. doi: 10.1111/j.1600-0501.2010.01918.x. [DOI] [PubMed] [Google Scholar]
- 2.Brkovic BM, Prasad HS, Rohrer MD, Konandreas G, Agrogiannis G, Antunovic D, et al. Beta-tricalcium phosphate/type I collagen cones with or without a barrier membrane in human extraction socket healing: clinical, histologic, histomorphometric, and immunohistochemical evaluation. Clin Oral Investig. 2012;16:581–590. doi: 10.1007/s00784-011-0531-1. [DOI] [PubMed] [Google Scholar]
- 3.Shi B, Zhou Y, Wang YN, Cheng XR. Alveolar ridge preservation prior to implant placement with surgical-grade calcium sulfate and platelet-rich plasma: a pilot study in a canine model. Int J Oral Maxillofac Implants. 2007;22:656–665. [PubMed] [Google Scholar]
- 4.Schulte W, Heimke G. [The Tübinger immediate implant] Quintessenz. 1976;27:17–23. [PubMed] [Google Scholar]
- 5.Botticelli D, Renzi A, Lindhe J, Berglundh T. Implants in fresh extraction sockets: a prospective 5-year follow-up clinical study. Clin Oral Implants Res. 2008;19:1226–1232. doi: 10.1111/j.1600-0501.2008.01620.x. [DOI] [PubMed] [Google Scholar]
- 6.de Molon RS, de Avila ED, Cirelli JA, Mollo FA, Jr, de Andrade MF, Filho LA, et al. A combined approach for the treatment of resorbed fresh sockets allowing immediate implant restoration: a 2-year follow-up. J Oral Implantol. 2015;41:712–718. doi: 10.1563/AAID-JOI-D-13-00140. [DOI] [PubMed] [Google Scholar]
- 7.de Molon RS, de Avila ED, de Barros-Filho LA, Ricci WA, Tetradis S, Cirelli JA, et al. Reconstruction of the alveolar buccal bone plate in compromised fresh socket after immediate implant placement followed by immediate provisionalization. J Esthet Restor Dent. 2015;27:122–135. doi: 10.1111/jerd.12154. [DOI] [PubMed] [Google Scholar]
- 8.Akimoto K, Becker W, Persson R, Baker DA, Rohrer MD, O'Neal RB. Evaluation of titanium implants placed into simulated extraction sockets: a study in dogs. Int J Oral Maxillofac Implants. 1999;14:351–360. [PubMed] [Google Scholar]
- 9.Botticelli D, Berglundh T, Persson LG, Lindhe J. Bone regeneration at implants with turned or rough surfaces in self-contained defects. An experimental study in the dog. J Clin Periodontol. 2005;32:448–455. doi: 10.1111/j.1600-051X.2005.00693.x. [DOI] [PubMed] [Google Scholar]
- 10.Yoon HC, Choi JY, Jung UW, Bae EK, Choi SH, Cho KS, et al. Effects of different depths of gap on healing of surgically created coronal defects around implants in dogs: a pilot study. J Periodontol. 2008;79:355–361. doi: 10.1902/jop.2008.070306. [DOI] [PubMed] [Google Scholar]
- 11.Carmagnola D, Berglundh T, Araújo M, Albrektsson T, Lindhe J. Bone healing around implants placed in a jaw defect augmented with Bio-Oss. An experimental study in dogs. J Clin Periodontol. 2000;27:799–805. doi: 10.1034/j.1600-051x.2000.027011799.x. [DOI] [PubMed] [Google Scholar]
- 12.Araújo MG, Linder E, Lindhe J. Bio-Oss collagen in the buccal gap at immediate implants: a 6-month study in the dog. Clin Oral Implants Res. 2011;22:1–8. doi: 10.1111/j.1600-0501.2010.01920.x. [DOI] [PubMed] [Google Scholar]
- 13.Araújo MG, Lindhe J. Ridge preservation with the use of Bio-Oss collagen: A 6-month study in the dog. Clin Oral Implants Res. 2009;20:433–440. doi: 10.1111/j.1600-0501.2009.01705.x. [DOI] [PubMed] [Google Scholar]
- 14.Han JY, Shin SI, Herr Y, Kwon YH, Chung JH. The effects of bone grafting material and a collagen membrane in the ridge splitting technique: an experimental study in dogs. Clin Oral Implants Res. 2011;22:1391–1398. doi: 10.1111/j.1600-0501.2010.02127.x. [DOI] [PubMed] [Google Scholar]
- 15.Carvalho AC, Queiroz TP, Okamoto R, Margonar R, Garcia IR, Jr, Magro Filho O. Evaluation of bone heating, immediate bone cell viability, and wear of high-resistance drills after the creation of implant osteotomies in rabbit tibias. Int J Oral Maxillofac Implants. 2011;26:1193–1201. [PubMed] [Google Scholar]
- 16.Queiroz TP, Hochuli-Vieira E, Gabrielli MA, Cancian DC. Use of bovine bone graft and bone membrane in defects surgically created in the cranial vault of rabbits. Histologic comparative analysis. Int J Oral Maxillofac Implants. 2006;21:29–35. [PubMed] [Google Scholar]
- 17.Queiroz TP, Souza FA, Guastaldi AC, Margonar R, Garcia-Júnior IR, Hochuli-Vieira E. Commercially pure titanium implants with surfaces modified by laser beam with and without chemical deposition of apatite. Biomechanical and topographical analysis in rabbits. Clin Oral Implants Res. 2013;24:896–903. doi: 10.1111/j.1600-0501.2012.02471.x. [DOI] [PubMed] [Google Scholar]
- 18.Sisti KE, Piattelli A, Guastaldi AC, Queiroz TP, de Rossi R. Nondecalcified histologic study of bone response to titanium implants topographically modified by laser with and without hydroxyapatite coating. Int J Periodontics Restorative Dent. 2013;33:689–696. doi: 10.11607/prd.1151. [DOI] [PubMed] [Google Scholar]
- 19.Souza FA, Queiroz TP, Guastaldi AC, Garcia-Júnior IR, Magro-Filho O, Nishioka RS, et al. Comparative in vivo study of commercially pure Ti implants with surfaces modified by laser with and without silicate deposition: biomechanical and scanning electron microscopy analysis. J Biomed Mater Res B Appl Biomater. 2013;101:76–84. doi: 10.1002/jbm.b.32818. [DOI] [PubMed] [Google Scholar]
- 20.Souza FA, Queiroz TP, Sonoda CK, Okamoto R, Margonar R, Guastaldi AC, et al. Histometric analysis and topographic characterization of cp Ti implants with surfaces modified by laser with and without silica deposition. J Biomed Mater Res B Appl Biomater. 2014;102:1677–1688. doi: 10.1002/jbm.b.33139. [DOI] [PubMed] [Google Scholar]
- 21.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage. 2012;20:256–260. doi: 10.1016/j.joca.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 22.de Molon RS, Morais-Camilo JA, Verzola MH, Faeda RS, Pepato MT, Marcantonio E., Jr Impact of diabetes mellitus and metabolic control on bone healing around osseointegrated implants: removal torque and histomorphometric analysis in rats. Clin Oral Implants Res. 2013;24:831–837. doi: 10.1111/j.1600-0501.2012.02467.x. [DOI] [PubMed] [Google Scholar]
- 23.dos Santos PL, Queiroz TP, Margonar R, Gomes de Souza Carvalho AC, Okamoto R, de Souza Faloni AP, et al. Guided implant surgery: what is the influence of this new technique on bone cell viability? J Oral Maxillofac Surg. 2013;71:505–512. doi: 10.1016/j.joms.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Esteves JC, Marcantonio E, Jr, de Souza Faloni AP, Rocha FR, Marcantonio RA, Wilk K, et al. Dynamics of bone healing after osteotomy with piezosurgery or conventional drilling - histomorphometrical, immunohistochemical, and molecular analysis. J Transl Med. 2013;11:221. doi: 10.1186/1479-5876-11-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Queiroz TP, Souza FA, Okamoto R, Margonar R, Pereira-Filho VA, Garcia Júnior IR, et al. Evaluation of immediate bone-cell viability and of drill wear after implant osteotomies: immunohistochemistry and scanning electron microscopy analysis. J Oral Maxillofac Surg. 2008;66:1233–1240. doi: 10.1016/j.joms.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 26.de Souza Faloni AP, Schoenmaker T, Azari A, Katchburian E, Cerri PS, de Vries TJ, et al. Jaw and long bone marrows have a different osteoclastogenic potential. Calcif Tissue Int. 2011;88:63–74. doi: 10.1007/s00223-010-9418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Avila ED, de Molon RS, de Assis Mollo F, Jr, de Barros LA, Capelozza Filho L, de Almeida Cardoso M, et al. Multidisciplinary approach for the aesthetic treatment of maxillary lateral incisors agenesis: thinking about implants? Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:e22–8. doi: 10.1016/j.oooo.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 28.de Barros LA, de Almeida Cardoso M, de Avila ED, de Molon RS, Siqueira DF, Mollo-Junior FA, et al. Six-year follow-up of maxillary anterior rehabilitation with forced orthodontic extrusion: achieving esthetic excellence with a multidisciplinary approach. Am J Orthod Dentofacial Orthop. 2013;144:607–615. doi: 10.1016/j.ajodo.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 29.Sisti KE, de Rossi R, Antoniolli AM, Aydos RD, Guastaldi AC, Queiroz TP, et al. Surface and biomechanical study of titanium implants modified by laser with and without hydroxyapatite coating, in rabbits. J Oral Implantol. 2012;38:231–237. doi: 10.1563/AAID-JOI-D-10-00030. [DOI] [PubMed] [Google Scholar]
- 30.Lioubavina-Hack N, Lang NP, Karring T. Significance of primary stability for osseointegration of dental implants. Clin Oral Implants Res. 2006;17:244–250. doi: 10.1111/j.1600-0501.2005.01201.x. [DOI] [PubMed] [Google Scholar]
- 31.Moriya K, Maruo Y, Minagi S. Does rotational strain at screw tightening affect the attainment or maintenance of osseointegration? Clin Oral Implants Res. 2006;17:451–458. doi: 10.1111/j.1600-0501.2005.01243.x. [DOI] [PubMed] [Google Scholar]
- 32.Wong RW, Rabie AB. Effect of bio-oss collagen and collagen matrix on bone formation. Open Biomed Eng J. 2010;4:71–76. doi: 10.2174/1874120701004010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antunes AA, Oliveira Neto P, de Santis E, Caneva M, Botticelli D, Salata LA. Comparisons between Bio-Oss(®) and Straumann(®) Bone Ceramic in immediate and staged implant placement in dogs mandible bone defects. Clin Oral Implants Res. 2013;24:135–142. doi: 10.1111/j.1600-0501.2011.02385.x. [DOI] [PubMed] [Google Scholar]
- 34.Zambon R, Mardas N, Horvath A, Petrie A, Dard M, Donos N. The effect of loading in regenerated bone in dehiscence defects following a combined approach of bone grafting and GBR. Clin Oral Implants Res. 2012;23:591–601. doi: 10.1111/j.1600-0501.2011.02279.x. [DOI] [PubMed] [Google Scholar]
- 35.Park IP, Kim SK, Lee SJ, Lee JH. The relationship between initial implant stability quotient values and bone-to-implant contact ratio in the rabbit tibia. J Adv Prosthodont. 2011;3:76–80. doi: 10.4047/jap.2011.3.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlsson L, Röstlund T, Albrektsson B, Albrektsson T. Implant fixation improved by close fit. Cylindrical implant-bone interface studied in rabbits. Acta Orthop Scand. 1988;59:272–275. doi: 10.3109/17453678809149361. [DOI] [PubMed] [Google Scholar]
- 37.Carmagnola D, Adriaens P, Berglundh T. Healing of human extraction sockets filled with Bio-Oss. Clin Oral Implants Res. 2003;14:137–143. doi: 10.1034/j.1600-0501.2003.140201.x. [DOI] [PubMed] [Google Scholar]
- 38.Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets. Part 1: histomorphometric evaluations at 9 months. J Periodontol. 2000;71:1015–1023. doi: 10.1902/jop.2000.71.6.1015. [DOI] [PubMed] [Google Scholar]
- 39.Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000;289:1501–1504. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- 40.Thorwarth M, Rupprecht S, Falk S, Felszeghy E, Wiltfang J, Schlegel KA. Expression of bone matrix proteins during de novo bone formation using a bovine collagen and platelet-rich plasma (prp)--an immunohistochemical analysis. Biomaterials. 2005;26:2575–2584. doi: 10.1016/j.biomaterials.2004.07.041. [DOI] [PubMed] [Google Scholar]