Abstract
Background:
After a subcutaneous injection fluid might leak out of the skin, commonly referred to as leakage or backflow. The objective was to examine the influence of needle design and injection technique on leakage after injections in the subcutaneous tissue of humans and pigs.
Method:
Leakage data were obtained from a post hoc analysis of clinical trial data and from a pig study. Data from the clinical study were used to determine leakage as a function of injection volume, speed and region. Data from the pig study were used to determine leakage as a function of needle wall thickness, needle taper, injection angle, and wait time from end of injection to withdrawal of needle from skin.
Results:
Leakage volume was positively related to injection volume. Injections in the abdomen caused less leakage than thigh injections. A 32G needle caused less leakage than a 31G and a 32G tip (tapered) needle, and a “straight in” 90° needle insertion angle caused less leakage than an angled (~45°) insertion. Wait times of minimum 3 seconds caused less leakage than immediate withdrawal of the needle after injection. Needle wall thickness and injection speed did not influence leakage.
Conclusions:
Leakage will be minimized using a thin needle, using 90° needle insertion in the abdomen, injecting maximum 800 µL at a time, and waiting at least 3 seconds after the injection until the needle is withdrawn from the skin.
Keywords: backflow, injection technique, insulin injections, leakage, pen needle
When administering insulin via subcutaneous injections, consistency of the injection and dose accuracy are essential; the user must receive the dialed and expected dose of insulin. One commonly known and well discussed event, which may occur after an injection, is the leakage of fluid out of the skin at the injection site, commonly referred to as either leakage or backflow.
Studies indicate that the amount of detected leakage is not of clinical significance.1-4 However, leakage influences the patient perception of insulin administration, because the patients are concerned whether they have received the correct dosage.5-7 Therefore, clinicians may be consulted by concerned patients to support their choice of injection technique and pen needle.
The present article investigates how different injection techniques and needle design factors potentially influence the volume and frequency of leakage following subcutaneous injections. Identified from a literature study of leakage, these factors were chosen to be injection region (abdomen or thighs), injection volume, injection speed, needle wall thickness, needle taper (outer shape of needle), needle insertion angle into the skin, and wait time after an injection until the needle is withdrawn from the skin. The 3 first mentioned factors were informed by analyzing unpublished leakage data from a previous clinical trial,8 and the 4 latter factors were investigated in an exploratory leakage study on pigs. See Table 1 for an overview of the identified factors potentially influencing leakage, and our choice of data to substantiate our recommendation pertaining to these factors.
Table 1.
Overview of the Identified Factors Potentially Influencing Leakage, and Our Choice of Data to Substantiate Our Recommendation Pertaining to These Factors.
| Investigated factor | Publications discussing the factor | Post hoc analysis of clinical trial data | Pig study |
|---|---|---|---|
| Injection region (abdomen/thighs) | 1, 2, 10, 11 | X | |
| Injection volume | 3, 8, 19-21 | X | |
| Injection speed | 9, 13, 14, 19, 21 | X | |
| Needle wall thickness | 26, 27 | X | |
| Needle taper | 28 | X | |
| Needle insertion angle into the skin | 3, 13, 14 | X | |
| Wait time after an injection | 13, 14, 19, 21-25 | X |
Methods
Leakage Study in Clinical Trial
The clinical trial was a single-center, 1-visit, double-blinded, randomized controlled trial, registered at ClinicalTrials.gov with number NCT01680328. Investigation of leakage was a secondary objective of the trial and the amount of leakage was a prespecified secondary endpoint, but only parts of leakage data were analyzed and included in the publication.8 The body of clinical leakage data collected was large, and intended for further analysis, and therefore suitable for a post hoc analysis of factors potentially influencing leakage.
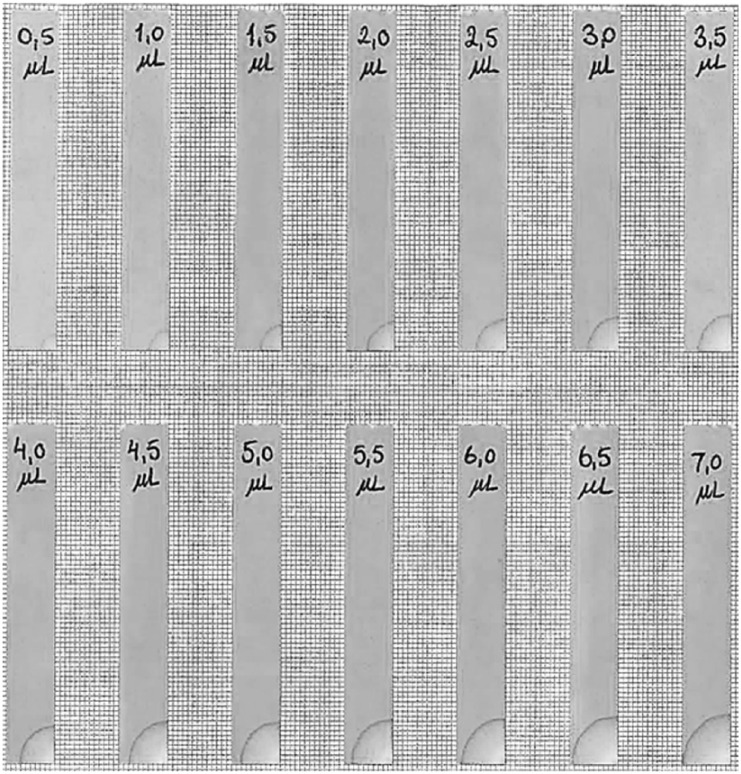
In brief, the trial included 82 injection experienced adult subjects (mean age 54.3 years ± standard deviation [SD] 11.9, 61% male, mean body mass index 26.4 kg/m2 ± SD 2.6) with type 1 or type 2 diabetes mellitus (mean diabetes duration 17.9 years ± SD 11.3, self-injecting for at least 6 months).8 Each subject received a total of 17 sodium chloride 9 mg/mL solution injections and 2 needle insertions (no injection), in the abdomen and thighs, and with different volume and speed combinations. Three injection speeds were tested (150, 300, and 450 µL/s) and 4 injection volumes were tested (400, 800, 1200, and 1600 µL). Injection speed was controlled using a programmable syringe pump (Harvard Apparatus, Holliston, MA, USA), and the needles were TSK STERiJECT 6 mm 30G luer lock needles (TSK Laboratory, Tochigi-Ken, Japan). To blind the injection speed and volume combinations for the patients, all needles were kept in the skin for 15-20 seconds before withdrawal.8 Two minutes after each injection, leakage was measured by placing a filter paper over the injection site until all liquid was absorbed onto the paper (pH sticks, Macherey-Nagel, Düren, Germany). The area of the wet spot on the filter paper served as a measure of the leakage and was estimated using a leakage evaluation scale for comparison (Figure 1).
Figure 1.
Extract of the leakage comparison scale used to quantify leakage in both the pig study and the clinical trial. With volume increments of 0.5 µL, saline was absorbed on pH filter strips from the corner. When dry, the filter strip was mounted on graph paper with 1 × 1 mm squares. When quantifying absorbed leakage from an injection, the graph paper lines are used as help lines when determining the amount of leakage. Figure not to scale. Full leakage comparison scale can be acquired from corresponding author.
Leakage Study on Pigs
The study was approved by the Animal Experiments Inspectorate, the Ministry of Food, Agriculture and Fisheries, Denmark. Two hundred injections (25 of each injection setup) of 400 µL sodium chloride 9 mg/mL solution were performed in the lateral part of the upper neck of 4 anesthetized pigs (Landrace Yorkshire Duroc pigs of approximately 100 kg). The sample size of 25 was chosen based on previous experience with leakage data from pig studies.9
Injections were given with NovoFine® 32G tip 6 mm ETW (extra-thin-walled) needles (Novo Nordisk A/S, Bagsværd, Denmark) using perpendicular (~90°) needle insertion, and with a 6-second wait time from end of injection to needle withdrawal from skin unless otherwise specified.
The injection speed was controlled by hand and the investigator making all injections was trained to deliver at a speed of approximately 100 µL/s.
The leakage after needle removal was collected and quantified using the same method as in the clinical trial.
To investigate the influence of needle wall thickness, taper, insertion angle, and wait time on leakage, the following injection setups were used:
Needle Wall Thickness
The first NovoFine 32G tip 6 mm needle (Novo Nordisk A/S, Bagsværd, Denmark) was thin-walled (TW), but was later marketed in an ETW version to increase the inner diameter and thereby flow in the needle. The wall thicknesses of the TW and ETW needles are 0.050 mm and 0.043 mm, respectively. These 2 needles were used to test the effect of needle wall thickness on leakage.
Needle Taper
The NovoFine 32G tip needle has a tapered cannula, which is 32G at the needle tip and 31G at the needle base. Thus, to test whether cannula tapering has an effect on leakage, and whether the amount may relate to the tip or base diameter, the tapered needle was tested against NovoFine 31G 6 mm needles (Novo Nordisk A/S, Bagsværd, Denmark) and custom-made 32G 6 mm needles identical to the NovoFine 32G tip needles apart from having a nontapered cannula (manufactured by Hart Needles, Sparta, MI, USA).
Needle Insertion Angle
Needles were inserted either perpendicular to the skin (90°) or in a handheld estimated 45° angle.
Wait Time
Wait times of 0 (immediate withdrawal), 3, 6, and 10 seconds after end of injection were tested to compare the wait time effect on leakage.
Statistical Methods
Statistical analysis was performed using SAS JMP 10.0.2 (SAS Institute, Cary, NC, USA). All data are presented as mean values and 95% confidence interval (CI) unless otherwise specified. A significance level of 5% was used throughout the study. Data of leakage volumes were analyzed by an ANOVA model with subject, volume, nested speed (volume), and speed × volume as fixed effects.
Results
In the clinical trial there were a total of 1392 injections and 164 needle insertions. Leakage occurred from 548 (39%) of the injections and from 8 (5%) of the insertions. The absolute leakage volumes from the injections ranged from 0 to 50 µL, and the relative leakage volumes ranged from 0 to 10% of the injected volume.
In the pig study, leakage appeared in 174 out of the 200 injections (87%). The largest amount of leakage was 12 µL (3% of the injected volume).
Injection Region
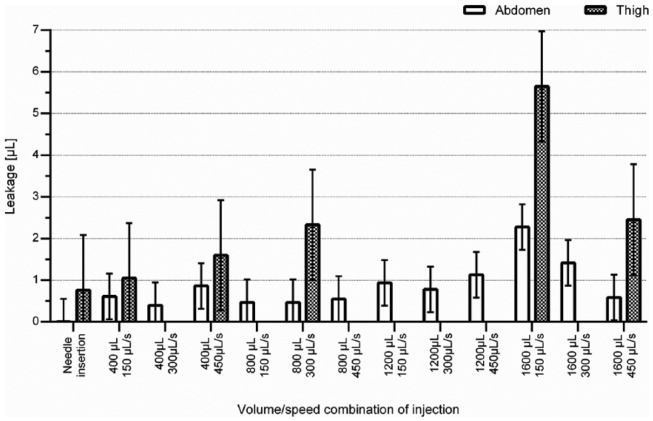
In the clinical trial leakage was related to injection region with abdominal injections causing both lower frequency and smaller amount of leakage than thigh injections (P < .0001) (Figure 2 and Table 2). Leakage was seen in 26% of abdominal injections and 44% of thigh injections (P < .0001). The 82 needle insertions without injections in the abdomen and thighs caused leakage in 1 and 7 cases, respectively.
Figure 2.
Leakage data from the clinical trial. Bars and error bars denote means and 95% confidence intervals (CI), respectively. All injections and needle insertions were performed with the same type of needle, and the needle was inserted in the skin for a total time of 15-20 seconds. Data quantities are seen in Table 2.
Table 2.
Leakage Data From Clinical Trial With Leakage Mean Volumes and 95% Confidence Interval (CI) for the Different Injection Setups.
| Level |
Region | n | Leakage mean (µL), [95% CI] | Letter | |
|---|---|---|---|---|---|
| Injection volume [µL] |
Injection speed[µL/s] | ||||
| Needle insertion | Abdomen | 82 | 0.01 [-0.54, 0.55] | D | |
| 400 | 150 | Abdomen | 82 | 0.61 [0.06, 1.16] | C, D |
| 400 | 300 | Abdomen | 81 | 0.40 [-0.16, 0.95] | C, D |
| 400 | 450 | Abdomen | 82 | 0.86 [0.31, 1.41] | B, C |
| 800 | 150 | Abdomen | 82 | 0.47 [-0.08, 1.02] | C, D |
| 800 | 300 | Abdomen | 82 | 0.47 [-0.08, 1.02] | C, D |
| 800 | 450 | Abdomen | 82 | 0.55 [0.00, 1.10] | C, D |
| 1200 | 150 | Abdomen | 82 | 0.93 [0.39, 1.48] | B, C |
| 1200 | 300 | Abdomen | 82 | 0.78 [0.23, 1.33] | B, C, D |
| 1200 | 450 | Abdomen | 82 | 1.13 [0.58, 1.68] | B, C |
| 1600 | 150 | Abdomen | 82 | 2.27 [1.73, 2.82] | A |
| 1600 | 300 | Abdomen | 82 | 1.41 [0.87, 1.96] | B |
| 1600 | 450 | Abdomen | 82 | 0.59 [0.04, 1.13] | C, D |
| Needle insertion | Thigh | 82 | 0.76 [-0.56, 2.08] | E | |
| 400 | 150 | Thigh | 82 | 1.05 [-0.27, 2.37] | E |
| 400 | 450 | Thigh | 82 | 1.60 [0.28, 2.92] | E |
| 800 | 300 | Thigh | 82 | 2.33 [1.01, 3.65] | E |
| 1600 | 150 | Thigh | 82 | 5.65 [4.32, 6.97] | F |
| 1600 | 450 | Thigh | 81 | 2.45 [1.12, 3.78] | E |
The numbers and percentages of injections causing leakage are given. Leakage from levels not connected by the same letter are significantly different (P < .05) (analysis performed separately for abdomen and thigh injections). The needle used for all injections and needle insertions were TSK laboratories 30G 6 mm.
Injection Volume
Injection volume had impact on the leakage8 so that larger injection volume caused larger absolute leakage volumes in both the abdomen and thighs (P < .0001). An injection volume of 1600 µL caused more leakage than 400 and 800 µL injections in both the abdomen and thighs (P < .001) (Figure 2 and Table 2). There was no statistical difference in leakage between injection volumes of 400, 800 and 1200 µL. In relative terms, injection volume did not affect leakage volume. Leakage frequency increased with increasing injection volume (P < .0001). However, no difference in frequency was seen between 400 and 800 µL injections (27% and 28% in the abdomen and 43% and 45% in the thighs, respectively). Similarly, there was no difference in frequency of leakage from injections of 1200 and 1600 µL in the abdomen (37% and 42%, respectively). Leakage frequency was 69% for 1600 µL injections in the thighs.
Injection Speed
Overall, the injection speed did not influence leakage volume or frequency. However, there was a significant interaction between injection volume and speed (P < .01). This implies that the effect on leakage volume of the injection volume is different for different levels of injection speed. In particular for injection volumes of 1600 μL, the amount of leakage was negatively related to injection speed in both the abdomen and the thighs, which was not the case for any of the other injection volumes.
Needle Wall Thickness
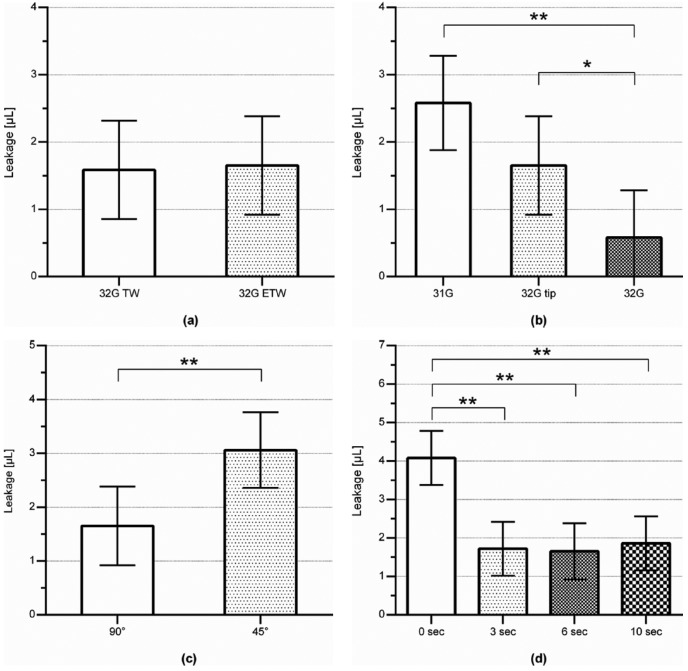
Needle wall thickness did not influence the amount or frequency of leakage after an injection (Figure 3a and Table 3, injection ID 1 and 2).
Figure 3.
Leakage data from the pig study. Bars and error bars denote mean and 95% CI, respectively. Asterisks denote a significant difference with *P < .05 or **P < .01. Data quantities are seen in Table 3. (a) Effect of needle wall thickness on the amount of leakage after injections. A NovoFine 32G tip 6 mm extra-thin-walled needle was tested against the same needle in the older thin-walled version. Both needles were inserted perpendicular to the skin and with 6 seconds wait time between end of injection and needle removal from skin. (b) Effect of needle taper on the amount of leakage after injections. Tapered NovoFine 32G tip 6 mm needles were tested against nontapered 6 mm needles in 32G and 31G. All needles were inserted perpendicular to the skin and with 6 seconds wait time between end of injection and needle removal from skin. (c) Effect of needle insertion angle in the skin on the amount of leakage after injections. The NovoFine 32G tip 6 mm needles were inserted either perpendicular to the skin (90°) or in a 45° angle, and with 6 seconds wait time between end of injection and needle removal from skin. (d) Effect of the wait time between end of injection and needle removal from skin on the amount of leakage after injections. All needles were NovoFine 32G tip 6 mm needles which were inserted perpendicular to the skin.
Table 3.
Leakage Data From Pig Study With Leakage Mean Volume and SD for the Different Injection Setups.
| Level | Needle gauge | Wall thickness | Insertion angle (°) | Wait time (sec) | n | Leakage mean (µL), [95% CI] | Letter |
|---|---|---|---|---|---|---|---|
| 1 | 32G tip | TW | 90 | 6 | 23 | 1.59 [0.86, 2.32] | C, D |
| 2 | 32G tip | ETW | 90 | 6 | 23 | 1.65 [0.92, 2.38] | C |
| 3 | 31G | TW | 90 | 6 | 25 | 2.58 [1.88, 3.28] | B, C |
| 4 | 32G | ETW | 90 | 6 | 25 | 0.58 [-0.12, 1.28] | D |
| 5 | 32G tip | ETW | 45 | 6 | 25 | 3.06 [2.36, 3.76] | B |
| 6 | 32G tip | ETW | 90 | 0 | 25 | 4.08 [3.38, 4.78] | A |
| 7 | 32G tip | ETW | 90 | 3 | 25 | 1.72 [1.02, 2.42] | C |
| 8 | 32G tip | ETW | 90 | 10 | 25 | 1.86 [1.16, 2.56] | C |
The numbers and percentages of injections causing leakage are given. Leakage from levels not connected by the same letter are significantly different. Injected volume for all injections were 400 µL with a speed of ~100 µL/s. All needles were NovoFine needles, except needle 4, which was a custom-designed needle designed to match the NovoFine 32G tip needle on all parameters except for not being tapered.
Needle Taper
Needle taper influenced volume of leakage after an injection; injections with the 32G straight needle caused less leakage than with the 31G straight needle (P < .01) and the 32G tip tapered needle (with 31G base) (P < .05), with estimated differences of 2.00 µL and 1.07 µL, respectively (Figure 3b and Table 3, injection ID 2, 3, and 4). Furthermore, the frequency of leakage was less with the 32G straight needle as opposed to the 32G tip tapered needle and the 31G straight needle (64%, 91%, and 96%, respectively) (P < .01).
Needle Insertion Angle
Needle insertion angle influenced leakage after an injection, with 45° insertion angle causing increased leakage compared to 90° insertion angle (P < .01) (Figure 3c and Table 3, injection ID 2 and 5). There was no difference in frequency of leakage between 45° and 90° injections.
Wait Time
Immediate withdrawal (wait time of 0 seconds) of the needle from the skin after the injection influenced the leakage (P < .0001) (Figure 3d and Table 3, injection ID 2, 6, 7, and 8). Immediate withdrawal caused more leakage than when waiting 3 (P < .01), 6 (P < .01), or 10 (P < .01) seconds, with estimated differences of 2.36 µL, 2.42 µL, and 2.22 µL, respectively. There was no difference in frequency of leakage between the different wait times.
Discussion
In the 2 studies it was investigated how injection technique, in terms of injection site, needle insertion angle, injection volume, injection speed and wait time, and needle design, in terms of wall thickness and needle taper, influence leakage after subcutaneous injections.
Leakage Influenced by Injection Technique
Typical injection sites for a person with diabetes are the abdomen, thighs, buttocks, and upper arms, but only injections in the abdomen and thighs have been investigated for a relation with insulin leakage. Clinical trials provide contradicting conclusions, since some have found that leakage is more likely to occur after injections in the thighs than in the abdomen,3,8,10 whereas others found no relation between injection site and leakage.2,11 Our data show that when injecting in the subcutaneous tissue of the abdomen, both the frequency and the amount of leakage are lower than after injections in the thighs. It is unknown why less leakage is seen in the abdomen than in the thighs. However, a previous study described that making a skinfold in the abdomen is easier than in the thighs,12 which may indicate important differences in tissue density, internal tissue pressure, or other mechanical factors of the subcutaneous layer and the skin.
Whether the needle insertion angle influences leakage has been discussed previously with contradicting recommendations.13,14 In the present study, an angled needle insertion of 45° caused more leakage than after an injection with a perpendicular needle insertion. This may be explained by the fact that when using an angled insertion, the deposit of insulin is situated closer to the dense dermal layer. The difference may be that a deeper subcutaneous insulin deposition displaces the soft subcutaneous tissue without much resistance, while a deposition closer to the much more resistant dermal layer may cause higher pressure and more fluid to be forced out of the needle puncture. Thus, using an angled insertion might correspond to using a shorter needle. A number of studies conclude that a shorter needle causes less or equal amount of leakage than when using a longer needle.2,4,15-18 However, in these studies investigators tested not only needles of different length but also of different diameter, which could potentially influence the results. Two prior studies isolated needle length as the varying parameter and found that when only varying the length, shorter needles cause more leakage.9,11 Therefore, the increased leakage for 45° insertions in our study could potentially be explained by the closer deposition to the dermal layer. Leakage frequency has been reported to be higher with perpendicular than with angled injections (65% for perpendicular and 59% for angled, P < .001).3 In our study, there was no difference in frequency of leakage between perpendicular and angled injections.
Larger injection volumes have been reported to cause more leakage in absolute terms.8,19-21 In 1 study, the volume of the injection did not affect the frequency of leakage.3 In accordance with most previous studies, we found that larger injection volumes cause larger absolute leakage.8 The relative amount of leakage did not differ for different injected volumes, which is also in line with a published study.21 The reason for the injection volume dependency could be an increased tissue pressure on the insulin deposition from the surrounding subcutaneous tissue. Thereby, a larger amount of leakage could be pushed out of the needle puncture. Although no difference in leakage volume was seen between 400, 800, and 1200 µL injections, the 1200 µL injections caused leakage more frequently.
The effect of wait time on leakage has been investigated by several research groups, resulting in that the recommended wait time has decreased over the years; from 30-60 seconds in 1991,19 to 20 seconds in 2006,13 down to 10 seconds in publications between 2010 and 2012,14,21-23 and 6-10 seconds in a 2011 publication.24 A study from 2010 concluded that there was no relationship between wait time (more than/less than 10 seconds) and the volume of leakage from the injection site.25 To our knowledge wait times less than 6 seconds have not been investigated prior to our study. Our data show that immediate withdrawal of the needle caused more leakage than waiting as little as 3 seconds before withdrawal. No differences were observed between wait times of 3, 6, and 10 seconds. Thus, the subcutaneous tissue may not need more than 3 seconds to even out the applied pressure of an injection.
In several publications it is advised to inject slowly to avoid leakage,13,14,19,21 but in an experimental approach on porcine skin, no relation between injection speed and amount of leakage was found.9 Overall, speed did not influence leakage in our study, but higher speed caused the numerically largest absolute leakage, except for the injection volume of 1600 μL for which the leakage was negatively related to injection speed. It should, however, be noted that regardless of injection speed and dose, all needles were in the skin for a total of 15-20 seconds to blind the injection speed and volume combination.8 Thus, the 1600 µL dose at the fastest speed of 450 µL/s had a short injection time (3.5 seconds) followed by a long wait time (11.5-16.5 seconds), while the 1600 µL dose at lowest speed had a long injection time (10.7 seconds injection) and a short wait time (4.3-9.3 seconds). This could explain the negative relation between injection speed and leakage seen for 1600 µL injections. Although the findings regarding wait time revealed that waiting times between 3 and 10 seconds did not influence leakage, it should be noted that the doses used to test for wait time effect on leakage was 400 µL. For a dose 4 times as large, waiting time might play an important role, wherefore the combination of dose volume, speed and wait time should be assessed in future studies.
Leakage Depending on Needle Design
Using 32G needles, needle wall thickness did not influence leakage, which is opposed to published results26,27 where patients self-reported leakage and voted in favor of thin walled needles for less leakage. However, the studies were not blinded for the experimental needle, why a bias might have been introduced.
In the pig study, 32G tip tapered needles, with a 31G needle base, caused more leakage than 32G nontapered needles, and numerically (nonsignificant) less leakage than the 31G nontapered needles. Hence, injection with a tapered needle causes an amount of leakage which quantitatively lies in between the amount of leakage from straight needles having the tapered needle’s tip and base diameters. Published literature conclude that when using 30G, 32G, and 34G needles, a thinner needle causes less leakage after an injection.9 Also according to the literature, needle taper does not influence leakage after injections28 when comparing a 33G tip tapered needle (28G base) to a 31G straight needle. Therefore, our finding that leakage after injection with a tapered needle corresponds to using a straight needle with a diameter size between the tip and base diameters is in agreement with published data. Again, these results support the claim that diameter is one of the most influential needle parameters when it comes to leakage after an injection.
Strengths, Limitations, and Future Work
Anesthesia is known to reduce blood pressure, and thus tissue pressure, which could potentially have influenced leakage in the pig study, but the anesthesia was light, so any effect is assumed minor and negligible. Furthermore, all experiments were conducted on pigs which all had received the same anesthesia, so a given effect hereof is expected to be the same for all leakage measurements in the pig study. Thus, the differences between the different injection setups should not have been affected by this, but possibly the absolute leakage volumes.
In the pig study, the subcutaneous injections caused leakage more frequently than in the clinical trial, roughly twice as often. This could be due to a denser adipose tissue in pigs than in humans caused by more fibrous tissue or different composition of the fatty acids in porcine versus human adipose tissue. It could also be due to the fact that different injection setups were used in the 2 studies in terms of, for example, injection speed, use of skin fold, needle type, and wait times. For instance, it is unknown whether using a skinfold influences leakage since it has both been argued that skinfold reduces leakage1,14,24 and has no effect on leakage.11,20 A limitation is that a representative skinfold lifting is not possible in pigs, which might be due to a possible denser dermal layer. However, the mean leakages in the clinical trial and the pig study are in same order of magnitude, so it is assumed that despite the skinfold limitation, the pig is a relevant model for leakage investigation. Thus, it could be interesting for future clinical studies to determine if the use of skinfold impacts leakage.
The clinical trial demonstrated generally larger inter- and intrasubject variations in leakage after injections than the pig study. Of the 1556 injections and needle insertions administered, 556 caused a leakage recorded as a volume > 0 μL. A leakage volume greater than 10 μL, which would correspond to 1 IU of a U100 insulin formulation, was seen in 43 cases (2.7%). Thus, the leakage amounts were generally low and can therefore be considered as of minor clinical relevance in most cases of insulin use. However, the clinical relevance can depend on other factors, for example, the potency of the injected drug, intended dose, small-volume and pediatric users, and whether the user of the drug is in a titration period. Thus, leakage should always be minimized or avoided, if possible.
Several methods of quantifying leakage after an injection have been used in prior studies, including weighing the leakage,3,18 using a comparison chart or scale for volume estimation,4,16 measuring or digitally quantifying the wet spot on filter paper,1,9,15 using questionnaires or visual analog scales comparing leakage from 2 or more injections,26,29,30 or by quantifying amount of insulin hormones using ELISA20 or γ counting of radioactively marked insulin.21 In both the clinical trial8 and present pig study, a leakage comparison scale was used as volume estimation of leakage after injection. The method was chosen because the scale method with the filter paper strips mounted on graph paper made it easy to discriminate by approximately 0.5 µL, and it was feasible to use.
When leakage is experienced by users, it is unknown whether the leakage fluid is a part of the injected insulin, interstitial fluid or blood, or a combination. The fact that leakage can occur without injection indicates that the leakage experienced by people with injection treatment might be a combination of the injected drug and extracellular fluid. To our knowledge the constituents of leakage have not been investigated in previous studies, so this could be the topic for future studies.
Conclusions
In conclusion, neither needle wall thickness nor injection speed affected leakage. To minimize leakage, an injection should be performed with perpendicular insertion of a thin needle in the abdomen, be less than 800 µL in volume, and the needle should be kept in the skin for 3 seconds after the end of dose.
Acknowledgments
Thank-you to Charlotte Jensen (Novo Nordisk A/S) for help with carrying out the pig study, the vets and animal caretakers at the pig facilities at Novo Nordisk A/S, and Tim Heise, Leszek Nosek, Sibylle Dellweg, and Eric Zijlstra (Profil Institute, Germany), Gert Nielsen, Berit Kjeldsen, Marianne Qvist (all employees at Novo Nordisk A/S), and all other people involved in the clinical trial.
Footnotes
Abbreviations: CI, confidence interval; ETW extra thin wall; G, gauge (needle diameter); GLP-1, glucagon-like peptide-1; mL, milliliter; mg, milligram; µL, microliter; TW, thin wall.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This study is part of the Danish industrial PhD program, sponsored by the Innovation Fund Denmark and Novo Nordisk A/S. KAP, MLJ, TS, NBM, and JK are all employees of Novo Nordisk A/S. MLJ, TS, NBM, and JK all hold stock in Novo Nordisk A/S.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The studies are part of KAP’s industrial PhD studies, which are funded by the Danish Innovation Fund as well as Novo Nordisk A/S.
References
- 1. Hanas R, Lytzen L, Ludvigsson J. Thinner needles do not influence injection pain, insulin leakage or bleeding in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2000;1:142-149. [DOI] [PubMed] [Google Scholar]
- 2. Birkebaek NH, Solvig J, Hansen B, Jorgensen C, Smedegaard J, Christiansen JS. A 4-mm needle reduces the risk of intramuscular injections without increasing backflow to skin surface in lean diabetic children and adults. Diabetes Care. 2008;31:e65. [DOI] [PubMed] [Google Scholar]
- 3. Hofman PL, Derraik JG, Pinto TE, et al. Defining the ideal injection techniques when using 5-mm needles in children and adults. Diabetes Care. 2010;33:1940-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergenstal RM, Strock ES, Peremislov D, Gibney MA, Parvu V, Hirsch LJ. Safety and efficacy of insulin therapy delivered via a 4mm pen needle in obese patients with diabetes. Mayo Clin Proc. 2015;90:329-338. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association. Adults living with type 2—insulin leakage. Available at: http://community.diabetes.org/t5/Adults-Living-with-Type-2/Insulin-leaking-out-of-skin-at-injection/td-p/137045. Accessed November 18, 2015.
- 6. Diabetes Daily. Insulin leaking out of your skin. Available at: http://www.diabetesdaily.com/forum/multiple-daily-injections-mdi/12507-insulin-leaking-out-of-your-skin/. Accessed November 18, 2015.
- 7. Diabetes Forums. Lantus insulin leakage? Available at: http://www.diabetesforums.com/forum/topic/48700-lantus-insulin-leakage/. Accessed November 18, 2015.
- 8. Heise T, Nosek L, Dellweg S, et al. Impact of injection speed and volume on perceived pain during subcutaneous injections into the abdomen and thigh: a single-centre, randomized controlled trial. Diabetes Obes Metab. 2014;16:971-976. [DOI] [PubMed] [Google Scholar]
- 9. Juul KA, Bengtsson H, Eyving B, et al. Influence of hypodermic needle dimensions on subcutaneous injection delivery—a pig study of injection deposition evaluated by CT scanning, histology, and backflow. Skin Res Technol. 2012;18:447-455. [DOI] [PubMed] [Google Scholar]
- 10. Van Doorn L, Alberda A, Lytzen L. Insulin leakage and pain perception with NovoFine 6 mm and NovoFine 12 mm needle lengths in patients with type 1 or type 2 diabetes. Diabetic Med. 1998;1:S50. [Google Scholar]
- 11. Kreugel G, Keers JC, Kerstens MN, Wolffenbuttel BH. Randomized trial on the influence of the length of two insulin pen needles on glycemic control and patient preference in obese patients with diabetes. Diabetes Technol Ther. 2011;13:737-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hofman P, Lawton S, Peart J, et al. An angled insertion technique using 6-mm needles markedly reduces the risk of intramuscular injections in children and adolescents. Diabetic Med. 2007;24:1400-1405. [DOI] [PubMed] [Google Scholar]
- 13. Hanas R. Side Effects of Insulin Treatment. Type 1 Diabetes in Children, Adolescents, and Young Adults: How to Become an Expert on Your Own Diabetes. London: Class Publishing; 2007. [Google Scholar]
- 14. Frid A, Hirsch L, Gaspar R, et al. New injection recommendations for patients with diabetes. Diabetes Metab. 2010;36(suppl 2):S3-S18. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz S, Hassman D, Shelmet J, et al. A multicenter, open-label, randomized, two-period crossover trial comparing glycemic control, satisfaction, and preference achieved with a 31 gauge × 6 mm needle versus a 29 gauge × 12.7 mm needle in obese patients with diabetes mellitus. Clin Ther. 2004;26:1663-1678. [DOI] [PubMed] [Google Scholar]
- 16. Hirsch LJ, Gibney MA, Albanese J, et al. Comparative glycemic control, safety and patient ratings for a new 4 mm × 32G insulin pen needle in adults with diabetes. Curr Med Res Opin. 2010;26:1531-1541. [DOI] [PubMed] [Google Scholar]
- 17. Strauss K, Hannet I, McGonigle J, et al. Ultra-short (5 mm) insulin needles: Trial results and clinical recommendations. Pract Diabetes Int. 1999;16:218-222. [Google Scholar]
- 18. Ignaut DA, Fu H. Comparison of insulin diluent leakage postinjection using two different needle lengths and injection volumes in obese patients with type 1 or type 2 diabetes mellitus. J Diabetes Sci Technol. 2012;6:389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Broadway CA. Commentary prevention of insulin leakage after subcutaneous injection. Diabetes Educ. 1991;17:90. [DOI] [PubMed] [Google Scholar]
- 20. Ross S, Jamal R, Leiter L, et al. Evaluation of 8 mm insulin pen needles in people with type 1 and type 2 diabetes. Pract Diabetes Int. 1999;16:145-148. [Google Scholar]
- 21. Wittmann A, Kover J, Kralj N, et al. Insulin leakage value in relation to pen needle length and administered dose after subcutaneous injection. Diabetes Technol Ther. 2010;12:587-590. [DOI] [PubMed] [Google Scholar]
- 22. Saltiel-Berzin R, Cypress M, Gibney M. Translating the research in insulin injection technique: implications for practice. Diabetes Educ. 2012;38:635-643. [DOI] [PubMed] [Google Scholar]
- 23. Kalra S, Balhara YP, Baruah MP, et al. Forum for injection techniques, India: the first Indian recommendations for best practice in insulin injection technique. Indian J Endocrinol Metab. 2012;16:876-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hansen B, Matytsina I. Insulin administration: selecting the appropriate needle and individualizing the injection technique. Expert Opin Drug Deliv. 2011;8:1395-1406. [DOI] [PubMed] [Google Scholar]
- 25. De Coninck C, Frid A, Gaspar R, et al. Results and analysis of the 2008-2009 Insulin Injection Technique Questionnaire survey. J Diabetes. 2010;2:168-179. [DOI] [PubMed] [Google Scholar]
- 26. Siegmund T, Blankenfeld H, Schumm-Draeger PM. Comparison of usability and patient preference for insulin pen needles produced with different production techniques: “thin-wall” needles compared to “regular-wall” needles: an open-label study. Diabetes Technol Ther. 2009;11:523-528. [DOI] [PubMed] [Google Scholar]
- 27. Aronson R, Gibney MA, Oza K, Berube J, Kassler-Taub K, Hirsch L. Insulin pen needles: effects of extra-thin wall needle technology on preference, confidence, and other patient ratings. Clin Ther. 2013;35:923-933. [DOI] [PubMed] [Google Scholar]
- 28. Asakura T, Seino H, Nunoi K, et al. Usability of a microtapered needle (TN3305) for insulin treatment in Japanese patients with diabetes mellitus: a comparative clinical study with a standard thin wall needle. Diabetes Technol Ther. 2006;8:489-494. [DOI] [PubMed] [Google Scholar]
- 29. Iwanaga M, Kamoi K. Patient perceptions of injection pain and anxiety: a comparison of NovoFine 32-gauge tip 6mm and Micro Fine Plus 31-gauge 5mm needles. Diabetes Technol Ther. 2009;11:81-86. [DOI] [PubMed] [Google Scholar]
- 30. Kreugel G, Beijer H, Kerstens M, Ter Maaten J, Sluiter W, Boot B. Influence of needle size on metabolic control and patient acceptance. Eur Diabetes Nurs. 2007;4:51-55. [Google Scholar]