Requirements for blood glucose monitoring systems (BGMS) intended to be used by lay persons are stipulated in the international standard ISO 15197.1 System accuracy, e.g., shall be evaluated by comparing blood glucose (BG) results measured with a BGMS to those of a comparison method. A new version of the standard was published in 2013;2 ISO 15197:2003 is valid until May 2016. In contrast to the previous version, ISO 15197:2013 prescribes tighter acceptable system accuracy criteria. While ISO 15197:2003 allowed deviations of ±0.83 mmol/L (15 mg/dL) from the comparison values at BG concentrations <4.2 mmol/L (75 mg/dL) and ±20% at BG concentrations ≥4.2 mmol/L, ±0.83 mmol/L and ±15% for BG concentrations <5.55 mmol/L (100 mg/dL) and ≥5.55 mmol/L, respectively, are the limits according to ISO 15197:2013. Both versions of the standard stipulate that 95% of the measured values have to be within these limits. The second minimum system accuracy criterion stipulated only by ISO 15197:2013 is that 99% of all measured values have to fall within zones A and B of the consensus error grid (CEG) for type 1 diabetes. The CEG describes clinical accuracy of a BGMS; zones A und B indicate little or no effect on clinical outcome.3 While ISO 15197:2003 demanded the evaluation of 1 reagent system lot, ISO 15197:2013 requests 3 lots.
In this study, accuracy of the CE-marked BGMS GlucoRx Nexus TD-4277 (Taidoc Technology Corp, New Taipei City, Taiwan) was assessed following ISO 15197:2013. The test strip package insert of the BGMS implies compliance with ISO 15197:2013 accuracy acceptance criteria. The study was performed at the Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm in compliance with the German Medical Devices Act and was approved by the ethics committee and the competent authority.
Capillary blood samples from different subjects were each measured with 3 lots of the BGMS and evaluated against the comparison method YSI 2300 STAT Plus™ glucose analyzer (YSI Incorporated, Yellow Springs, OH, USA) (glucose oxidase method). The BGMS were purchased from a pharmacy in the United Kingdom by the funder of the study and were set up, adjusted and maintained according to the instructions for use. Daily measurements with control solution were performed. Regular internal and external quality control measures were performed as required by the German national standard to verify trueness and precision of the comparison method and daily quality control measurements were performed following internal standard operating procedures.
The limits of the ISO 15197:2013 standard were fulfilled by 92.5% (lot 1), 93.5% (lot 2), and 90% (lot 3) (n = 200 results for each lot) (Figure 1), so that compliance with ISO 15197:2013 requirements could not be shown for this BGMS. The acceptance criterion for the CEG was fulfilled. The system met the minimum system accuracy criteria of ISO 15197:2003 (96% to 98.5% within the limits). All 3 lots showed a considerable negative bias (calculated according to Bland and Altman)4 with respect to the comparison method (–6.3% [lot 1], –7.9% [lot 2], and −7.2% [lot 3]).
Figure 1.
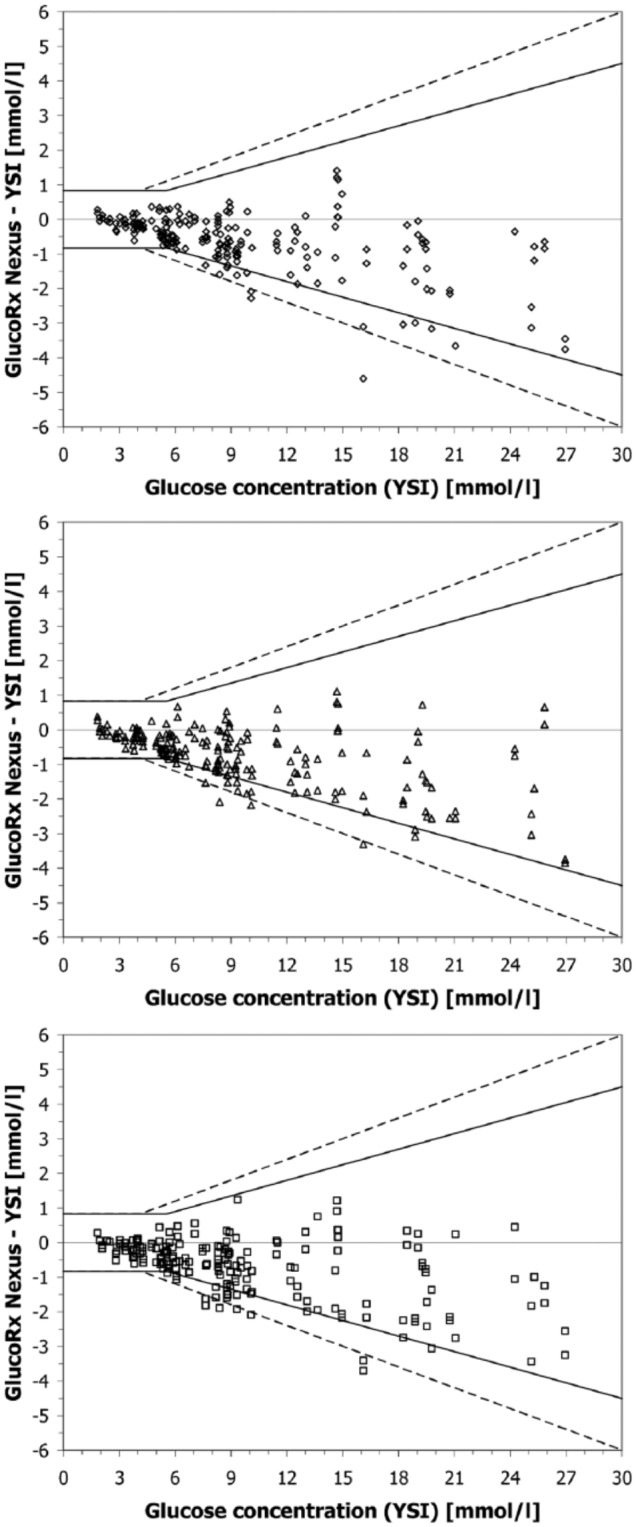
Absolute differences between BGMS results and comparison measurement results for lot 1 (top), lot 2 (middle), and lot 3 (bottom). For each lot, 200 data points (measurement of 100 samples in duplicate) are shown. Solid lines indicate system accuracy limits of ISO 15197:2013; dashed lines indicate limits of ISO 15197:2003.
In contrast to the information given in the package insert, in this study the investigated BGMS did not meet system accuracy requirements of ISO 15197:2013.
Footnotes
Abbreviations: BG, blood glucose; BGMS, blood glucose monitoring system; CEG: consensus error grid.
Declaration of Conflicting Interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by Roche Diagnostics GmbH, Mannheim, Germany.
References
- 1. International Organization for Standardization. In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. EN ISO 15197:2003 E. [Google Scholar]
- 2. International Organization for Standardization. In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. ISO 15197:2013 (E). [Google Scholar]
- 3. Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23(8):1143-1148. [DOI] [PubMed] [Google Scholar]
- 4. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307-310. [PubMed] [Google Scholar]


