Abstract
Objectives:
Pen devices offer advantages compared with vial and syringe (VaS). The purpose of this article was to evaluate efficacy of pen devices compared to VaS.
Methods:
A systematic review of literature was performed in 8 different databases. References were independently screened and selected. Primary observational or experimental studies comparing pen devices with VaS for insulin administrations were included. Studies on specific populations were excluded. Risk of bias was evaluated using appropriate tools. Data on glycosylated hemoglobin (HbA1c), hypoglycemia, adherence, persistence, patient preference, and quality of life (QOL) were collected. Meta-analysis was performed when appropriate. Heterogeneity and risk of publication bias were evaluated. Otherwise, descriptive analyses of the available data was done.
Results:
In all, 10 348 articles were screened. A total of 17 studies were finally selected: 7 experimental and 10 analytical. The populations of the included articles were mainly composed of adults with type 2 diabetes mellitus. Important risk of bias was found in all of the articles, particularly experimental studies. Meta-analyses were performed for HbA1c, hypoglycemia, adherence and persistence. Pen device showed better results in mean HbA1c change, patients with hypoglycemia, adherence and persistence compared to VaS. No difference was observed in number of patients achieving <7% HbA1c. Preference studies showed a tendency favoring pen devices, however nonvalidated tools were used. One QoL study showed improvements in some subscales of SF-36.
Conclusions:
There is evidence that pen devices offer benefits in clinical and, less clearly, patient-reported outcomes compared to VaS for insulin administration. However, these results should be taken with caution.
Keywords: diabetes mellitus, equipment and supplies, hemoglobin A, glycosylated, insulin, medication adherence, patient preference
Since the first application of a pancreatic extract in 19221 to the modern continuous subcutaneous insulin infusion pumps (CSII),1,2 different options have been developed for diabetic patients. Pen devices have attained a wide diffusion,1 despite limited evidence comparing their effectiveness against the traditional vial and syringe (VaS).2 Our aim was to perform a systematic review of literature on the effectiveness and safety profiles of these 2 options for insulin administration.
Methods
We followed the Colombian Agency for Technology Evaluation (IETS) guidelines,3 based on Canadian, European, and Argentinean guidelines, as well as PRISM group recommendations.4
We performed a systematic review of MEDLINE, EMBASE, Clinical Trials.gov, LILACS, Cochrane Database of Systematic Reviews, Cochrane Register of Controlled Trials, DARE, and International Clinical Trials Registry Platform.
Terms used and adapted for each database were (diabet*) AND (Insulin) AND (needles OR syringes OR “disposable equipment” OR “self administration” OR “drug delivery systems” OR “jet injections” OR “equipment design” OR devices).
Clinical studies, either analytical or experimental designs, on diabetic patients of any age, with indication for insulin therapy, comparing pen devices and VaS in terms of glycemic control, hypoglycemia, adherence, or quality of life (QoL) were included. Studies on specific clinical subpopulations (ie, gestational diabetes, patients with kidney failure) or using other designs (ie, case reports and series) were excluded. Selection was made by 2 independent investigators; full-text versions were obtained for data extraction.
Risk of bias was assessed using Cochrane’s risk of bias evaluation tool for experimental studies,5 and Scottish Intercollegiate Guidelines Network (SIGN) tool for analytical observational studies.6
Population characteristics, glycated hemoglobin (HbA1c), hypoglycemic episodes, treatment adherence, persistence, patient preference, and QoL data were extracted.
Data were reported using means, medians or proportions. Measures of effect were expressed as risk ratio (RR) or odds ratio (OR). Confidence intervals (CI) and standard deviations (SD) were obtained if available. This process was performed separately by 2 research team members with expertise in systematic reviews.
Meta-analyses were performed when homogeneity allowed, using Review Manager 5.3. Heterogeneous data results were reported and analyzed individually.
Chi-square and I2 tests were assessed. Significance level of P < .1 for χ2 test was considered consistent with heterogeneity, which was then stratified according to I2 value.7 If heterogeneity was detected, sensitivity analyses were performed.
Funnel plots for meta-analysis were visually inspected. Egger test was used if appropriate.8
An expert committee of 3 endocrinologists and an internal medicine specialist assisted the research team. They validated the original methodology, helped select the outcomes, and defined a variation >0.4% in HbA1c for glycemic control as clinically significant.9
Results
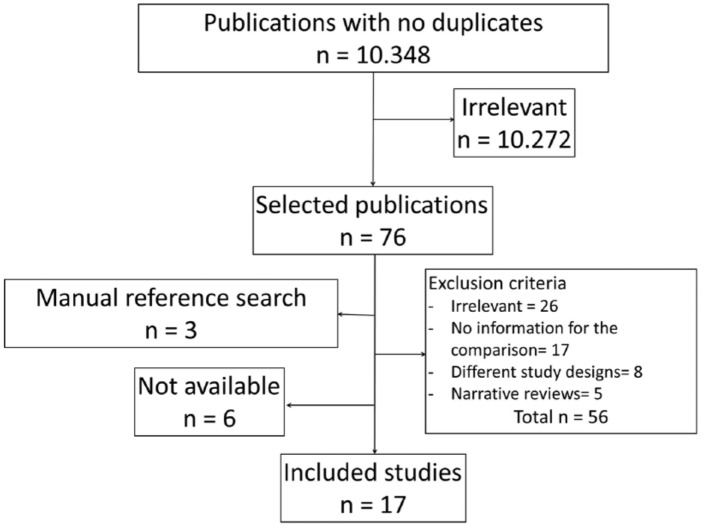
Of 10 348 initial references, 17 were finally included for data extraction (Figure 1): 10 retrospective cohort studies, 6 crossover randomized clinical trials, and a parallel nonrandomized clinical trial.
Figure 1.
Flow chart or literature search and article selection.
Most studies (13 of 17) were performed in the United States, with mostly type 2 diabetes mellitus (T2DM) patients, with mean age between 45 and 65. All studies reported initial HbA1c, levels, which on average were higher than 7%, some of them reaching even 10%. Some studies10-18 assessed exclusively insulin-naïve patients. Insulins used were mostly basal. Glargine was the most frequent (Table 1).
Table 1.
Characteristics of Included Studies and Their Populations.
| Study | Study type | Country | Sample size | Diabetes type | Insulin used | Outcomes | Max follow-up |
|---|---|---|---|---|---|---|---|
| Ahmann et al10 | RCOES | USA | 405 | 2 | Glargine | HbA1c, HyG, Pref | 40 weeks |
| Asche et al11 | RCAS | USA | 19 882 | 1 & 2 | Aspart | HyG | 1 year |
| Baser et al12 | RCAS | USA | 1064 | NS | Analogues | Adh | 1 year |
| Buysman et al13 | RCAS | USA | 1876 | 2 | NPH, detemir | Adh, Pers | 1 year |
| Cheen et al19 | RCAS | Singapore | 955 | NS | Premixed human and analogue | Adh, Pers | 2 years |
| Coscelli et al20 | RCOES | Italy | 60 | 1 & 2 | NPH, premixed | Hyg | 6 weeks |
| Davis et al14 | RCAS | USA | 3842 | 2 | Glargine | HbA1c, HyG, Pers | 1 year |
| Grabner et al15 | RCAS | USA | 2531 | 2 | Glargine | HbA1c, Hyg, Adh, Pers | 1 year |
| Kadiri et al21 | RCOES | Morocco | 96 | 2 | Premixed | Pref | 12 weeks |
| Korytkowski et al22 | RCOES | USA | 121 | 1 & 2 | Aspart 70/30 | Pref | 4 weeks |
| Lee et al23 | RCAS | USA | 1156 | 2 | Aspart, aspart 70/30 | HyG, Adh | 2 years |
| Lee et al24 | NROPES | Taiwan | 65 | 1 & 2 | NS | QoL | 12 weeks |
| Lee et al16 | RCAS | USA | 829 | 2 | Lispro | HyG, Adh | 2 years |
| Seggelke et al17 | RCOES | USA | 31 | 1 & 2 | Glargine | HbA1c, Pref | 12 weeks |
| Shelmet et al25 | RCOES | USA | 79 | 1 & 2 | NPH, NPH/human insulin | Pref | 6 weeks |
| Xie et al26 | RCAS | USA | 3893 | 2 | Glargine | HbA1c, HyG, Adh, Pers | 1 year |
| Xie et al18 | RCAS | USA | 1308 | 2 | Glargine | HbA1c, HyG, Pers | 1 year |
The SIGN tool showed an acceptable quality, with deficiencies in blinding of the evaluator on patients’ status. Baser12 had a low quality score, related to the lack of explicit ways to consider confounding factors and to not reporting confidence intervals in the results; they reported, however, standard deviations.
Experimental studies had high risk of bias; the main issues were nonblinded design and insufficient explanation of randomization method.
Glycated Hemoglobin
Lee et al24 found no difference between pen devices and VaS in HbA1c change after 12-week follow-up (–0.6 ± 0.3 vs −0.3 ± 0.2; P = .452). Seggelke et al’s17 crossover study, with two 12-week treatment periods with pen or VaS, found a statistical difference favoring pen devices (7.1 ± 1.6 vs 8.5 ± 2; P < .01). Ahmann et al10 reported no difference between both groups in the percentage of patients that achieved HbA1c < 7% (37.7% vs 37%; P = .89) after 40-week follow-up.
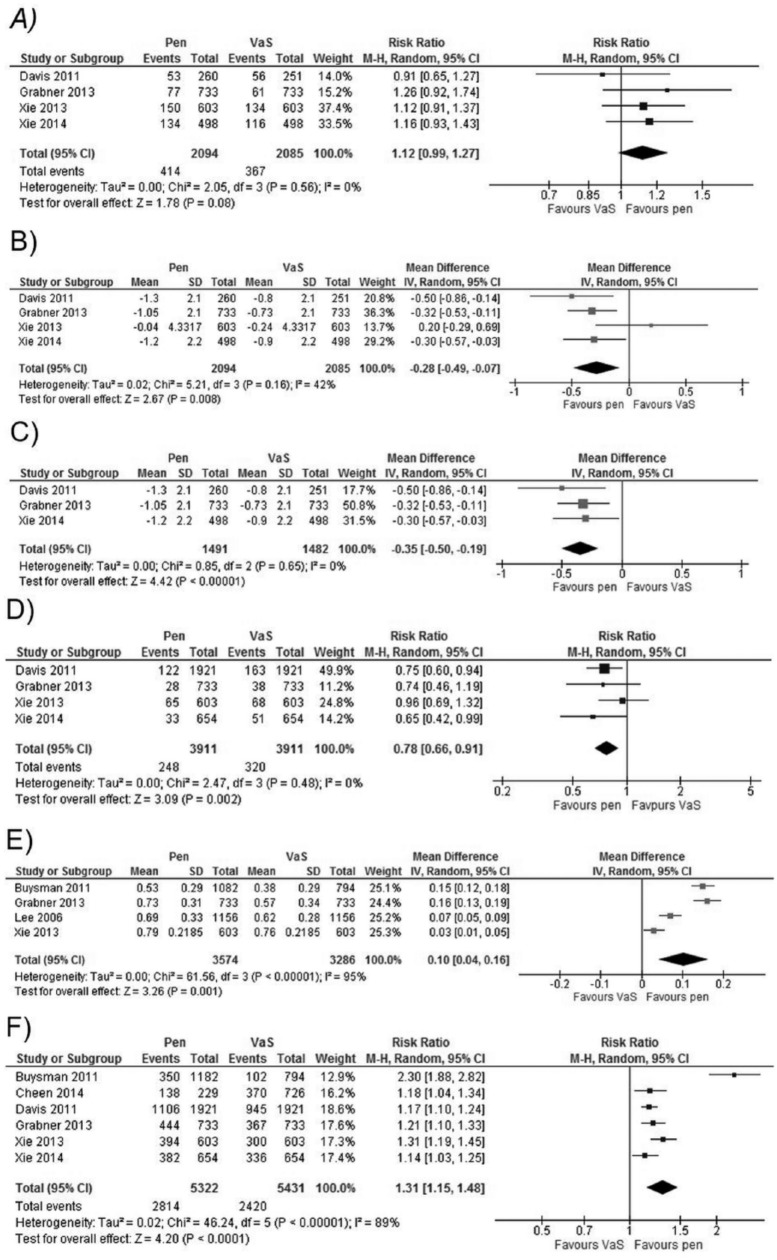
Four studies14,15,18,26 presented results at 12 months. Meta-analysis showed a non–statistically significant trend toward pen devices in the percentage of patients who reached HbA1c<7% (Figure 2A) without significant heterogeneity. There was a statistically significant difference for the mean change in HbA1c level, favoring pen devices, but it did not reach the clinical significance threshold (Figure 2B). This analysis had an I2 = 47%, showing moderate heterogeneity with a nonsignificant χ2. We identified that previous use of insulin could be a relevant factor. We performed a subanalysis excluding the study with this population feature, which showed a bigger difference and less heterogeneity favoring pen devices compared to the complete analysis (Figure 2C). The study that had patients with previous insulin use26 showed a non–statistically significant difference. In conclusion, in the long term a slight advantage for pen devices in glycemic control is present.
Figure 2.
Forest plots of meta-analysis for the comparisons between pen devices and vial and syringe (VaS). (A) Percentage of patients achieving HbA1c<7% at 12 months. (B) Mean hBA1c change at 12 months. (C) Mean HbA1c change at 12 months, only studies with insulin-naïve patients. (D) Percentage of patients suffering at least 1 episode of hypoglycemia after 12 months. (E) Mean change for medication possession ratio (MPR) after 12 months. (F) Percentage of persistent patients after 12 months.
Hypoglycemia
Coscelli et al,20 in an experimental, randomized, crossover study with a 6-week follow-up, found no difference in mild or moderate hypoglycemia rate per patient-week of exposure (mild: 0.18 vs 0.15; moderate 0.03 vs 0.03; no P value provided) for pen and VaS, respectively. Ahmann et al10 did not find significant differences in the percentage of patients with any hypoglycemia episode (26.1% vs 29.1%, P = .5381), symptomatic hypoglycemia (22.4% vs 22.4%, P = 1), or severe hypoglycemia (1.2% vs 2.4%, P = .4191), for pen and VaS, respectively, after 6 weeks of follow-up. Lee et al,16 in an observational, analytical, retrospective cohorts study, did not report statistically significant difference between both arms regarding the rate of hypoglycemic episodes per patient (0.03 vs 0.02; P = .539) after 6 months of follow-up, for pen and VaS, respectively. Ahmann et al10 reported non–statistically significant differences in the number of patients with any hypoglycemia episode (47.8% vs 47.7%, P = .99), symptomatic hypoglycemia (34.8% vs 39.9%, P = .79), or severe hypoglycemia (4.4% vs 7.2%, P = .2958) at 30 weeks. Four studies14,15,18,26 reported hypoglycemia results at 12 months and a meta-analysis was performed. We found a statistical significant difference favoring pen devices, without heterogeneity (Figure 2D).
Data regarding hospital admissions related to hypoglycemia were available in 2 studies. Asche11 analyzed data from 2 claims databases, and reported statistically significant differences favoring pen devices for the mean number of hypoglycemia-related admissions (0.87 ± 4.7 vs 1.27 ± 8.12, P < .001; and 0.31 ± 2.1 vs 0.58 ± 3.66, P < .001), as well as for suffering ≥1 hypoglycemic episode (vial vs pen; OR = 1.354, 95% CI: 1.191-1.541; and OR = 1.442, 95% CI: 1.234-1.683). Lee et al23 designed a retrospective cohort study with a 24-month follow-up. He reported a reduction in the number of hypoglycemia episodes requiring emergency room visits (OR = 0.44, 95% CI: 0.21-0.92), physician visits (OR = 0.39, 95% CI: 0.24-0.64) and any other health-related admissions (OR = 0.38, 95% CI: 0.20-0.71). There was a favorable trend toward pen device in hypoglycemic episodes requiring hospitalization (OR = 0.88, 95% CI: 0.47-1.66) and outpatient visits (OR = 0.79, 95% CI: 0.31-2.01). In conclusion, pen devices seem to lower hypoglycemia events compared to VaS in the long term.
Preference
This outcome was measured using several nonvalidated questionnaires, in different time horizons. Ahmann et al10 randomized 405 patients to either pen device or VaS, swapping the interventions after 2 weeks and measuring preference using the Insulin Injection Preference Questionnaire (score ranging from 1 to 5; being 5 the best score). 85.4% of patients marked 5 for pen device in general preference, compared to 7.3% in VaS arm. Least square mean difference in general preference score was 2.3 (standard error 0.07; P < .001) in favor of pen device. Coscelli et al20 applied an author-developed questionnaire, reporting that 88% of patients considered the pen faster to use, 86% rated it as easier for dose measurement, 55% claimed less pain and 90% preferred it for long-term management, after 6 weeks of treatment. Kadiri et al21 evaluated the treatment acceptability in 78 patients using a nonvalidated survey. After 12 weeks of usage, he reported that 62.8% of the pen device group considered it easier to use, compared with 32.6% in the VaS group; 89.5% of patients assured they would prefer pen devices for insulin application. Korytkowski et al22 used a nonvalidated score system and reported that 74% of patients preferred continuing their treatment with a pen device, compared to 20% of the vial group. The majority of patients assessed pen devices, as easy-to-use, discrete, and reliable method for correct dose administration and achievement of glycemic control. They also used the Diabetes Treatment Satisfaction Questionnaire and reported no differences between the interventions (31.8 points vs 31.1 points; no P value reported) after a 4-week follow-up. Seggelke et al17 assessed preference with 3 nonvalidated questions (score: 0 to 6, 6 being “very satisfied”), after 3 months of treatment. They evidenced statistically significant difference in favor of pen in general satisfaction (5.5 ± 0.6 vs 3.1 ± 0.1; P < .0001), likelihood of recommending the method (5.4 ± 0.4 vs 3.0 ± 0.9; P < .0001), and likelihood of continuing using the method (5.8 ± 0.4 vs 2.7 ± 0.7; P < .0001). Shelmet et al27 used a nonvalidated questionnaire after 6 weeks, and reported that 82% of patients treated with pen preferred their method compared with 10% of VaS patients. Furthermore, the majority rated the pen as easier to use, more reliable, less painful, and better suited for everyday acceptability. In conclusion, pen is generally the preferred insulin administration method when compared to VaS.
Adherence
Adherence was assessed using medication possession ratio (MPR), which estimates the proportion of days in which the patient has available medication in the observation period. It ranges from 0 to 1, indicating null or complete adherence, respectively.13,15,23,26 Proportion of days covered (PDC) is calculated in a similar fashion to MPR.16 Four studies13,15,23,26 assessed final MPR at 12-month follow-up, and showed statistically significant difference favoring pen, but had considerable heterogeneity (I2 = 95%) (Figure 2E). When insulin-naïve and nonnaïve populations were compared, and a greater difference was seen in the former population.13,15 Low heterogeneity was estimated in this subanalysis. A nonnaïve population23,26 showed a less marked trend, with important heterogeneity (I2 = 80%; χ2 significant).
Baser et al12 reported that mean change in MPR in patients who exchanged vial for pen device was 0.22 ± 0.01, compared with 0.13 ± 0.36 for those who continued on vial (P = .0011). Two studies evaluated the proportion of patients with MPR > 0.80: Lee et al23 showed difference favoring pen (0.546 vs 0.361; P < .01), and Buysman et al,13 using a logistic regression model, found an OR = 1.39 (95% CI 1.04-1.85) favoring pen, after adjusting for age, gender, and comorbidities. Two studies evaluated adherence at 24 months. Lee et al16 evidenced a difference in PDC, favorable to pen (54.6% vs 45.2%; P < .001), while Cheen et al19 did not find statistically significant differences in MPR between groups (86% ± 23.2 vs 83.8% ± 26.9; P = .266). In this study, mean MPR was high in this Singaporean population, recruited in a specialized care center. In conclusion, pen devices show better MPR values compared to VaS. In highly specialized settings, differences could be minimal.
Persistence
Persistence is defined as number of days until discontinuation of the medication; percentage of patients not discontinuing medication is another form of this outcome,13-15,18,19,26 with discontinuation defined as absence of medication claims after a certain threshold of days. Six studies with a follow-up of 12 months were found,13-15,18,19,26 with statistically significant difference in favor of pen (Figure 2F), but important heterogeneity (I2 = 89%) was detected. A subanalysis was performed using the studies with similar definitions of discontinuation threshold.14,15,18,26 The favorable tendency for pen devices was maintained, with an I2 of 40% and a nonsignificant χ2.
Quality of Life
Lee et al24 recruited patients with previous use of insulin, assigning 32 patients to pen and 33 to VaS. Short Form Health Survey (SF-36) was used to assess quality of life. Statistically significant differences favoring pen were found regarding change from baseline in physical component scores (+3.9 ± 1.9 vs −1.0 ± 1.3; P = .037), physical role scores (+16.4 ± 9.4 vs −18.2 ± 8.4; P = .008), and general health status score (+9.8 ± 4.0 vs −2.5 ± 3.3; P = .021). Other component scores did not show significant differences.
Publication Bias
Visual inspection of funnel plots detected asymmetry toward pen for the persistency analysis. This matches the important heterogeneity detected in that meta-analysis. No statistical analysis was performed given the insufficient number of studies included.
Discussion
All 17 studies that compared pen devices with VaS could be considered to have methodological flaws. Blinding, for example, is difficult when medical devices are being tested; differences in important outcomes like mortality or cardiovascular complications, would require a combination of larger samples and a longer follow-up. Risk of bias might be a concern. However it is sometimes unavoidable given the nature of the devices and outcomes tested, especially in patient-reported outcomes. Even with these limitations, our review shows interesting results.
This systematic review used currently accepted methodologies for reference search and data extraction. Outcomes of interest are reported in diverse forms, and with different time horizons, which makes integrating information somewhat more difficult.
Results in HbA1c reduction showed no difference in short follow-ups, as well as in percentage of patients achieving HbA1c<7% at 12 months. However, a difference was found in mean HbA1c change at 12 months, with limited clinical relevance. The absence of difference in the proportion of patients achieving their glycemic goal might be explained by the combination of initial HbA1c much greater than 7% and an overall modest decrease in HbA1c between interventions. The influence of HbA1c in microvascular outcomes of diabetes has been clearly defined, so a small reduction might not represent evident benefits for individual patients but could be significant on a population-level. Hypoglycemia results showed a clear tendency of pen devices to reduce the percentage of patients affected. The combination of these 2 elements could potentially be beneficial. One possible explanation for this is increased dose accuracy with pen devices. A recent systematic review28 found 5 studies that showed evidence of increased insulin dose accuracy with different pen devices compared to VaS especially when administering <5 UI. Authors suggest that this could imply better glycemic control and lower hypoglycemia rate. Their conclusions are consistent with our results but the link between dose accuracy and clinical outcomes should be further explored.
Adherence and persistence show better results in patients with pen devices. Caution should be taken with these results since they are indirect measures of possession of medication not necessarily implying adequate use. Anyway, MPR was not designed for determining adherence in dynamic dosing medications such as insulin.29 Preference toward pen devices was uniform in all studies analyzed, but they used nonstandardized tools.
Quality of life was addressed in only 1 study, using SF-36. It showed better results with pen devices, in general and physical health components.
This study has some important limitations. Since most studies are from high-income countries, a selection bias might be in place and results may not be applicable to different international contexts. Many sources of heterogeneity might influence results such as local cultural differences, patient training, and schemes of insulin administration. We could argue that a difference in favor of pen devices would be even greater in impoverished places. However, sensitivity analysis showed that previous history of insulin use might have been the main source of heterogeneity.
In 2009, Molife et al30 published a review of the literature focusing in patient-reported outcomes in comparative studies of insulin therapy with pen versus VaS. They included 41 studies published between 1990 and 2008 and evaluated preference, acceptability, satisfaction, ease of use, convenience, pain, management, and dosage. The review concluded that the available evidence showed a marked tendency of the measured variables in favor of the pen. Pen devices were also associated with a better social acceptability leading to an improved adherence. This review emphasized the lower perception of pain with the use of pen devices. The authors argued that this factor is essential to adherence since the perception of big barriers to commitment such as pain can overcome the potential benefits of treatment.31 This review also estimated that 60% to 80% of patients using VaS make mistakes related to dosage. Pen devices could address this issue given its ease of use.
Another systematic review32 on adherence included 17 studies and reported that the use of pen devices correlates with better adherence. On the other hand, according to the practice guidelines of DM from NICE33,34 pen devices have made it possible to avoid issues of hygiene in the application and of calculation of the correct dose. NICE recommends that patients should be provided with the most convenient application system considering their preference.
The present study identifies gaps in the scientific literature that deserve further investigation. New strategies to measure adherence in a more direct and reliable way is one of them. We need to design experimental studies with higher methodological rigor. This would provide high-quality evidence for decision making with less risk of bias. Another interesting topic is the validation of patient-reported outcome tools. Having high-quality device- or disease-specific questionnaires would facilitate the production of valuable information for decision makers.
Conclusions
Pen devices for insulin administration seem to be associated with mild benefits in clinical variables like HbA1c and hypoglycemia. The simultaneous presence of these effects can be synergic for improved diabetes care. Adherence and patient preference are also positively correlated with the use of pen devices. It is necessary to underline the significant risk of bias of the included studies. Problems related to validity and standardization of measures for adherence, persistence and preference is another issue. Priorities for future investigation should be generating higher quality of evidence for comparisons between pen devices and VaS and assessing new tools to measure adherence and preference in a more reliable way.
Footnotes
Abbreviations: Adh, adherence; CI, confidence interval; HbA1c, glycated hemoglobin; HyG, hypoglycemia; IETS, Instituto de evaluación de teconologás en salud; MPR, medication possession ratio; NROPES, nonrandomized open parallel experimental study; NS, not specified; OR, odds ratio; PDC, proportion of days covered; Pers, persistence; Pref, preference; QoL, quality of life; RCAS, retrospective cohorts analytical study; RCOES, randomized crossover open experimental study; RR, risk ratio; SD, standard deviation; SF-36, Short Form 36 Health Survey; SIGN, Scottish Intercollegiate Guidelines Network; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; VaS, vial and syringe.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Eli Lilly Interamerica, Bogota, Colombia.
References
- 1. Fry A. Insulin delivery device technology 2012: where are we after 90 years? J Diabetes Sci Technol. 2012;6(4):947-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Selam JL. Evolution of diabetes insulin delivery devices. J Diabetes Sci Technol. 2010;4(3):505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Díaz M, Peña E, Mejía A, Flórez I. Manual metodológico para la elaboración de evaluaciones de efectividad, seguridad y validez diagnóstica de tecnologías en salud- IETS. 2014. [Google Scholar]
- 4. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(7):264-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Higgins J, Green S. Assessing risk of bias in included studies. In: Cochrane Handbook for Systematic Reviews of Interventions. 2011. Available at: http://handbook.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm.
- 6. Scottish Intercollegiate Guidelines Network. Critical appraisal: Notes and checklists. 2014. Available at: http://www.sign.ac.uk/methodology/checklists.html.
- 7. Higgins JP, Green S. 9.5.2 Identifying and measuring heterogeneity. In: Cochrane Handbook for Systematic Reviews of Interventions. 2011. Available at: http://handbook.cochrane.org/chapter_9/9_5_2_identifying_and_measuring_heterogeneity.htm.
- 8. Higgins J, Green S. 10.4.3.1 Recommendations on testing for funnel plot asymmetry. In: Cochrane Handbook for Systematic Reviews of Interventions. 2011. Available at: http://handbook.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm.
- 9. US Department of Health and Human Services, FDA, CDER. Guidance for industry diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention. 2008. Available at: http://www.fda.gov/downloads/Drugs/Guidances/ucm071624.pdf.
- 10. Ahmann A, Szeinbach SL, Gill J, Traylor L, Garg SK. Comparing patient preferences and healthcare provider recommendations with the pen versus vial-and-syringe insulin delivery in patients with type 2 diabetes. Diabetes Technol Ther. 2014;16:76-83. [DOI] [PubMed] [Google Scholar]
- 11. Asche CV, Luo W, Aagren M. Differences in rates of hypoglycemia and health care costs in patients treated with insulin aspart in pens versus vials. Curr Med Res Opin. 2013;29(10):1287-1296. [DOI] [PubMed] [Google Scholar]
- 12. Baser O, Bouchard J, DeLuzio T, Henk H, Aagren M. Assessment of adherence and healthcare costs of insulin device (FlexPen) versus conventional vial/syringe. Adv Ther. 2010;27:94-104. [DOI] [PubMed] [Google Scholar]
- 13. Buysman E, Conner C, Aagren M, Bouchard J, Liu F. Adherence and persistence to a regimen of basal insulin in a pre-filled pen compared to vial/syringe in insulin-naïve patients with type 2 diabetes. Curr Med Res Opin. 2011;27:1709-1717. [DOI] [PubMed] [Google Scholar]
- 14. Davis SN, Wei W, Garg S. Clinical impact of initiating insulin glargine therapy with disposable pen versus vial in patients with type 2 diabetes mellitus in a managed care setting. Endocr Pract. 2011;17(6):845-852. [DOI] [PubMed] [Google Scholar]
- 15. Grabner M, Chu J, Raparla S, Quimbo R, Zhou S, Conoshenti J. Clinical and economic outcomes among patients with diabetes mellitus initiating insulin glargine pen versus vial. Postgrad Med. 2013;125(3):204-213. [DOI] [PubMed] [Google Scholar]
- 16. Lee LJ, Li Q, Reynolds MW, Pawaskar MD, Corrigan SM. Comparison of utilization, cost, adherence, and hypoglycemia in patients with type 2 diabetes initiating rapid-acting insulin analog with prefilled pen versus vial/syringe. J Med Econ. 2011;14(1):75-86. [DOI] [PubMed] [Google Scholar]
- 17. Seggelke SA, Hawkins RM, Gibbs J, et al. Effect of glargine insulin delivery method (Pen Device Versus Vial/Syringe) on glycemic control and patient preferences in patients with type 1 and type 2 diabetes. Endocr Pract. 2014;20(6):536-539. [DOI] [PubMed] [Google Scholar]
- 18. Xie L, Zhou S, Pinsky B., Buysman EK, Baser O. Impact of initiating insulin glargine disposable pen versus vial/syringe on real-world glycemic outcomes and persistence among patients with type 2 diabetes mellitus in a large managed care plan: a claims database analysis. Diabetes Technol Ther. 2014;16(9):567-575. [DOI] [PubMed] [Google Scholar]
- 19. Cheen HH, Lim SH, Huang MC, Bee YM, Wee HL. Adherence to premixed insulin in a prefilled pen compared with a vial/syringe in people with diabetes in Singapore. Clin Ther. 2014;36(7):1043-1053. [DOI] [PubMed] [Google Scholar]
- 20. Coscelli C, Lostia S, Lunetta M, Nosari I, Coronel GA. Safety, efficacy, acceptability of a pre-filled insulin pen in diabetic patients over 60 years old. Diabetes Res Clin Pract. 1995;28(3):173-177. [DOI] [PubMed] [Google Scholar]
- 21. Kadiri A, Chraibi A, Marouan F, et al. Comparison of NovoPen 3 and syringes/vials in the acceptance of insulin therapy in NIDDM patients with secondary failure to oral hypoglycaemic agents. Diabetes Res Clin Pract. 1998;41(1):15-23. [DOI] [PubMed] [Google Scholar]
- 22. Korytkowski M, Bell D, Jacobsen C, et al. A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25(11):2836-2848. [DOI] [PubMed] [Google Scholar]
- 23. Lee W., Balu S, Cobden D, Joshi AV, Pashos CL. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: An analysis of third-party managed care claims data. Clin Ther. 2006;28(10):1712-1725. [DOI] [PubMed] [Google Scholar]
- 24. Lee IT, Liu HC, Liau YJ, Lee WJ, Huang CN, Sheu WH. Improvement in health-related quality of life, independent of fasting glucose concentration, via insulin pen device in diabetic patients. J Eval Clin Pr. 2009;15:699-703. [DOI] [PubMed] [Google Scholar]
- 25. Shelmet J, Schwartz S, Cappleman J, et al. Preference and resource utilization in elderly patients: InnoLet versus vial/syringe. Diabetes Res Clin Pract. 2004;63(1):27. [DOI] [PubMed] [Google Scholar]
- 26. Xie L, Zhou S, Wei W, et al. Does pen help? A real-world outcomes study of switching from vial to disposable pen among insulin glargine-treated patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2013;15(3):230-236. [DOI] [PubMed] [Google Scholar]
- 27. Shelmet J, Schwartz S, Cappleman J, Peterson G, Skovlund S, Lytzen L. Preference and resource utilization in elderly patients: InnoLet versus vial/syringe. Diabetes Res Clin Pract. 2004;63(1):27-35. [DOI] [PubMed] [Google Scholar]
- 28. Luijf YM, DeVries JH. Dosing accuracy of insulin pens versus conventional syringes and vials. Diabetes Technol Ther. 2010;12(suppl 1):S73-S77. [DOI] [PubMed] [Google Scholar]
- 29. Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records. Description and validation. Med Care. 1988;26(8):814-823. [DOI] [PubMed] [Google Scholar]
- 30. Molife C, Lee LJ, Shi L, Sawhney M, Lenox SM. Assessment of patient-reported outcomes of insulin pen devices versus conventional vial and syringe. Diabetes Technol Ther. 2009;11(8):529-538. [DOI] [PubMed] [Google Scholar]
- 31. Becker MH, Maiman LA. Sociobehavioral determinants of compliance with health and medical care recommendations. Med Care. 1975;13(1):10-24. [DOI] [PubMed] [Google Scholar]
- 32. Davies MJ, Gagliardino JJ, Gray LJ, Khunti K, Mohan V, Hughes R. Real-world factors affecting adherence to insulin therapy in patients with type 1 or type 2 diabetes mellitus: a systematic review. Diabet Med. 2013;30(5):512-524. [DOI] [PubMed] [Google Scholar]
- 33. NICE. NICE guidelines (CG87) type 2 diabetes. 2009. Available at: https://www.nice.org.uk/guidance/cg87/resources/guidance-type-2-diabetes-pdf.
- 34. NICE. NICE guidelines (CG15) type 1 diabetes. 2004; Available at: http://www.nice.org.uk/guidance/cg15/evidence.