Abstract
Background:
The psychosocial impact of the bionic pancreas (BP) was assessed among children attending diabetes camp.
Methods:
Nineteen children were randomly assigned for 5 days to the BP condition and 5 days to the control condition in a crossover design.
Results:
Significant reductions in hypoglycemic fear and regimen burden were found. Children felt less burdened or worried about diabetes and felt freer to do things they enjoyed while using the BP. Children wished the BP responded to out of range numbers faster and expressed annoyance about carrying around the necessary equipment.
Conclusions:
Children may experience improved psychosocial outcomes following use of BP while expressing key areas of user concern. Future studies in less controlled environments with larger sample sizes can determine if these findings are generalizable to other groups.
Keywords: artificial pancreas, automated insulin delivery, bionic pancreas, diabetes camp, psychosocial
A number of advances in automated insulin delivery (AID) systems have recently been made, with the goal of making tight glycemic control easier, safer, and less burdensome to achieve.1-3 Typically, AID systems consist of 3 specific parts: a drug-infusion system (with 1 or 2 hormones), a continuous glucose monitor (CGM), and a control algorithm, which makes automated dosing decisions about when and how much of the hormone(s) to administer. A number of research teams are developing these systems, and all show the superiority of AID systems over standard pump therapy with increased time spent within glucose targets, and/or reduced hypoglycemia and/or better over-night blood glucose control.4-12
Five studies have reported on the use of AID systems in a summer camp setting, where AID use can be closely monitored.13-17 Two studies assessed overnight closed-loop systems, each using the system for 2-3 nights (ages 10-20 years).13,14 A third camp study15 assessed a wide age-range of campers, including teens as well as young adults (ages 15-31 years) who used a hybrid closed-loop system for 6 days. Two camp studies16,17 employed a random-order crossover design where teens (ages 12-20 years) and preteens (ages 6-11 years) used the bionic pancreas (BP) for 5 days and their personal insulin pump for 5 days. While all 5 of these studies demonstrated the safety and efficacy of AID systems in camp settings, only one17 collected data on the psychosocial impact of these systems.
The impact of AID systems on patient burden and emotional well-being must be considered, as the success of any technology is contingent on whether or not the patient is willing to use it. For example, in a recent review of studies assessing the psychosocial aspects of diabetes technology, while 292 abstracts were reviewed, only 9 met inclusion criteria for in-depth review.18 Of those, only 3 were related to AP systems.19-21 Van Bon et al19 explored patient’s perceptions (who were currently using insulin pump therapy) regarding future AP systems. The majority reported they intended to use an AP system and believed it would be useful, easy to use, and worthy of trust. Barnard et al20 explored the perceptions of people with diabetes as well as parents of children with diabetes regarding expectations about AP systems. Over 90% of respondents reported that they intend to use AP systems when they are available. Concerns/barriers to use included the size, visibility and potential lack of effectiveness of the systems. Benefits included using technology that could minimize diabetes burden and improve psychosocial functioning. Finally, Bevier et al21 assessed the perceptions of individuals who had already participated in an AP trial. Future acceptance of AP technology was strongly associated with prior technology acceptance, and more than 85% of respondents were interested in using an AP system once it was commercially available. Clearly, the ongoing use of any AID system will depend on the patient’s (and/or family’s) perception that the technology is safe, reliable, and effective and can adapt to real-life experiences and events without increasing the burden of caring for the disease.
As a first step toward addressing this gap in knowledge, the current report describes the psychosocial impact of the BP system, in particular, on preteens 6-11 years old attending diabetes summer camp (Camp Joslin and the Clara Barton Camp).17
Methods
Participants
The camp provided information about the study to all who enrolled in camp, the study information was posted on the Children With Diabetes Website, and families independently contacted the study team asking to enroll in any studies for which their children were eligible.17 The full study protocol is available in the appendix to the study article.17,22 Nineteen children (mean age 9.8 ± 1.6; range 6.5-11.9 years) participated in this study. Approximately 68% were female, 90% were non-Hispanic Caucasian, and the mean HbA1c at baseline was 7.8 ± 0.8%. All had type 1 diabetes (T1D) for at least 1 year and were on insulin pump therapy for ≥ 6 months.
Study Design Overview
All participants were assigned for 5 days to the BP and 5 days to their own insulin pump with ordering randomly assigned in a crossover design. Participants had no restrictions on their activities or food intake in either study period. Study staff were available to assist with any technical problems with the BP during the camp sessions. Each camper completed questionnaires 3 times: prior to the first study arm, at the end of the first study arm, and at the end of the second study arm. The mean total number of plasma glucose checks (scheduled and unscheduled) per participant was 50.7 (SD 12.3) with the BP and 52.5 (SD 8.7) during the control period. Participants stayed in the same cabins, engaged in the same activities, and ate the same meals as nonparticipant campers.17
Measures
Four psychosocial measures were assessed at all time points. Fear of hypoglycemia was assessed by the Hypoglycemia Fear Survey,23 a 25-item measure assessing both worries about low blood sugars (eg, worrying about “not recognizing that my blood sugar is low”) and behaviors to prevent lows (eg, “I keep blood sugars high when I will be alone for a while”). Items are rated on a 5-point scale from 0 = never to 5 = almost always, and scores could range from 0 to 100. Diabetes specific emotional distress was assessed via the Child version of the Problem Areas in Diabetes survey,24 which was adapted for a lower reading level from the Teen version of the Problem Areas in Diabetes Survey.25 This 26-item survey assesses how much distress children experience with such items as “feeling sad when I think about having diabetes,” “feeling stressed out by what I have to do every day for my diabetes,” or “feeling that my blood sugars keep going up and down, no matter how hard I try.” Items are rated on a 6-point scale from 1 = not a problem to 6 = a big problem, and scores could range from 26 to 156. Perceptions regarding regimen burden is a 20-item measure, developed for this study, which asks respondents to report on how much children feel bothered by their current diabetes regimen. Items are rated on a 4-point scale from 1 = not bothered at all to 4 = very bothered, and scores could range from 20 to 40. Example items include “not knowing how what I eat changes my blood sugars,” “having to figure out how much insulin to take to correct a high number,” and “treating a low blood sugar when I’m not hungry” (Cronbach’s alpha for this measure = .88). The Experience with the BP Questionnaire was also developed for this study, and the 38 items were based on interviews conducted with individuals who had participated in previous BP trials about their experience regarding the BP. This questionnaire was administered immediately following completion of the BP arm of the trial and assessed both positive and negative experiences with the BP, including blood glucose management, device burden, and overall satisfaction. Example items include “Carrying everything with me all of the time was annoying” “It was easier to do the things I wanted to do,” “I wish the Bionic Pancreas treated my low blood sugars faster,” and “I spent much less time thinking about my diabetes.” Items were rated on a 5-point scale (1 = strongly disagree, 5 = strongly agree), and scores could range from 38 to 190. In total, children spent between 15 and 20 minutes completing the questionnaire battery.
Data Analysis
Descriptive analyses were computed to review score distributions. Independent t-tests and chi-square analyses were used to examine ordering effects of the groups at baseline. Repeated measures ANOVA models were employed to examine group differences in the degree of change from study baseline to the end of the control follow-up period and from baseline to the end of the BP follow-up period, and the difference in the change between these 2 follow-up periods. Post hoc analyses were conducted using Fisher’s least significant difference (LSD) test.
Results
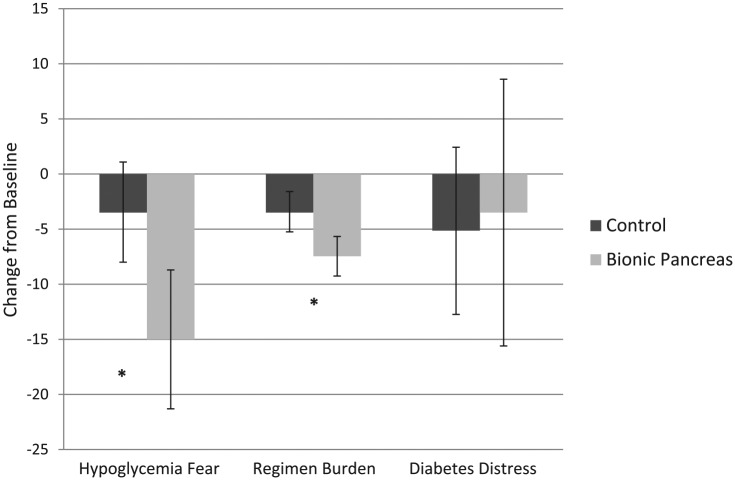
Significant reductions in hypoglycemic fear and regimen burden were found following use of the BP compared to control (Table 1, Figure 1), but no differences in diabetes-specific emotional distress were observed. Regarding BP experiences, participants endorsed many positive aspects of the technology (indicating agreement or strong agreement) including feeling less burdened or worried about diabetes (90% felt more relaxed; 84% spent less time thinking about diabetes; 79% felt diabetes was less annoying), feeling more free (95% felt they could engage in more activities that they enjoyed; 79% found it easier to make food choices), and worrying less about high (90%) and low (84%) blood sugar levels. The most common concern included wishing the BP responded to lows (53%) or highs (42.1%) faster, the annoyance of carrying around the necessary equipment (31.6%) and having to change the glucagon daily (31.6%). Overall, the majority of participants (73.7%) endorsed that they wanted to continue using the BP after the conclusion of the study.
Table 1.
Changes in Psychosocial Outcomes by Study Condition.
| Hypoglycemia fear | Regimen burden | Diabetes distress | |
|---|---|---|---|
| Descriptive (mean, SE) | |||
| Baseline | 33.50 (7.81) | 33.75 (2.25) | 56.00 (6.80) |
| Control | 30.00 (5.82) | 30.26 (2.01) | 50.86 (5.66) |
| Bionic pancreas | 18.50 (6.29)bc | 26.29 (1.98)bc | 52.49 (14.14) |
| Analysis of change (F, P) | |||
| Time | 7.82 (.03) | 8.48 (.003) | 0.18 (.84) |
| Ordering | 2.56 (.18) | 1.37 (.28) | 0.08 (.79) |
| Time × ordering | 0.58 (.61) | 0.42 (.53) | 0.49 (.66) |
Note: Mean total summed scores are presented for each outcome. Significant differences (P < .05) between conditions are denoted as follows: aChange from baseline to control; bChange from baseline to bionic pancreas; cChange between control and bionic pancreas.
Figure 1.
Changes in psychosocial outcomes by condition.
Discussion
Our results show that children attending a diabetes camp found the BP helpful in reducing their fear of hypoglycemia, decreasing their sense of regimen burden, decreasing their worries about out-of-range blood sugar levels, and improving their overall freedom to engage in the activities that they enjoyed. Decreased burden was a key patient goal in previous studies regarding psychosocial impact of AP systems.19,20 Concerns about the BP included wishing the system responded to out-of-range blood sugar levels more quickly and the annoyance of carrying a number of devices around. These concerns are consistent with previous studies as well.20 The lack of change in diabetes-specific emotional distress may in part be attributed to the low level of distress reported by the campers at baseline, as their baseline score was 1 full standard deviation below the previously reported mean of this measure (Figure 1),24 perhaps in part due to being in a safe and supportive camp environment. Results provide critical initial support that children may experience improvements in psychosocial outcomes following use of BP and point to key areas of user concern that can be considered when introducing an AID system such as the BP. For example, the bulkiness of the BP device used in this study will likely be addressed before a more integrated system is commercially available. Limitations include the fact that 90% of participants were Caucasian (although consistent with camp demographics from around the United States),26 and the sample included only those who chose to attend diabetes camp. The fact that parents did not participate in the trial (the children were attending an overnight camp) also narrows the focus to child-report data only. Further studies are warranted in less controlled environments with larger sample sizes to determine if these findings are generalizable to other samples and/or other AID systems, and to assess the impact of the BP on additional psychosocial outcomes (eg, parental and family conflict).
Acknowledgments
The authors wish to thank the children and families who participated in this study. The Perceptions Regarding Regimen Burden Scale and the Experience with the Bionic Pancreas Questionnaire are available by contacting the corresponding author, Jill Weissberg-Benchell, at jwbenchell@luriechildrens.org
Footnotes
Abbreviations: AID, automated insulin delivery system; BP, bionic pancreas, CGM, continuous glucose monitor; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Haidar A, Legault L, Messier V, Mitre T, Leroux C, Rabase-Lhorel R. Comparison of dual-hormone artificial pancreas, single hormone artificial pancreas, and conventional insulin pump therapy for glycemic control in patients with type 1 diabetes. Lancet Diabetes Endocrinol. 2015;3(1):17-26. [DOI] [PubMed] [Google Scholar]
- 2. Shah V, Shoskes A, Tawfik B, Garg S. Closed-loop system in the management of diabetes: past, present and future. Diabetes Technol Ther. 2014;16(8):477-490. [DOI] [PubMed] [Google Scholar]
- 3. Thabit H, Lubina-Solomon A, Stadler M, et al. Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicenter, randomized crossover study. Lancet Diabetes Endocrinol. 2014;2(9):701-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Breton M, Farre A, Bruttomesso D, et al. Fully integrated artificial pancreas in type 1 diabetes modular closed loop glucose control maintains near normoglycemia. Diabetes. 2012;61:2230-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castle J, Engle J, Youssef J, et al. Novel use of glucagon in a closed-loop system for prevention in hypoglycemia in type 1 diabetes. Diabetes Care. 2010;33:1282-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cobelli C, Renard E, Kovachev B. Artificial pancreas: past, present, future. Diabetes. 2011;60:2672-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El-Khatib F, Russell S, Nathan D, Sutherlin R, Damiano E. A bio-hormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;4:1374-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weinzimer S, Steil G, Swan K, Dziura J, Kurtz N, Tamborlane W. Fully automated closed loop control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31:934-939. [DOI] [PubMed] [Google Scholar]
- 9. Hovorka R, Allen J, Elleri D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomized cross over trial. Lancet. 2010;375:743-751. [DOI] [PubMed] [Google Scholar]
- 10. Atlas E, Nimri R, Miller S, Grunberg E, Phillip M. MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes Care. 2010;33:1072-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Del Favero S, Place J, Kropff J, et al. Multicenter outpatient dinner/overnight reduction of hypoglycemia and increased time of glucose in target with a wearable artificial pancreas using modular model predictive control in adults with type 1 diabetes. Diabetes Obes Metab. 2015;17(5):468-476. [DOI] [PubMed] [Google Scholar]
- 12. Nimri R, Muller I, Atlas E, et al. MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care. 2014;37(11):3025-3032. [DOI] [PubMed] [Google Scholar]
- 13. Ly T, Breton M, Keith-Hynes P, et al. Overnight glucose control with an automated, unified safety system in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Care. 2014;37:2310-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;386:824-933. [DOI] [PubMed] [Google Scholar]
- 15. Ly T, Roy A, Grossman B, et al. Day and night closed loop control using the integrated Medtronic hybrid closed loop system in type 1 diabetes at diabetes camp. Diabetes Care. 2015;38:1205-1211. [DOI] [PubMed] [Google Scholar]
- 16. Russell S, El-Khatib F, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;3714:313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Russell S, Hillard M, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol. 2016;4(3):233-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barnard K, Hood K, Weissberg-Benchell J, Aldred C, Oliver N, Laffel L. Psychosocial assessment of artificial pancreas (AP): commentary and review of existing measures and their applicability in AP research. Diabetes Technol Ther. 2015;17(4):295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Bon A, Brouwer T, von Basum G, Hoekstra J, DeVries J. Future acceptance of an artificial pancreas in adults with type 1 diabetes. Diabetes Technol Ther. 2011;13(7):731-736. [DOI] [PubMed] [Google Scholar]
- 20. Barnard K, Pinsker J, Oliver N, Astle A, Dassau E, Kerr D. Future artificial pancreas technology for type 1 diabetes: what do users want? Diabetes Technol Ther. 2015;17(5):311-315. [DOI] [PubMed] [Google Scholar]
- 21. Bevier W, Fuller S, Fuller R, et al. Artificial pancreas (AP) clinical trial participants’ acceptance of future AP technology. 2014;16(9):590-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russell S, Hilliard M, Balliro C, et al. Appendix for: outpatient glycemic control using a bionic pancreas in pre-adolescent children with type 1 diabetes. Lancet Diabetes Endocrinol. 2016. [Google Scholar]
- 23. Gonder-Frederick L, Nyer M, Shepard J, Vajda K, Clarke W. Assessing fear of hypoglycemia in children with type 1 diabetes and their parents. Diabetes Manage. 2011;1(6):627-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weissberg-Benchell J, Rychlik K. Assessing the impact of diabetes camp. Abstract presented at: 75th Scientific Sessions, American Diabetes Association; June 2015; Boston, MA. [Google Scholar]
- 25. Weissberg-Benchell J, Antisdel-Lomaglio J. Diabetes-specific emotional distress among adolescents: feasibility, reliability and validity of the problem areas in diabetes-teen version. Pediatr Diabetes. 2011;12(4):341-344. [DOI] [PubMed] [Google Scholar]
- 26. Weissberg-Benchell J, Rychlik K. Assessing the impact of camp. Abstract published from: 75th Annual Scientific Sessions, American Diabetes Association; 2015; Boston, MA. [Google Scholar]