Abstract
Background
This study aims to determine the synergistic effects of the chemotherapeutic drug melphalan and the phytoconstituents extracted from Pinus kesiya Royle ex Gordon (Simaosong) in human cancer cells.
Methods
P. kesiya twigs extracted from 50 % ethanol–water were evaluated alone (6–500 µg/mL) and in combination with melphalan (0.75–15 µg/mL). The cytotoxic effects of single extract or extract and melphalan combination were examined by a neutral red assay to investigate their antiproliferative and apoptosis induction effects in the U937 and HepG2 cell lines. Nuclei morphological change and DNA fragmentation were examined by DNA nuclei staining with 4´6-diamidino-2-phenylindole (DAPI) and agarose gel electrophoresis, respectively. The chemical constituents of the P. kesiya extract were assessed using gas chromatography–mass spectrometry (GC–MS) analysis. The synergistic effects of different IC50 ratios of the P. kesiya extract and melphalan combination were analyzed in each cancer cell line. The dose reduction index (DRI) was calculated to determine the extent of concentration reduction in the combination treatment compared with the concentration of each single treatment.
Results
The IC50 ratios for melphalan to P. kesiya extract that caused 75 % antiproliferation could be reduced after combination. This response was greater in the U937 cells than in the HepG2 cells (all P < 0.001). Melphalan and P. kesiya extract had a similar effect on apoptosis induction both singly and in combination. P. kesiya extract synergized the antiproliferation and apoptosis induction effects of melphalan.
Conclusions
Combining the P. kesiya extract with melphalan reduced toxicity while retaining the therapeutic efficacy of melphalan.
Background
Specific and selective apoptosis induction in targeted chemotherapy is limited due to multidrug resistance and intolerably severe side effects [1, 2]. Synergistic combinations of two or more agents could overcome the toxicity and side effects associated with the high doses of chemotherapeutic drugs in monotherapy [3]. Several herbal plant extracts are rich sources of bioactive constituents that inhibit, reverse, or retard tumorigenesis [3, 4]. Additionally, some research on herbal synergistic action indicates that the whole herb produces a better effect than any single isolated active ingredient [5, 6].
The rosin of Pinus kesiya Royle ex Gordon (Simaosong) contains szemaoenin (diterpenoid) isopimaric acid, abiet-13(14)-en-8,12-epoxy-18-oic acid, abiet-8,11,13-trien-15-hydroxy-18-oic acid, pimarol, isopimarol, abiet-8,11,13-trien-18-oic acid, and 15-hydroxyabietic acid [7]. The main compounds found in P. kesiya turpentine are α- and β-pinene [8]. The needle oil is rich in α- and β-pinene, citronellol, bornyl acetate, β-phellandrene, camphene, and β-caryophyllene [9]. The needles of P. kesiya contain dichlorobenzene isomer, 1,4-cineol, α-terpinene, o-cimene and imonene enantiomers [10, 11], and monoterpenes [12].
P. kesiya is used to relieve flatulence, stomachache, and cough in complementary and alternative medicine [13]. The 50 % ethanol–water crude extract of the woody twigs of P. kesiya has an apoptotic-induction effect on both the human hepatocellular carcinoma HepG2 and the human leukemic U937 cell lines because of its cytotoxicity [14, 15]. Many studies indicate that this effect arises from the whole crude extract with its lower activity of isolated individual compounds [3–6]. The synergistic effect of the whole extract of P. kesiya plus the chemotherapeutic drug melphalan is of interest as it may expand the use of this herbal plant. Melphalan is an alkylating anticancer drug, leading to DNA cross-linking, DNA damage, and finally apoptosis of cancer cells [16]; however, melphalan resistance and highly toxic side effects in other tissues limit its use [17, 18]. We hypothesized that plant extracts that exhibited an anticancer activity in vitro might enhance the antiproliferative activity of melphalan, which would permit the use of a lower dosage and reduced side effects. This study aims to determine the synergistic effects of P. kesiya and melphalan phytoconstituents in human cancer cells.
Methods
Plant extraction
P. kesiya woody twigs were collected and taxonomically authenticated by Assistant Professor Thaweesak Thitimetharoach. The species of samples were determined using the Flora of Thailand [19] and the Thai Forest Bulletin [20]. The voucher (TT-OC-SK-910) was deposited at the Herbal Herbarium, Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen Province, Thailand. The 50 % ethanol–water extract of P. kesiya twig was prepared as previously reported [14, 15]. Dried plants were cut and macerated with 50 % ethanol–water (1 kg:6 L) for 7 days with occasional manual shaking. The solvent was filtered, distilled by a rotary evaporator (RV 8 V, IKA, Germany) below 40°C, and freeze-dried to obtain the crude extract. The percent yield of the 50 % ethanol–water extract of P. kesiya was 4.3 %. Stock solution of P. kesiya extract was freshly prepared in dimethyl sulfoxide (DMSO) to make 100 mg/mL stock solution and further diluted with culture media to create a working solution (10 to 500 µg/mL).
Gas chromatography–mass spectrometry (GC–MS) analysis
GC–MS analysis was performed as described by Weerapreeyakul et al. [21] on an Agilent 6890 N gas chromatograph (Agilent Technologies, China) coupled to an Agilent 5973 N mass selective detector (Agilent Technologies, USA) to determine the extract composition for further standardization. Capillary GC analysis was performed using a DB-5 ms (3 m × 0.25 mm id, 0.25 µm) capillary column from Agilent Technologies (J&W Scientific, USA) with helium as the carrier gas. The column initially flowed at 80 °C for 6 min at a rate of 2 mL/min and an average velocity of 52 cm/s. The temperature was raised to 280 °C (at a rate of 5 °C/min) for 24 min. The total runtime was 70 min. The injector temperature was maintained at 250 °C and the injection volume was set at 2.0 µL in the splitless mode. The interface temperature was held at 280 °C. Mass spectra were scanned from m/z 50.0 to m/z 500.0 at a rate of 1.5 scans/s with a threshold of 150. The electron impact ionization energy was 70 eV. The chemical components of the crude extracts were identified from the chromatograms and mass spectra using the Wiley 7 N.l database (Agilent Technologies, USA).
Cell culture
The human leukemic (U937) cell line was cultured in RPMI 1640. The human hepatocellular carcinoma (HepG2) cell line and normal African green monkey kidney epithelial (Vero) cell line were cultured in DMEM (GIBCO, Invitrogen Corporation, USA). Both media were supplemented with 10 % fetal bovine serum (GIBCO, Invitrogen Corporation), 100 units/mL penicillin and 100 µg/mL streptomycin (GIBCO, Invitrogen Corporation). The cells were cultured at 37 °C in a humidified atmosphere containing 5 % CO2.
Antiproliferative effect
The antiproliferative activity of the P. kesiya extract in the leukemia (U937), hepatocellular carcinoma (HepG2), and normal (Vero) cell lines was assessed by neutral red assay [14, 15]. Briefly, 100 µL of U937 cells at a density of 5 × 105 cells/mL and 100 µL of HepG2 or Vero cells at a density of 3 × 105 cells/mL were independently seeded in 96-wells plates and incubated for 24 h. After cell growth, cells were treated with various concentrations (10 to 500 µg/mL) of the P. kesiya crude extract or melphalan (purity 95 %, Sigma–Aldrich Chemie GmbH, Germany) dissolved in DMSO (United States Biological, USA). The maximum final concentration of the compound was 500 µg/mL to maintain 1 % v/v DMSO with a cytotoxicity <10 % compared with the untreated cells. After treated cells were exposed to the test compound for 24 h, cells were stained directly with a final concentration of 50 µg/mL neutral red dye (Sigma Chemicals Co., USA) and incubated for another 2 h. The neutral red stained viable cells were dissolved by 0.33 % hydrogen chloride in isopropanol and detected using a colorimetric-based method. The absorbance was measured at 520 and 650 nm (reference wavelength) by a spectromicroplate reader (TECAN, Grödig, Austria). A plot of percentage cytotoxicity vs. test compound concentration was used to calculate the IC50.
Apoptosis induction effect
Nuclei morphological study by 4´6-diamidino-2-phenylindole (DAPI) staining assay
The apoptosis effect of the plant crude extracts was primarily determined by fluorescent dye staining and DAPI to identify the condensation and fragmentation of nuclear DNA [14, 15]. Briefly, the cancer cells (HepG2 or U937) were treated with various concentrations of the test compounds (6 to 500 µg/mL of P. kesiya and 0.75 to 15 µg/mL of melphalan) for 24 h, after which the culture medium was removed and the cells were washed with fresh medium. Cells were then fixed by cold methanol. DAPI dye (Sigma–Aldrich Chemie GmbH, Germany) was then added to stain the nuclei DNA for 1 h. The excess dye was removed and 1X PBS (pH 7.4; 10 mM) was added to glycerin (at a 1:1 ratio). The DAPI staining assay was performed in triplicate in independent experiments. An inverted fluorescence microscope (Nikon eclipse 80i, Kanagawa, Japan) was used to record images of the DAPI staining. The average percentage of apoptotic cells was calculated from three independent wells with 10 eye views per well under the inverted fluorescence microscopy at a magnitude of 40×.
Analysis of DNA fragmentation
DNA fragmentation was analyzed [14, 15]. Briefly, after cancer cells were treated with various concentrations of P. kesiya extracts (60, 150, 300, 450, and 500 µg/mL) and melphalan (15 µg/mL) for 24 h, they were collected and washed with fresh medium. Then, the cell suspension was transferred to microcentrifuge tubes and centrifuged (Daihan Scientific, Seoul, Korea) at 300×g for 5 min to collect the cell pellet. The DNA in the cell pellet was extracted using a FlexiGene DNA kit (QIAGEN GmbH, Germany) then 2 µg of the DNA was analyzed using electrophoresis on 2 % agarose gels (Bio-Rad, USA) containing 0.1 mg/mL ethidium bromide (AppliChem, USA). DNA was mixed with loading dye (SibEnzyme Ltd., Russia) and the gel electrophoresed in 0.5X Tris–Borate-EDTA buffer (pH 8.3; 40 mMTris base, 45 mM boric acid, 1 mM EDTA; Sigma–Aldrich Chemie GmbH, Germany) at 250 V for 1 min and 20 V for 4 h. After electrophoresis, the DNA fragments were analyzed by In Genius L gel documentation (Syngene, USA).
Enhancement of anticancer effect of P. kesiya extract in vitro
The enhanced chemosensitivity of melphalan combined with P. kesiya on the U937 and HepG2 cell lines was determined as per Chou and Talalay [22]. The antiproliferation assay on the melphalan and P. kesiya extract combination treatment against the HepG2 cancer cell line after 24 h was performed using the same method as for melphalan or P. kesiya extract alone. The cells were treated with the P. kesiya extract and melphalan was added subsequently. Different concentrations of the P. kesiya extract and melphalan combination treatment were used (Table 1). Briefly, the melphalan concentration was fixed at 1 × IC50 and the P. kesiya concentration was varied at 0.02, 0.05, 0.1, 0.2, 0.5, 1, 1.5, and 2 × IC50 in the U937 and HepG2 cell lines. In another series, the P. kesiya extract concentration was fixed at 1 × IC50 and the melphalan concentration was varied at 0.05, 0.1, 0.2, 0.5, 1, 1.5, and 2 × IC50 in both cancer cell lines. The maximum concentration that could be used in the experiment to maintain the percentage DMSO at less than 1 % v/v was 500 µg/mL, instead of a 2 × IC50 concentration of P. kesiya (600 µg/mL), the concentration used in this study was only 500 µg/mL. After 24 h exposure, the percentage of antiproliferation for each treatment was calculated.
Table 1.
Concentration ratios of P. kesiya extract and melphalan combinations used in the combination anticancer study
| IC50 Ratio in U937 | ||||||||||||||
| IC50 of P. kesiya (µg/mL) | 0.02 (6) | 0.05 (15) | 0.1 (30) | 0.2 (60) | 0.5 (150) | 1 (300) | 1.5 (450) | 2 (500a) | 1 (300) | 1 (300) | 1 (300) | 1 (300) | 1 (300) | 1 (300) |
| IC50 of melphalan (µg/mL) | 1 (15) | 1 (15) | 1 (15) | 1 (15) | 1 (15) | 1 (15) | 1 (15) | 1 (15) | 0.05 (0.75) | 0.1 (1.5) | 0.2 (3) | 0.5 (7.5) | 1.5 (22.5) | 2 (30) |
| IC50 Ratio in HepG2 | ||||||||||||||
| IC50 of P. kesiya (µg/mL) | 0.02 (1.1) | 0.05 (2.75) | 0.1 (5.5) | 0.2 (11) | 0.5 (27.5) | 1 (55) | 1.5 (83) | 2 (110) | 1 (55) | 1 (55) | 1 (55) | 1 (55) | 1 (55) | 1 (55) |
| IC50 of melphalan (µg/mL) | 1 (40) | 1 (40) | 1 (40) | 1 (40) | 1 (40) | 1 (40) | 1 (40) | 1 (40) | 0.05 (2) | 0.1 (4) | 0.2 (8) | 0.5 (20) | 1.5 (60) | 2 (80) |
The experimental IC50 concentrations of P. kesiya in U937 and HepG2 cells were 299.0 ± 5.2 and 52.0 ± 5.8 µg/mL, respectively
The experimental IC50 concentrations of melphalan in U937 and HepG2 cells were 15.0 ± 1.0 and 37.7 ± 9.8 µg/mL, respectively
aThe maximum concentration used in the experiment to keep percentage DMSO at <1 % v/v was 500 µg/mL
The combined effects were analyzed to determine whether the enhanced growth inhibitory effect was antagonistic, additive, or synergistic. The combination index (CI) was calculated (adapted from the multiple-drug effect analysis based on the median effect principle and the isobologram technique developed by Chou [22] and Eid et al. [23]). The CI took into account both the potency and shape of the dose–effect curve, which is given by Eq. (1):
| 1 |
CI > 1, = 1, and <1 correspond to synergism, addition, and antagonism, respectively. The denominators (Dx)1 and (Dx)2 are the doses of a single treatment of the first and second compound designated as 1 and 2, respectively. Additionally, (D)1 and (D)2 are the doses of the compounds 1 and 2 in the combination treatment that exhibited x % antiproliferation.
The calculated dose reduction index (DRI) was used to determine the extent of dose reduction in the combination treatment compared with the dose of each single treatment. For the combination effect, DRI was defined as . The relationship between DRI and CI was represented as: .
The same antiproliferation experiment was performed on the normal Vero cells to provide a comparison to determine the cytotoxic effect of combining the compounds against the normal cells. The synergistic apoptotic effects of the combination of P. kesiya extract and melphalan were evaluated against the U937 and HepG2 cells by DAPI staining and DNA fragmentation assay.
Statistical analysis
Experiments were performed in triplicate and the results were expressed as a mean ± standard deviation from three independent experiments. Statistical analysis was performed by IBM SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA). A two-way analysis of variance (ANOVA) test followed by Fisher’s least significant difference test for multiple comparisons of independent experiments were used to determine the effective enhancement of the inhibition growth by P. kesiya and melphalan in various cell lines. The significance level was set at P < 0.05. Statistical differences of percentage apoptotic cells between the P. kesiya treated group and the positive control group were compared by one-way ANOVA followed by Tukey’s honest significant difference test with a significance level of P < 0.05.
Results
Antiproliferative effects of P. kesiya extract and melphalan singly versus in combination on U937 and HepG2 cell lines
The antiproliferative effects of P. kesiya extract and melphalan singly or in combination were investigated in U937 and HepG2 cell lines. The IC50 values of P. kesiya in U937 and HepG2 were 299.0 ± 5.2 µg/mL and 52.0 ± 5.8 µg/mL, respectively. The IC50 values of melphalan in U937 and HepG2 were 15.0 ± 1.0 µg/mL and 37.7 ± 9.8 µg/mL, respectively. Both melphalan and P. kesiya extract caused significantly greater antiproliferation than was observed in untreated cancer cells (control). P. kesiya extract exerted potent antiproliferation in the HepG2 cells at an IC50 value of 52.0 ± 5.8 µg/mL. P. kesiya extract had a stronger selectivity against the two cancer cell lines than melphalan. A significant enhanced antiproliferative effect occurred with the combination when treating human U937 and HepG2 cell lines (Table 2). A two-way ANOVA was conducted that examined the effect of IC50 ratio and cell types on percentage antiproliferation. There was a statistically significant interaction between the effects of IC50 ratio and cell types on percentage antiproliferation, F(26, 84) = 42.654, P < 0.001. A two-way ANOVA detected significant differences in the antiproliferative activity of the various IC50 ratios (all P < 0.001) and among the various cell lines (P < 0.001). Significantly higher antiproliferative activities were observed at P. kesiya to melphalan concentration ratios of 0.2:1, 0.5:1, 1:1, 1.5:1, 2:1, 1:0.2, 1:0.5, 1:1.5, and 1:2 compared with normal Vero cells (P < 0.001). When the concentration of melphalan was constant, an enhanced antiproliferative effect was observed at P. kesiya to melphalan concentration ratios of 0.05:1 and 0.5:1 in the U937 and HepG2 cell lines, respectively. Alternatively, when P. kesiya was constant, the enhanced antiproliferative effect was observed at melphalan to P. kesiya concentration ratios of 0.1:1 and 0.2:1 in the U937 and HepG2 cell lines, respectively.
Table 2.
Antiproliferation of P. kesiya extract and melphalan combination in U937, HepG2, and Vero cell lines
| IC50 Ratio | % Antiproliferation | |||
|---|---|---|---|---|
| P. kesiya | Melphalan | U937 | HepG2 | Vero |
| 0 | 1 | 50 | 50 | 50a |
| 1 | 0 | 50 | 50 | Inactiveb |
| 1 | 1 | 100 ± 7.0 | 95.7 ± 12.2 | 6.8 ± 1.9 |
| 0.02 | 1 | 46.0 ± 5.7 | Nd | Nd |
| 0.05 | 1 | 59.8 ± 5.3 | Nd | Nd |
| 0.1 | 1 | 76.6 ± 13.5 | Nd | 1.0 ± 3.5 |
| 0.2 | 1 | 79.3 ± 3.7 | 43.7 ± 2.9 | 4.0 ± 6.1 |
| 0.5 | 1 | 100 ± 7.0 | 82.1 ± 4.9 | 7.3 ± 1.9 |
| 1.5 | 1 | 100 ± 2.2 | 93.4 ± 1.6 | 16.4 ± 4.7 |
| 2 | 1 | 100 ± 11.3 | 100 ± 3.1 | 24.7 ± 9.1 |
| 1 | 0.05 | 46.8 ± 9.5 | 34.2 ± 4.4 | Nd |
| 1 | 0.1 | 51.8 ± 5.5 | 46.0 ± 9.1 | Nd |
| 1 | 0.2 | 73.0 ± 4.2 | 60.7 ± 16.4 | 0 ± 3.3 |
| 1 | 0.5 | 76.4 ± 3.6 | 65.1 ± 9.1 | 1.8 ± 1.2 |
| 1 | 1.5 | 100 ± 2.0 | 100 ± 10.5 | 8.6 ± 1.4 |
| 1 | 2 | 100 ± 3.4 | 100 ± 4.9 | 9.5 ± 1.9 |
Two-way ANOVA detected significant antiproliferative activity differences among various IC50 ratios (P < 0.001) and among various cell lines (P < 0.001). A significant two-way interaction was observed between IC50 ratio and various cell lines (P < 0.001)
Nd not determined
aIC50 value of melphalan in Vero cells equals 59.9 ± 3.2 µg/mL
bInactive when IC50 of P. kesiya in Vero cells was >500 µg/mL
Melphalan alone exhibited strong antiproliferation in normal Vero cells at an IC50 value of 59.9 ± 3.2 µg/mL, while P. kesiya extract alone was inactive in normal Vero cells. When treating the Vero cells, the antiproliferation of the combination treatment was <25 % when the concentration of melphalan was constant (Table 2). The result showed an antagonistic effect of P. kesiya extract on the antiproliferation of melphalan in Vero cells. In the presence of a 1 × IC50 concentration of P. kesiya, the concentration of melphalan used could be as high as 2 × IC50, producing only 9.5 ± 1.9 % antiproliferation against Vero cells.
Synergistic effect of melphalan and P. kesiya extract combination analyzed in U937 leukemic and HepG2 hepatocellular carcinoma cell lines
The CIs of the combined effect of P. kesiya and melphalan in the U937 and HepG2 cell lines at IC75 and IC90 values are presented in Table 3 and Fig. 1. Our results indicated that the combination of P. kesiya and melphalan exhibited high synergistic effects in U937 and HepG2 cells at concentrations producing 75 and 90 % antiproliferation (IC75 and IC90). As observed from the higher synergistic effect (low CI value), the combination therapy was more effective on the U937 cells than on the HepG2 cells.
Table 3.
Dose reduction index (DRI) and combination index (CI)
| % Antiproliferation | Cell lines | Combination treatment | DRI | CI | |
|---|---|---|---|---|---|
| P. kesiya | Melphalan | ||||
| 90 % (IC90) | U937 | Fixed [P. kesiya] = IC50 | 2.3 | 7.8 | 0.56 |
| Fixed [melphalan] = IC50 | 6.5 | 5.5 | 0.33 | ||
| HepG2 | Fixed [P. kesiya] = IC50 | 2.0 | 3.6 | 0.79 | |
| Fixed [melphalan] = IC50 | 2.4 | 3.2 | 0.74 | ||
| 75 % (IC75) | U937 | Fixed [P. kesiya] = IC50 | 1.8 | 8.7 | 0.66 |
| Fixed [melphalan] = IC50 | 9.1 | 3.8 | 0.37 | ||
| HepG2 | Fixed [P. kesiya] = IC50 | 1.6 | 3.8 | 0.90 | |
| Fixed [melphalan] = IC50 | 2.7 | 2.4 | 0.79 | ||
DRI and CI of the combination treatment of P. kesiya/melphalan in U937 or in HepG2 cells at the concentrations that produced 90 and 75 % antiproliferation
Fig. 1.
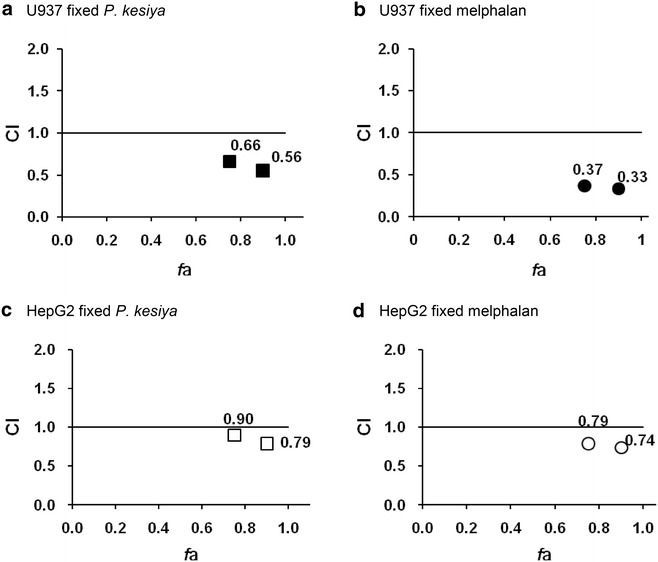
Isobolograms of the plot between combination index (CI) and fa in U937 (black symbol) and HepG2 (white symbol). fa = fraction affected by D (i.e., percentage antiproliferation/100). Isobolograms of the combination of compounds when P. kesiya was fixed (a, c; square), and when melphalan was fixed (b, d; circle) in each cancer cell lines
Extent of dose reduction in the combination treatment compared with single doses of each treatment
Table 3 shows the decreased concentration of melphalan or P. kesiya extract in the combination therapy to produce 90 and 75 % antiproliferation in U937 cells and HepG2 cells. The concentration of melphalan (when combined with a fixed concentration of P. kesiya extract) necessary to inhibit cancer growth by 90 % (IC90) represents a 7.8- and 3.6-fold decrease in U937 and HepG2 cells, respectively. By comparison, the concentration of P. kesiya extract (when combined with a fixed concentration of melphalan) needed for the IC90 represents a 6.5- and 2.4-fold decrease in U937 and HepG2 cells, respectively. A similar DRI trend (i.e., a greater melphalan dose reduction) for U937 cells compared with HepG2 cells was observed at 75 % antiproliferation. Dose reduction also depended on the type of cancer cells: 90 and 75 % cell death occurred when the P. kesiya extract concentration was fixed. The doses for melphalan per dose of P. kesiya extract could be reduced in the U937 cells (10.55:300 = 0.04:1 and 6.6:300 = 0.02:1) more than in the HepG2 cells (33.7:55.0 = 0.61:1 and 23.7:55.0 = 0.43:1). The synergistic effect appears to be greater for the melphalan because a greater potential dose reduction could be achieved; thus, melphalan concentrations could be significantly reduced.
Apoptotic induction effect of single treatments and of P. kesiya extract and melphalan combination treatment
Apoptosis induction by P. kesiya extract and melphalan when used singly or in combination was evaluated in HepG2 and U937 cell lines using a method based on the DAPI staining assay (Table 4) and DNA fragmentation assays (Fig. 2). In the control group, the nuclei were roundish and homogeneously stained by DAPI; in contrast, the apoptotic nuclei in the treated U937 and HepG2 cells were irregularly shaped, small, detached, and had apoptotic bodies. At a 1 × IC50 concentration, P. kesiya extract induced 42.5 ± 4.8 and 39.7 ± 2.6 % apoptosis in the U937 and HepG2 cells, respectively; melphalan induced 43.1 ± 16.3 and 53.0 ± 6.1 % apoptosis, respectively. The combination therapy of P. kesiya extract and melphalan was significantly more effective in inducing apoptosis against U937 and HepG2 cells.
Table 4.
Effects of P. kesiya and melphalan on apoptosis induction in U937 and HepG2 cells
| IC50 ratio | % Apoptotic cells | ||
|---|---|---|---|
| IC50 of P. kesiya | IC50 of Melphalan | U937 | HepG2 |
| 0 | 1 | 43.1 ± 16.3 | 53.0 ± 6.1 |
| 1 | 0 | 42.5 ± 4.8b | 39.7 ± 2.6a |
| 1 | 1 | 100a | 100a |
| 0.1 | 1 | 77.0 ± 1.9a | 66.7 ± 3.1a |
| 0.2 | 1 | 100a | 74.9 ± 12.3a |
| 0.5 | 1 | 100a | 92.8 ± 1.3a |
| 1.5 | 1 | 100a | 98.3 ± 3.0a |
| 2 | 1 | 100a | 100a |
| 1 | 0.05 | 38.6 ± 6.1a | 62.4 ± 3.2a |
| 1 | 0.1 | 48.4 ± 3.8a | 87.9 ± 3.3a |
| 1 | 0.2 | 61.3 ± 3.3a | 90.3 ± 6.0a |
| 1 | 0.5 | 100a | 100a |
| 1 | 1.5 | 100a | 100a |
| 1 | 2 | 100a | 100a |
One-way ANOVA of percentage apoptotic cells compared with single treatment by melphalan at 1 × IC50 in cancer cells
aSignificant difference (P < 0.001)
bNon-significant difference (P ≥ 0.001)
Fig. 2.
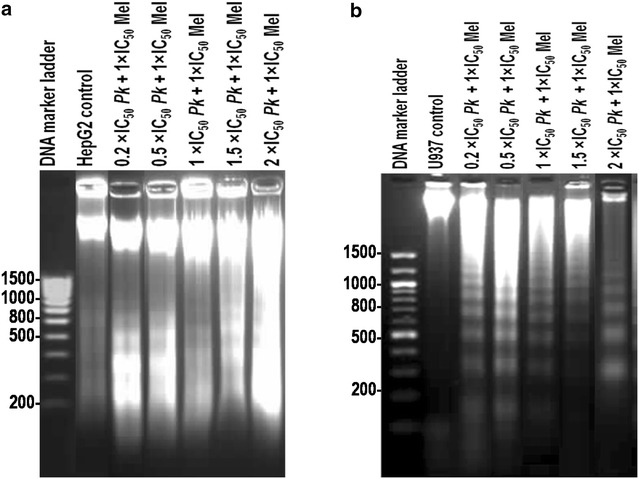
DNA fragments after combination treatment of P. kesiya (Pk) extract with melphalan in HepG2 (a) and U937 (b) cell lines
DNA fragmentation was determined according to whether the synergistic apoptosis induction in both cancer cells occurred toward the late stage of apoptosis, as previously observed for single therapy [15]. The combined treatment of P. kesiya with melphalan resulted in DNA laddering in both the HepG2 and U937 cells compared with the control cells (Fig. 2).
GC–MS analysis of P. kesiya extract
GC–MS analysis of P. kesiya extract was performed to obtain its characteristic fingerprint (Table 5). The GC peaks obtained were used for further chemical constituent identification and revealed several compounds:podocarpa-8,11,13-trien-15-oic acid, neopine, rosin acid, pimaric acid, oleic acid, pyrocatecol, and vanillin (Table 5).
Table 5.
Identified compounds and their relative distribution in P. kesiya
| Retention time (min) | % of Total area | Assigned compounds |
|---|---|---|
| 11.074 | 2.48 | Pyrocatecol (1,2-benzenediol or brenzcatechin) |
| 16.412 | 2.66 | trans-Sobrerol |
| 16.703 | 1.88 | Vanillin |
| 29.551 | 1.55 | Palmitic acid |
| 32.878 | 4.49 | Oleic acid |
| 35.650 | 4.16 | Benzoyl isocyanate |
| 36.096 | 4.77 | Pimaric acid |
| 37.170 | 6.52 | Rosin acid |
| 37.907 | 20.16 | Podocarpa-8,11,13-trien-15-oic acid, 13-isopropyl |
| 38.525 | 4.31 | 2,3-dimethoxy-5-[2-(3-hydroxy-4-methoxyphenyl)ethenyl]phenol or Combretastatin A3 |
| 41.788 | 5.98 | Neopine |
GC–MS data of the 50 % ethanol–water crude extract of P. kesiya
Discussion
To our knowledge, this is the first report of a synergistic effect of P. kesiya extract on melphalan anticancer activity via the apoptosis induction mechanism in vitro. The U937 cells were only sensitive to melphalan, based on the lower IC50 value; however, the HepG2 cells were sensitive to both P. kesiya extract and melphalan. A wide chemopreventive index was observed in the combination treatment, probably because the cytotoxic effect was lower in the normal Vero cells. P. kesiya extract’s potent antiproliferation effect in the HepG2 cell line and high selective antiproliferation effect in both cancer cell lines requires further investigation.
Drug and herb combinations could significantly decrease the toxicity and chemoresistance of drugs and increase their efficacy [24, 25] i.e., increasing the efficacy of the therapeutic effect; decreasing the dosage to avoid toxicity; reducing the development of drug resistance; and providing selective synergism against a target (or efficacy synergism) vs. host (or toxicity antagonism). The ability of herbal extracts to enhance anticancer activity might also arise from the potentiation of pharmacokinetics, wherein one ingredient enhanced the therapeutic effect of another (active ingredient or drug) by modulating its pharmacokinetic properties (i.e., absorption, distribution, metabolism, and/or excretion) [24, 25]. The administration of multiple therapeutic agents is often associated with additional toxicities, which can be life threatening; however, a combination treatment with a non-toxic herbal extract like P. kesiya might provide superior benefits.
Previous work indicated that, in addition to the compounds identified here in P. kesiya extract (Table 5), gallic acid, chlorogenic acid, caffeic acid, vanillin, and coumaric acid in the 50 % ethanol–water extract of P. kesiya exhibited an anticancer effect [14]. The main compounds found in various parts of Pinaceae were terpenes, such as α- and β-pinene [26]. Rosin is composed of a complex mixture of different compounds, including resin acids such as abietic acid, plicatic acid, and pimaric acid. Resin acids might be derived from terpenes through partial oxidation and undergo isomerization in the presence of strong acids or with heat [27]. Hence, terpenes such as α- and β-pinene might undergo thermal conversion into resin acids under the GC–MS condition studied. One previous study reported that α-pinene expressed both apoptosis induction and antimetastatic activity against melanoma cells [26]. A previous work has also shown that citronellol—found in the family Pinaceae—inhibited an efflux P-gp protein at an IC50 value of 504 µM [28].
Conclusions
Combining the P. kesiya extract with melphalan reduced toxicity while retaining the therapeutic efficacy (reduced approximately 4–9 fold) of melphalan.
Authors’ contributions
NW designed the study. SM performed the experiments. NW and SM collected and analyzed data. SM, NW and SB analyzed and interpreted data. SM and NW wrote the manuscript. NW and SB revised manuscript. All the authors read and approved the final manuscript.
Acknowledgements
We thank (a) The Office of the Higher Education Commission, Thailand, under the Strategic Scholarships for Thai Doctoral Degree Programs (CHE-PhD-THA-RG 3/2549), (b) Khon Kaen University for financial support, (c)The authors thank the Plant Genetics Conservation Project under the Royal Initiation of her Royal Highness Princess Maha Chakri Sirindhorn, The Bureau of The Royal Household for permission to conduct the research and Electricity Generating Authority of Thailand (EGAT) for field support and (d) Mr. Bryan Roderick Hamman and Mrs. Janice Loewen-Hamman for assistance with the English-language presentation.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethylsulfoxide
- FBS
fetal bovine serum
- HepG2
human hepatocellular carcinoma cell line
- NR
neutral red
- PBS
phosphate buffer solution
- NCI
National Cancer Institute (USA)
- U937
human leukemic cell line
- Vero
normal African green monkey kidney epithelial cell line
Contributor Information
Natthida Weerapreeyakul, Email: natthida@kku.ac.th.
Sasipawan Machana, Email: sasipawan.machana@gmail.com.
Sahapat Barusrux, Email: sahapat@kku.ac.th.
References
- 1.Au JLS, Panchal N, Li D, Gan Y. Apoptosis: a new pharmacodynamic endpoint. Pharm Res. 1997;14:1659–1671. doi: 10.1023/A:1012159208559. [DOI] [PubMed] [Google Scholar]
- 2.Lee C, Raffaghello L, Longo VD. Starvation, detoxification, and multidrug resistance in cancer therapy. Drug Resist Updates. 2012;15:114–122. doi: 10.1016/j.drup.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinmai K, Chunlaratthanabhorn S, Ngamkitidechakul C, Soonthornchareon N, Hahnvajanawong C. Synergistic growth inhibitory effects of Phyllanthus emblica and Terminalia bellerica extracts with conventional cytotoxic agents: doxorubicin and cisplatin against human hepatocellular carcinoma and lung cancer cells. World J Gastroentero. 2008;14:1491–1497. doi: 10.3748/wjg.14.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang SN, Singh C, Nall D, Meeker D, Shankar S, Srivastava RK. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J Mol Signal. 2010;5:14. doi: 10.1186/1750-2187-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner H, Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16:97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Machana S, Weerapreeyakul N, Barusrux S, Thumanu K, Tanthanuch W. Synergistic anticancer effect of the extracts from Polyalthia evecta caused apoptosis in human hepatoma (HepG2) cells. Asian Pac J Trop Biomed. 2012;2:589–596. doi: 10.1016/S2221-1691(12)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ya C, Ming-Hua Q, Kun G, Lin Z, ZhongRong L. A new abietane diterpenoid from the rosin of Pinus kesiya var. langbianensis (Pinaceae) Acta Botanica Yunnanica (ABY) 2006;3:323–325. [Google Scholar]
- 8.Jingkai D, Lisheng D, Yuanfen Y, Yu W, Handong S. Chemical constituents of the turpentine of Pinus kesiya var. langbianensis. Yunnan Zhiwu Yanjiu. 1983;5:224–246. [Google Scholar]
- 9.Koukos PK, Papadopoulou KI, Patiaka DT, Papagiannopoulos AD. Chemical composition of essential oils from needles and twigs of balkan pine (Pinus peuce Grisebach) grown in Northern Greece. J Agric Food Chem. 2000;48:1266–1268. doi: 10.1021/jf991012a. [DOI] [PubMed] [Google Scholar]
- 10.Mateus EP, Gomesdasilva MD, Ribeiro AB, Marriott PJ. Qualitative mass spectrometric analysis of the volatile fraction of creosote-treated railway wood sleepers by using comprehensive two-dimensional gas chromatography. J Chromatogr. 2008;1178:215–222. doi: 10.1016/j.chroma.2007.11.069. [DOI] [PubMed] [Google Scholar]
- 11.Mateus E, Baratab RC, Zrostlíková J, Gomesdasilva MDR, Paiva MR. Characterization of the volatile fraction emitted by Pinus spp. by one- and two-dimensional chromatographic techniques with mass spectrometric detection. J Chromatogr. 2010;1217:1845–1855. doi: 10.1016/j.chroma.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 12.Hiltunen R, Löyttyniemi K. Monoterpene composition of needle oil in Pinus kesiya Royle ex Gordon. Seloste: Pinus kesiya-männyn neulasöljyn monoterpeenikoostumus. Commun Inst For Fenn. 1978;94:1–9. [Google Scholar]
- 13.Hynniewta SR, Kumar Y. Herbal remedies among the Khasi traditional healers and village folk Mekhalaya. Indian J Traditional Knowl. 2008;7:581–586. [Google Scholar]
- 14.Machana S, Weerapreeyakul N, Barusrux S, Nonpanya A, Sripanidkulchai B, Thitimetharoch T. Cytotoxic and apoptotic effects of six herbal plants against the human hepatocarcinoma (HepG2) cell line. Chin Med. 2011;6:39. doi: 10.1186/1749-8546-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machana S, Weerapreeyakul N, Barusrux S, Thumanu K, Tanthanuch W. FTIR microspectroscopy discriminates anticancer action on human leukemic cells by extracts of Pinus kesiya; Cratoxylum formosum spp. pruniflorum and melphalan. Talanta. 2012;93:371–382. doi: 10.1016/j.talanta.2012.02.058. [DOI] [PubMed] [Google Scholar]
- 16.Cloutier JF, Castonguay A, O’Connor TR, Drouin R. Alkylating agent and chromatin structure determine sequence context-dependent formation of alkylpurines. J Mol Biol. 2001;306:169–188. doi: 10.1006/jmbi.2000.4371. [DOI] [PubMed] [Google Scholar]
- 17.Lialiaris T, Lyratzopoulos E, Papachristou F, Simopoulou M, Mourelatos C, Nikolettos N. Supplementation of melatonin protects human lymphocytes in vitro from the genotoxic activity of melphalan. Mutagenesis. 2008;23:347–354. doi: 10.1093/mutage/gen020. [DOI] [PubMed] [Google Scholar]
- 18.Boegsted M, Holst JM, Fogd K, Falgreen S, Sørensen S, Schmitz A, Bukh A, Johnsen HE, Nyegaard K, Dybkaer K. Generation of a predictive melphalan resistance index by drug screen of B-cell cancer cell lines. PLoS ONE. 2011;6:e19322. doi: 10.1371/journal.pone.0019322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phengklai C. Pinaceae/Cephalotaxaceae/Cupressaceae. Fl Thai. 1972;2:193–196. [Google Scholar]
- 20.Phengklai C. Studies in flora of Thailand : Pinaceae. Thai For Bull. (Bot.) 1973;7:1–4. [Google Scholar]
- 21.Weerapreeyakul N, Nonpunya A, Barusrux S, Thitimetharoch T, Sripanidkulchai B. Evaluation of the anticancer potential of six herbs against a hepatoma cell line. Chin Med. 2012;7:15. doi: 10.1186/1749-8546-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 23.Eid SY, El-Readi MZ, Wink M. Synergism of three-drug combinations of sanguinarine and other plant secondary metabolites with digitonin and doxorubicin in multi-drug resistant cancer cells. Phytomedicine. 2012;19:1288–1297. doi: 10.1016/j.phymed.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- 25.Feng-Zhu JJ, Ma X, Cao ZW, Li YX, Chen YZ. Mechanisms of drug combinations from interaction and network perspectives. Nat Rev Drug Discov. 2009;8:111–128. doi: 10.1038/nrd2683. [DOI] [PubMed] [Google Scholar]
- 26.Matsuo AL, Figueiredo CR, Arruda DC, Pereira FV, Scutti JAB, Massaoka MH, Travassos LR, Sartorelli P, Lago JHG. α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem Biophys Res Commun. 2011;411:449–454. doi: 10.1016/j.bbrc.2011.06.176. [DOI] [PubMed] [Google Scholar]
- 27.Parimal K, Khale A, Pramod K. Resins from herbal origin and a focus on their applications. IJPSR. 2011;2:1077–1085. [Google Scholar]
- 28.Yoshida N, Takagi A, Kitazawa H, Kawakami J, Adachi I. Inhibition of P-glycoprotein-mediated transport by extracts of and monoterpenoids contained in Zanthoxyli Fructus. Toxicol Appl Pharm. 2005;209:167–173. doi: 10.1016/j.taap.2005.04.001. [DOI] [PubMed] [Google Scholar]


