Abstract
There is limited understanding of the etiology and temporal relations of chronic pain, sleep complaints, and depression/anxiety. Several models have been proposed by which sleep disruption represents a common mechanism for the comorbidity of these symptoms. The goals of this study were to 1) clarify the boundaries of these domains and to 2) examine the relations of these symptoms over time following exposure to stressful and potentially traumatic experiences during a combat deployment. We found support for three distinct domains of sleep complaints, internalizing symptoms, and physical complaints. We tested two competing models that have been proposed in the literature, controlling for negative and positive emotionality. Internalizing symptoms strongly mediated the relation between sleep complaints and pain (total effect = .15, direct effect = -.05). The study suggests that increases in sleep complaints immediately following deployment increase the risk of internalizing symptoms and pain several years after deployment.
Keywords: chronic pain, insomnia, lassitude, internalizing disorders
Lower back pain and major depressive disorder are the top two causes of disability in the United States, followed closely by other musculoskeletal disorders and anxiety (US Burden of Disease Collaborators, 2013). These disabling conditions rarely occur in isolation. Over the past 25 years, research has demonstrated that chronic pain and depression/anxiety co-occur at greater than chance levels (Banks & Kerns, 1996; McWilliams, Cox, & Enns, 2003). These conditions also show a high comorbidity with insomnia (Daley, Morin, LeBlanc, Gregoire, & Savard, 2009; Daley, et al., 2008; Smith, Huang, & Manber, 2005; Wilson, Eriksson, D'Eon, Mikail, & Emery, 2002). Patients with chronic pain who report both sleep complaints and depression report the greatest levels of distress, pain, and impaired functioning (Asih, Neblett, Mayer, Brede, & Gatchel, 2014; Emery, Wilson, & Kowal, 2014; Wilson, et al., 2002). Together, chronic pain, sleep complaints, and depression/anxiety disorders form a triad of distress that represents great personal costs for sufferers, as well as significant societal costs and economic burden on the healthcare system.
Research in this area has gradually progressed from documenting the overlap of pain, sleep complaints, and depression/anxiety to exploring the temporal sequence of these conditions. Recent longitudinal evidence suggests that sleep complaints are a more consistent predictor of next day pain than pain is of sleep complaints (Affleck, Urrows, Tennen, Higgins, & Abeles, 1996; Bigatti, Hernandez, Cronan, & Rand, 2008; Finan, Goodin, & Smith, 2013; Tang, Goodchild, Sanborn, Howard, & Salkovskis, 2012). Similarly, sleep complaints are a stronger predictor of depression in patients with chronic pain than depression is of sleep problems (Campbell, et al., 2013).
In accordance with these findings, several theoretical models have been proposed in which dysregulation of sleep-wake processes represents a common risk factor for both chronic pain and depression/anxiety. Walker and colleagues suggest that sleep plays a crucial role in regulating emotional brain networks by facilitating the inhibitory effect of the medial-prefrontal cortex on the amygdala (Goldstein & Walker, 2014; Walker & van der Helm, 2009; Yoo, Gujar, Hu, Jolesz, & Walker, 2007). This theory would suggest that extended sleep difficulties disrupt affective processes, potentially resulting in chronic states of depression and anxiety. Conversely, adequate amounts of high quality sleep could strengthen emotion regulation, reducing the risk for developing depression/anxiety symptoms (Ong, et al., 2013; Steptoe, O'Donnell, Marmot, & Wardle, 2008). Although this theoretical model does not directly incorporate pain, O'Brien et al. (2010) suggest that sleep disturbances result in impaired affective processes, which in turn, lead to increases in pain-related distress and functional impairment. They used structural equation modeling (SEM) in a cross-section design and found that depression and anxiety symptoms mediated the effect of poor sleep on pain (O'Brien, et al., 2010).
An alternative model is one in which the influence of sleep complaints on depression/anxiety symptoms is mediated by pain. Vitiello, Rybarczyk, Von Korff, and Stepanski (2009) suggest that behaviors used to cope with chronic pain lead to dysregulation of sleep-wake cycles, which in turn lowers the pain threshold and amplifies the transmission of pain signals, leading to greater pain and ultimately increased negative cognitions and emotions. Similarly, Smith, Quartana, Okonkwo, and Nasir (2009) suggest that sleep disturbance increases hyperalgesia and decreases central pain inhibitory systems, directly resulting in increases in pain severity and indirectly resulting in mood disturbance. In support of this theory, Campbell et al. (2013) reported regression models using a prospective cohort design in which pain interference has a small mediating effect on the relation between sleep and depression.
The current study was designed to provide an empirical test of these two proposed models by examining the relations and mediational paths of sleep complaints, depression/anxiety symptoms, and pain complaints across time within the same sample. Although much work has been done to document the overlap of symptoms, very few prospective longitudinal studies have been conducted encompassing all three symptom domains, making it difficult to draw conclusions about directionality and mediation. In addition, previous work has not been conducted within the theoretical framework of the models reviewed earlier, limiting the ability to formulate and test specific hypotheses about the etiology and relations of symptoms. Finally, many studies utilize limited assessment batteries, often with one instrument per construct, and it is unclear if these instruments represent unique or overlapping domains. Given the moderate to high correlations among pain, sleep complaints, and depression/anxiety measures, it is important to clarify the boundaries of these domains to determine if sleep complaints and pain are independent constructs or a proxy for depression/anxiety symptoms. Although the working assumption has been that these three symptom domains are independent, there is a possibility that they represent nonspecific manifestation of general distress.
Some initial research has begun to establish the independence of these domains (Asih, et al., 2014; Koffel, et al., 2015; O'Brien, et al., 2010), but very little empirical work has been done in this area, particularly within the framework of basic temperament dimensions. Temperament dimensions are often utilized to account for symptom overlap in psychopathology (Clark & Watson, 1991; Clark, Watson, & Mineka, 1994; Watson, Clark, & Carey, 1988), and this framework would be helpful when examining the relations of pain, sleep complaints, and depression/anxiety symptoms. Depression and anxiety disorders are thought to share a substantial component of general distress represented by high levels of neuroticism/negative emotionality (N/NE), whereas low levels of pleasurable engagement represented by extraversion/positive emotionality (E/PE) are specific to depression and social anxiety (Mineka, Watson, & Clark, 1998; Naragon-Gainey, Watson, & Markon, 2009; Watson, Gamez, & Simms, 2005). If pain and sleep complaints have substantial components of N/NE and to a lesser extent, E/PE, they can be expected to show strong relations with depression/anxiety symptoms, limiting the extent to which they can be considered unique symptom dimensions.
In this study, we utilize a longitudinal prospective study design with a sample of National Guard Soldiers. This sample and study design makes it possible to investigate interactions of pain, sleep complaints, and anxiety/depression symptoms following exposure to stressful and potentially traumatic experiences during a combat deployment. Although extensive measurement of pain, sleep complaints, and internalizing symptoms was not feasible due to time constraints, multiple measures of each construct were collected, allowing us to begin to examine the overlap of these domains. In accordance with recent structural models of psychopathology, the construct of anxiety/depression will be referred to as internalizing to reflect the shared component of general distress (Krueger, 1999; Watson, 2005).
Controlling for baseline symptom levels and the temperament dimensions of N/NE and E/PE to account for symptom overlap due to shared traits, we examine the relation of symptoms over time, within the context of the theoretical models discussed earlier. We tested two models: 1) sleep complaints predict pain, mediated by internalizing symptoms and 2) sleep complaints predict internalizing symptoms, mediated by pain. Prior to testing these models, we examined the discriminant validity of physical complaints (including pain), sleep complaints, and internalizing symptoms to clarify the boundaries of these constructs and to establish that they represent distinct domains rather than manifestations of general distress. We hypothesized that we would be able to identify three distinct domains of sleep complaints, pain, and internalizing symptoms. Further, we hypothesized that sleep complaints immediately following deployment would be a significant predictor of both pain and internalizing symptoms 2 years following deployment, with evidence of significant mediation.
Methods
Participants and Procedures
Data were obtained as part of the Readiness and Resilience in National Guard Soldiers (RINGS) project, a prospective longitudinal study of 522 National Guard Brigade Combat Team Soldiers deployed to Iraq from March 2006 to July 2007. Details of the RINGS protocol are provided in other publications (Polusny, et al., 2011). Briefly, participants completed surveys approximately 1 month prior to combat deployment to Iraq (pre-deployment) and were mailed surveys 2-3 months after their return from Iraq, 1 year later, and 2 years later. Of the entire pre-deployment cohort, 424 (81%) returned surveys 2-3 months post-deployment, 343 (66%) returned surveys 1 year post-deployment, and 296 (57%) returned surveys 2 years post-deployment. The time points of interest in this study are pre-deployment, 2-3 months post deployment, and 2 years post-deployment. Study procedures were approved by the human subject research review boards of the Army, Minneapolis Veterans Affairs Health Care System, University of Minnesota, and the relevant Army National Guard Commands.
There were no significant differences between post-deployment responders and non-responders on most demographic variables and pre-deployment internalizing symptoms and personality traits. However, non-responders tended to be younger (t = -5.18, p < .001) and unmarried (t = -2.48, p < .05). There were no differences between responders and non-responders on pre-deployment measures of sleep complaints and pain. In addition, internalizing symptoms at 2-3 months post-deployment and report of injury during deployment was not related to responder status at 2 year follow-up for pain and internalizing measures. Participants in the baseline cohort were mostly male (88.3%) and white (92.8%), under 30 years of age (59.8%), and enlisted rank (90.1%).
Measures
Patient Health Questionnaire (PHQ-15)
General physical complaints, including pain and sleep complaints, were measured at pre-deployment and 2-years post deployment using the PHQ-15 (Kroenke, Spitzer, & Williams, 2002), a somatic symptom subscale of the PHQ that measures 15 distinct symptoms that account for more than 90% of the physical complaints reported in outpatient medical settings. The original instructions ask participants to rate how much they have been bothered by each symptom on a 3-point Likert scale. Due to time constraints, the version used in this study was modified to ask participants “during the past month, have you often been bothered by,” utilizing a dichotomous yes, no response for each item.
The PHQ-15 is a well-accepted measure of somatic symptoms with good internal reliability (Kroenke, et al., 2002). It has also shown evidence of discriminant validity when compared to symptoms of depression (Kroenke, et al., 2002). Evidence suggests that there are lower order dimensions within a general factor of physical complaints (Kroenke, Spitzer, deGruy, & Swindle, 1998; Witthoft, Hiller, Loch, & Jasper, 2013), with support for up to four specific factors representing pain symptoms (i.e., musculoskeletal and headaches), gastroenterological symptoms (i.e., stomach pain and nausea), cardio-pulmonary symptoms (i.e., chest pain, dizziness), and sleep-related symptoms (i.e., sleep problems, feeling tired) (Witthoft, et al., 2013).
Prior to data analyses, we excluded three items due to low base rates of endorsement (item 4, menstrual problems; item 5, pain during sexual intercourse; item 9, fainting spells). When submitting the PHQ-15 items to exploratory factor analysis in our sample, we found a structure very similar to that reported in Witthoft et al. (2013). As such, we used the subscales reported in Witthoft et al. (2013) in the current study. Coefficient alphas ranged from .81 to .84 for the entire instrument and from .57 to .73 for subscales across study time points.
Sleep Complaints scale
The RINGS study did not contain validated sleep measures, but did include items contained within the Beck Depression Inventory-II (BDI-II) (Beck, Steer, & Brown, 1996) that assessed loss of energy (e.g., “I have less energy than I used to”), changes in sleeping pattern (e.g., “I sleep a lot more than usual” or “I sleep a lot less than usual”), and tiredness/fatigue (e.g., “I get more tired or fatigued more easily than usual”). Sleep items were summed to create a measure of daytime and nighttime sleep disturbance, which has been utilized in previous analyses with this data (Koffel, Polusny, Arbisi, & Erbes, 2013). Validity data for this measure are not available. Coefficient alpha ranged from .72 to .82 across time points for the study sample, suggesting adequate reliability given the broad domain measured by this scale.
Beck Depression Inventory-II (BDI-II)
Depression symptom severity scores were obtained at all pre- and post-deployment time points from the BDI-II (Beck, et al., 1996), a 21-item measure, with each item rated on a 4-point scale from 0 to 3. Respondents indicate how they have been feeling over the past two weeks. This instrument has been shown to have good reliability and construct validity (Beck, et al., 1996; Quilty, Zhang, & Bagby, 2010; Ward, 2006; Whisman, Perez, & Ramel, 2000). The BDI-II was scored excluding the sleep-related items contained in the Sleep Complaints scale. Coefficient alpha ranged from .89 to .93 across time points.
PTSD Checklist-Civilian and Military Versions (PCL-C, PCL-M)
To obtain a measure of PTSD symptom severity, participants completed the PCL-C at pre-deployment and the PCL-M at all post-deployment time points (Weathers, Litz, Herman, Huska, & Keane, 1993). This instrument measures the 17 symptoms included in the diagnostic criteria for PTSD in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR; American Psychiatric Association, 2000). On the PCL-C, participants are asked to choose a response from a 5-point scale (ranging from not at all to extremely) that best describes how much they have been bothered by each symptom in the past month as it relates to a stressful experience. On the PCL-M, the instructions refer to a stressful military experience. Items are summed to provide a total score of PTSD symptom severity (Weathers, et al., 1993). The scale has demonstrated good reliability and convergent validity with self-report and interview measures of PTSD (Blanchard, Jones-Alexander, Buckley, & Forneris, 1996). The instrument was scored excluding two sleep-related items (“Repeated disturbing dreams of the stressful experience” and “Trouble falling or staying asleep”) to avoid inflating the relations between sleep complaints and psychopathology. Coefficient alpha ranged from .91 to .95 across time points.
Minnesota Multiphasic Personality Inventory -2 (MMPI-2) Psychopathology Five Scales (PSY-5)
Personality traits were assessed pre-deployment using abbreviated versions of the PSY-5 scales (Harkness, McNulty, & Ben-Porath, 1995). The PSY-5 model consists of five dimensions of personality traits spanning normal and abnormal functioning. This study reports data from the Negative Emotionality/Neuroticism (NEGE) and Introversion/Low Positive Emotionality (INTR) scales, which are used as markers of N/NE and E/PE, respectively. Due to time constraints associated with pre-deployment data collection, shortened versions of these scales were used. Items on these shortened scales were selected rationally by the third author (P.A.A.). The number of items and coefficient alphas for the abbreviated scales are as follows: NEGE had 23 of the original 33 items and a coefficient alpha of .82; INTR had 20 of the original 34 items and a coefficient alpha of .62.
Statistical Analyses
To determine if physical complaints (including pain), sleep complaints, and internalizing symptoms represented distinct constructs, we submitted pre-deployment and 2 year follow-up measures to two separate principal factor analyses with promax rotation in SAS. We used an oblique rotation to model the moderate to large correlations that have been obtained among these measures in the past. The prior communality estimate was calculated using squared multiple correlations (SMCs). The goal in these analyses was to extract the greatest number of factors that were interpretable and distinct from one another.
Subsequent analyses were conducted using SEM with maximum likelihood estimation in Mplus (version 7; Muthen & Muthen, 2012). Following recommendations for missing data that is missing at random or completely at random (Schafer & Graham, 2002), missing data was addressed with Full Information Maximum Likelihood (MLR) estimation. The SEM analyses involved several steps. First, we developed a measurement model in which latent variables were defined by observed indicators. The latent variables in this model were internalizing symptoms at pre-deployment and 2 years post-deployment (defined by total scores on the BDI-II and PCL). It was not possible to have a latent sleep variable due to limits of data collection at post-deployment. In addition, we focused these analyses on the specific PHQ Pain subscale rather than a more general physical complaints factor to more closely align with previous work.
In the next step, two structural models were estimated. Sleep complaints and pain were treated as observed variables using the Sleep Complaints scale at pre-deployment and 2-3 months post-deployment and the PHQ Pain scale at pre-deployment and 2 years post-deployment. To test the two proposed mediational models, pain was regressed onto internalizing symptoms and sleep complaints (see Figure 1) and internalizing symptoms were regressed onto pain and sleep complaints (see Figure 2). Post-deployment sleep complaints, pain, and internalizing symptoms were each regressed onto their respective pre-deployment measures to control for baseline symptom levels. Covariance among error terms for the same measures at different time points were estimated. In addition, pre-deployment symptoms were regressed onto the observed variables of NEGE and INTR to 1) examine the extent to which pain, sleep complaints, and internalizing symptoms show differential relations to these basic temperament dimensions and 2) to control for symptom overlap due to nonspecific, general distress.
Figure 1.
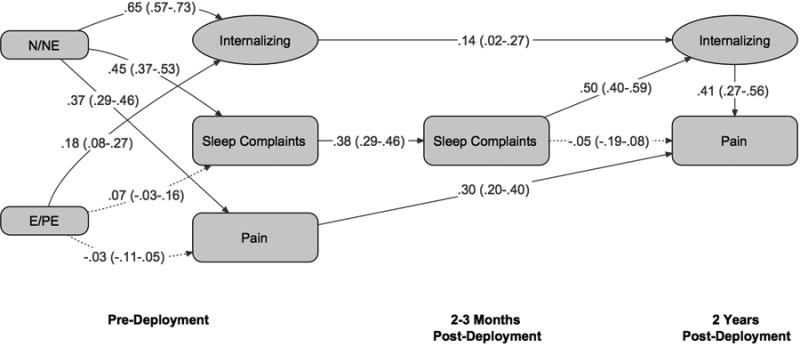
Standardized path coefficients generated using structural equation modeling for the model in which internalizing is a mediator. 95% confidence intervals are given in parentheses. n = 498. Significant paths and estimates have solid lines (p < .05). N/NE = Neuroticism/negative emotionality. E/PE = Extraversion/positive emotionality.
Figure 2.
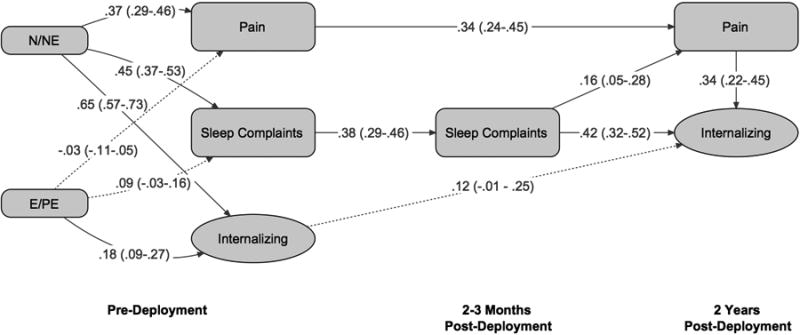
Standardized path coefficients generated using structural equation modeling for the model in which pain is a mediator. 95% confidence intervals are given in parentheses. n = 498. Significant paths and estimates have solid lines (p < .05). N/NE = Neuroticism/negative emotionality. E/PE = Extraversion/positive emotionality.
Three different fit indices were used to evaluate these models, including the comparative fit index (CFI), the standardized root-mean-square residual (SRMR), and the root-mean-square error of approximation (RMSEA). In general, fit is considered acceptable if CFI is .90 or greater and SRMR and RMSEA are .10 or less (Finch & West, 1997; Hu & Bentler, 1998). However, more stringent cutoffs for these indices have been recommended, including values of .95 for CFI, .08 for SRMR, and .06 for RMSEA (Hu & Bentler, 1999). In these analyses, we consider CFI values of .90 or greater to reflect an adequate fit and values of .95 or greater to reflect an excellent fit. Similarly, SRMR and RMSEA values of .10 or less are interpreted as representing an adequate fit and values of .06 or less represent an excellent fit.
Results
Preliminary Analyses
Table 1 presents the means and standard deviations for physical complaints, sleep complaints, and internalizing symptom measures at pre-deployment and 2 years post deployment in a subset of 244 participants who had complete data at both time points. Paired-sample t-tests indicated statistically significant increases on all symptom measures from pre- to post-deployment, suggesting that the combat deployment had a direct, detrimental effect on symptom levels. Cohen's d was calculated and effect sizes are shown in Table 1. There were medium effect sizes for internalizing symptoms, with small effect sizes for the remaining symptom measures.
Table 1. Means (SDs) of Sleep Complaints, Internalizing Symptoms, and Physical Complaints.
| Pre-deployment | 2-3 Months Post-deployment | 2 Years Post-deployment | t | d | |
|---|---|---|---|---|---|
| Sleep Complaints scale (range, 0-9) | 1.48 (1.60) | 2.05 (1.61) | 5.09* | .33 | |
| PCL-C, M (range, 15-75)† | 22.06 (8.01) | 30.06 (13.52) | 9.06* | .58 | |
| BDI-II (range, 0-54)† | 4.31 (5.47) | 8.72 (8.24) | 8.09* | .52 | |
| PHQ Sleep (range, 0-2) | .66 (.82) | 1.13 (.86) | 7.11* | .45 | |
| PHQ Pain (range, 0-3) | 1.09 (1.04) | 1.57 (1.10) | 6.22* | .40 | |
| PHQ Cardio Pulmonary (range, 0-4) | .37 (.81) | .76 (1.17) | 4.93* | .31 | |
| PHQ Gastroenterological (range, 0-3) | .52 (.82) | .81 (.99) | 4.19* | .27 |
Note. n = 244.
p < .01.
PCL-C, M = PTSD Checklist-Civilian and Military Versions. BDI-II = Beck Depression Inventory-II. PHQ = Patient Health Questionnaire.
sleep-related items removed.
For the 495 participants who had complete data at pre-deployment, physical complaints, sleep complaints, and internalizing scales at pre-deployment were submitted to exploratory factor analyses, initially revealing the presence of a large and relatively broad factor that was defined by all the scales (factor loadings of .53 and above) and accounted for 98% of the total variance pre-deployment. In the next step, two factors representing internalizing symptoms and physical complaints were extracted, with sleep measures loading moderately on both factors. These factors were moderately correlated at .65 and accounted for all of the common variance. When three factors were extracted, they represented internalizing symptoms, physical complaints, and sleep complaints.
Table 2 presents the factor loadings from the three-factor solution at pre-deployment, as well as the inter-factor correlations. Internalizing symptoms is defined by scales measuring symptoms of depression and PTSD. Physical complaints is defined by scales measuring pain gastroenterological symptoms, and cardio-pulmonary symptoms. Sleep complaints is defined by scales measuring insomnia and lassitude (e.g., fatigue, sleepiness). It is important to note that although there are relatively few markers for each dimension, the three-factor structure is clear and distinct, with limited cross-loadings. It should also be noted that the sleep scales cohere to form an independent factor, but do have moderate loadings across factors, most likely due to shared method variance (i.e., sleep scales were derived from the BDI-II and PHQ-15). Correlations among factors ranged from .55 to .64. This three factor structure was replicated in the post-deployment data. These findings suggest that self-reported symptoms of internalizing disorders, physical complaints, and sleep complaints represent distinct constructs rather than manifestations of nonspecific factors, such as general psychological distress.
Table 2. Factor structure of Internalizing Symptoms, Physical Complaints, and Sleep Complaints.
| Scales | Factor I (Internalizing) | Factor II (Physical Complaints) | Factor III (Sleep Complaints) |
|---|---|---|---|
| PCL-C, M | .77 | .09 | -.04 |
| BDI-II | .75 | -.03 | .18 |
| PHQ Pain | .05 | .60 | .06 |
| PHQ Cardio Pulmonary | .21 | .53 | -.05 |
| PHQ Gastroenterological | -.06 | .52 | .17 |
| Sleep Complaints scale | .29 | .03 | .58 |
| PHQ Sleep | .03 | .32 | .50 |
| Sleep Complaints | (.64) | ||
| Internalizing | (.55) | ||
| Physical Complaints | (.63) |
Note. n = 495 (pre-deployment). Factor loadings of |.40| and greater are highlighted. PCL-C, M = PTSD Checklist-Civilian and Military Versions. BDI-II = Beck Depression Inventory-II. PHQ = Patient Health Questionnaire.
Structural Equation Modeling
We examined the standardized factor loadings and 95% confidence intervals for the measurement model prior to investigating the relationships in the structural model to ensure that the latent variables were adequately defined by the specified indicators. All loadings were consistently high in the predicted direction and significant at p < .001, suggesting that the scales were good markers of their respective factors.
Prediction of Pain by Sleep Complaints Mediated by Internalizing Symptoms
The model in which internalizing symptoms are hypothesized as the mediator is shown in Figure 1. The fit indices suggested an excellent fit overall (χ2 = 79.37, df = 23), with CFI of .96 and SRMR of .05. RMSEA suggested an adequate fit at .07. Figure 1 also shows the standardized parameter estimates and 95% confidence intervals for the relationships among the predictors and the latent constructs, including the direct effects of sleep complaints on pain and the indirect effect of sleep complaints on pain, mediated by internalizing symptoms. The majority of regression coefficients were significant, with the exception of the path from sleep complaints at 2-3 months post-deployment to pain at 2 years post-deployment and the paths from E/PE to both pain and sleep complaints. As was hypothesized, sleep complaints significantly predicted internalizing symptoms, which in turn significantly predicted pain (regression coefficients ranged from .41 to .50), after controlling for temperament dimensions. The direct path from sleep complaints to pain was non-significant.
Pre-deployment symptoms had moderate to strong intercorrelations ranging from .26 to .61. In particular, the sleep complaints scale was strongly related to the latent factor internalizing symptoms at .61, most likely due to shared method variance. It is important to note that N/NE was strongly related to internalizing symptoms (regression coefficient = .65), with more moderate relations with pain and sleep complaints (around .40). In contrast, the trait of E/PE showed small relations with all baseline symptoms (regression coefficients ranged from -.03 to .18).
We also calculated the standardized coefficients of the direct, indirect, and total effects of sleep complaints on pain. It is noteworthy that sleep complaints have a significant total effect on pain (.15, p < .01) and a significant indirect effect (.21. p < .01), but the direct effect of sleep complaints on pain is rendered insignificant with the inclusion of internalizing symptoms as a mediator (-.05), suggesting strong evidence of mediation.
Prediction of Internalizing Symptoms by Sleep Complaints Mediated by Pain
The model in which pain is hypothesized as a mediator is shown in Figure 2. Once again, the majority of fit indices suggest an excellent fit (χ2 = 76.59, df = 23), with CFI at .96 and SRMR at .05. RMSEA suggested an adequate fit at .07. Figure 2 shows the standardized parameter estimates and 95% confidence intervals for the relationships among the predictors and the latent constructs, including the direct effects of sleep complaints on internalizing symptoms and the indirect effect of sleep complaints on internalizing symptoms, mediated by pain. As in the earlier model, the majority of regression coefficients were significant, with the exception of the path from pre-deployment internalizing symptoms to post-deployment internalizing symptoms and the paths from E/PE to pain and sleep complaints. Sleep complaints significantly predicted pain, which in turn significantly predicted internalizing symptoms (regression coefficients ranged from .16 to .34), controlling for baseline temperament dimensions. Sleep complaints also had a significant direct effect on internalizing disorders (regression coefficient = .42). As in the previous model, the pre-deployment symptom variables had moderate to strong intercorrelations ranging from .26 to .61. Once again, N/NE was primarily related to internalizing symptoms (regression coefficient = .65) and had more moderate relations with sleep complaints and pain. E/PE had small relations with all symptom dimensions.
Sleep complaints had significant indirect (.06, p < .01) effects on internalizing symptoms. The total effect of sleep complaints on internalizing symptoms was significant (.47, p < .01), with a reduced but significant direct effect (.42, p < .01) when pain was included as a mediator, suggesting some degree of mediation.
Discussion
This study found support for three distinct domains of internalizing symptoms, physical complaints, and sleep complaints. The two tested models provided an excellent fit to the data, suggesting that increases in sleep complaints immediately following deployment predict increases in pain and internalizing symptoms several years after deployment. There was strong evidence that the relation between sleep and pain is mediated by internalizing symptoms.
One of the main goals of this study was to examine the relative independence of sleep, pain, and internalizing symptom measures to determine if they represent distinct constructs. Using factor analysis, we found support for a three factor model of internalizing symptoms, physical complaints, and sleep complaints. These three factors were moderately related, but clearly distinct, suggesting that they represent unique underlying systems (e.g., sleep-wake system, pain system, internalizing disorder/affective system). Moreover, findings from the SEM models suggest that nonspecific general distress (i.e., N/NE) is primarily related to internalizing symptoms and has more modest relations with pain and sleep complaints. Although N/NE most likely accounts for some of the overlap in symptoms and was controlled for in the SEM analyses, it is clear that pain and sleep complaints have differential relations with temperament dimensions compared to internalizing symptoms and contain a smaller component of general distress.
Another primary goal was to empirically test two theoretical models within the same sample using SEM: 1) disturbed sleep predicts self-reported pain, mediated by internalizing symptoms and 2) disturbed sleep predicts internalizing symptoms, mediated by pain. Both models provided an overall excellent fit for the data, with the majority of fit indices surpassing the most stringent cut-offs. In the first model, there was strong evidence of mediation, with a non-significant direct path from sleep complaints to pain and significant indirect paths from sleep complaints to pain via internalizing symptoms. The second model provided evidence of some degree of mediation, with a significant direct path from sleep complaints to internalizing symptoms. The magnitude of this effect (i.e., total effect) was reduced with the inclusion of a significant indirect path from sleep complaints to internalizing symptoms through pain (total effect = .47, direct effect = .42). It is important to note that although the indirect effect was significant, it was smaller in magnitude than the indirect effect in the first model (.06 vs. .21). Internalizing symptoms appear to be a stronger mediator of the sleep-pain relationship than pain is a mediator of the sleep-internalizing relationship, providing support for the theory of emotional dysregulation following sleep disturbance (O'Brien, et al., 2010; Walker & van der Helm, 2009). However, the significant pathways in both models suggest that sleep complaints may result in both affective distress and reduced pain thresholds, with increases in pain and internalizing symptoms exerting bidirectional effects on each other.
The findings from this study have important implications for the development of effective, multimodal treatments for chronic pain and internalizing disorders. We found that changes in sleep complaints predict changes in pain and internalizing symptoms. This suggests sleep may be a promising treatment target for patients struggling with depression, anxiety, and/or chronic pain. There has been growing evidence that psychological therapy for insomnia leads to improvements in secondary outcomes, including pain and internalizing symptoms (Koffel, Koffel, & Gehrman, in press; Tang, 2009). Further work is needed to determine which patients are likely to benefit from sleep interventions and to delineate modifiable treatment targets that could improve both pain and mood outcomes. Our finding that internalizing symptoms mediate the relation between sleep complaints and pain suggests that emotional dysregulation may be an important mechanism of action to target within the context of behavioral sleep treatments for patients with chronic pain.
It is our hope that the current findings will help lay the groundwork for future studies examining the cause and effect of sleep, pain, and psychopathology. Future work in this area falls into two distinct but related categories. First, additional structural work is necessary to clarify where sleep and pain complaints fit within integrated conceptual models of personality and psychopathology. It is clear from the current work and a large body of previous research that mapping the overlap of temperament and psychopathology can inform our understanding of the etiology and co-occurrence of symptoms. The next steps will be to 1) locate self-reported pain and physical complaints within these models to determine if somatic symptoms represent an independent factor relative to internalizing and externalizing or if they are best conceptualized as a lower-order dimension of these broad classes and 2) continue mapping sleep complaints onto higher order factors of psychopathology including fear disorders (e.g., panic disorder, social phobia) and externalizing disorders. It will be important to utilize more nuanced measures of sleep as previous work has suggested that specific sleep complaints such as insomnia and lassitude have differential relations with pain, depression, and anxiety (Koffel, et al., 2015; Koffel & Watson, 2009); this information may be lost by using broad, nonspecific sleep measures. These questions can be addressed using structural techniques (e.g., factor analysis, SEM) and comprehensive and well-designed assessment batteries, with replication across clinical (e.g., patients with chronic pain, patients with sleep disorders, patients with psychological disorders) and nonclinical samples.
A second area for future research involves utilizing prospective, longitudinal designs to examine sleep-wake processes as an underlying neurobiological mechanism for the overlap of psychopathology and pain. Building on the current design, which focused on worsening sleep following stressful events, future studies can examine if improvements in sleep over the course of sleep treatment predict improvement in secondary outcomes such as pain and psychopathology and whether improvements in outcomes are preceded by changes in emotion regulation (e.g., mean levels and stability of affect). Within the context of a randomized clinical trial, future studies can shed light on the mechanism of action linking sleep-wake processes to downstream effects on pain and psychopathology and would help clarify the mediating role of emotion regulation in this relationship.
There are several limitations from the current study that should be noted. Given the limitations of pre-deployment data collection, it was not possible to administer full-length, dedicated instruments measuring sleep disturbance and pain. As such, our analyses utilized shorter, more general measures of these constructs. It is possible that using items from the BDI-II to define the Sleep Complaints scale inflated the correlation among internalizing symptoms and sleep complaints. Although we used structural analyses to establish the relative independence of both internalizing symptoms and sleep complaints within this sample prior to conducting the SEM analyses and controlled for shared temperament dimensions in the SEM analyses, it will be important for future studies to replicate findings with multiple, full-length measures of each construct. In addition, the concurrent and criterion validity of the sleep scale used in this study have not been examined in relation to commonly used sleep measures and sleep disorder diagnoses.
The longitudinal, prospective data utilized in this study provided a robust test of mediation and we were able to test alternative models with mediators as outcomes and vice versa to gain a clearer understanding of these interactions. However, due to the designs of the original study, post-deployment time points had different assessment batteries; as a result measures of the proposed mediator and outcomes variables were taken at the same time point, although ideally the mediator would precede the outcome. In addition, the data in this study were based on self-report obtained from questionnaire measures. It is well established that discrepancies exist between self-reported and objectively measured sleep. Next steps include replicating these findings using a multi-method approach, including interview and objective sleep measures.
Finally, the theories tested in this study focus on sleep disruption as a risk factor for downstream effects on pain and affective systems. It is important to note that this does not preclude the possibility of alterations in the pain system having downstream effects on sleep and mood. Indeed, it is clear from the research that disturbances in both the pain and sleep systems have at least some degree of impact on each other (Smith & Haythornthwaite, 2004; Smith, et al., 2005). Although sleep represents a promising target for prevention and early intervention, it is likely that intervention and treatment within either system would be beneficial. Continuing to examine the structure and interrelations of pain, sleep complaints, and internalizing symptoms will provide an important framework for a progressive research program and development of efficacious clinical interventions for patients who are suffering from this triad of symptoms.
Acknowledgments
This research was supported by grants to Melissa A. Polusny from the Minnesota Medical Foundation (Grant #3662-9227-06) and Department of Defense Congressionally Directed Medical Research Program (W81XWH-07-2-0033), a grant to Christopher R. Erbes from the Department of Veterans Affairs Health Services Research and Development (RRP 08-385), as well as a grant to Paul A. Arbisi from the University of Minnesota Press. This material is the result of work supported with resources and the use of facilities at the Minneapolis VA Health Care System, Minneapolis, MN.
Footnotes
Author Contributions: E.K., E.E.K., C.R.E., M.A.P., & P.A.A. developed the study concept. E.K., C.R.E., M.A.P., & P.A.A. contributed to the study design. E.K. performed the data analysis and interpretation. E.K. drafted the paper and all authors provided critical revisions. All authors approved the final version of the paper for submission.
Contributor Information
Erin Koffel, Center for Chronic Disease Outcomes Research, Minneapolis Veteran Affairs Health Care System, Department of Psychiatry, University of Minnesota, Minneapolis, MN.
Erin E. Krebs, Center for Chronic Disease Outcomes Research, Minneapolis Veteran Affairs Health Care System, University of Minnesota Medical School, Minneapolis, MN
Paul A. Arbisi, Minneapolis Veteran Affairs Health Care System, Departments of Psychology and Psychiatry, University of Minnesota, Minneapolis, MN
Christopher R. Erbes, Center for Chronic Disease Outcomes Research, Minneapolis Veteran Affairs Health Care System, Department of Psychiatry, University of Minnesota, Minneapolis, MN
Melissa A. Polusny, Center for Chronic Disease Outcomes Research, Minneapolis Veteran Affairs Health Care System, Department of Psychiatry, University of Minnesota, Minneapolis, MN
References
- Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68:363–368. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. text revision. Washington, DC: Author; 2000. [Google Scholar]
- Asih S, Neblett R, Mayer TG, Brede E, Gatchel RJ. Insomnia in a chronic musculoskeletal pain with disability population is independent of pain and depression. Spine Journal. 2014;14:2000–2007. doi: 10.1016/j.spinee.2013.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: A diathesis-stress framework. Psychological Bulletin. 1996;119:95–110. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory manual. 2nd. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bigatti SM, Hernandez AM, Cronan TA, Rand KL. Sleep disturbances in fibromyalgia syndrome: Relationship to pain and depression. Arthritis and Rheumatism. 2008;59:961–967. doi: 10.1002/art.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behaviour Research and Therapy. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- Campbell P, Tang N, McBeth J, Lewis M, Main CJ, Croft PR, et al. The role of sleep problems in the development of depression in those with persistent pain: A prospective cohort study. Sleep. 2013;36:1693–1698. doi: 10.5665/sleep.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology. 1994;103:103–116. [PubMed] [Google Scholar]
- Daley M, Morin CM, LeBlanc M, Gregoire JP, Savard J. The economic burden of insomnia: Direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32:55–64. [PMC free article] [PubMed] [Google Scholar]
- Daley M, Morin CM, Leblanc M, Gregoire JP, Savard J, Baillargeon L. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Medicine. 2008 doi: 10.1016/j.sleep.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Emery PC, Wilson KG, Kowal J. Major depressive disorder and sleep disturbance in patients with chronic pain. Pain Research and Management. 2014;19:35–41. doi: 10.1155/2014/480859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. Journal of Pain. 2013;14:1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch JF, West SG. The investigation of personality structure: Statistical models. Journal of Research in Personality. 1997;31:439–485. [Google Scholar]
- Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annual Review of Clinical Psychology. 2014;10:679–708. doi: 10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness AR, McNulty JL, Ben-Porath YS. The personality psychopathology five (PSY-5): Constructs and MMPI-2 scales. Psychological Assessment. 1995;7:104–114. [Google Scholar]
- Hu L, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3:424–453. [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Koffel E, Koffel J, Gehrman P. A meta-analysis of group Cognitive Behavioral Therapy for Insomnia. Sleep Medicine Reviews. doi: 10.1016/j.smrv.2014.05.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffel E, Kroenke K, Bair M, Leverty D, Polusny MA, Krebs EE. Sleep complaints predict pain outcomes: Analysis of data from a randomized trial. Manuscript submitted for publication. 2015 doi: 10.1037/hea0000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffel E, Polusny MA, Arbisi PA, Erbes CR. Pre-deployment daytime and nighttime sleep complaints as predictors of post-deployment PTSD and depression in National Guard troops. Journal of Anxiety Disorders. 2013;27:512–519. doi: 10.1016/j.janxdis.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Koffel E, Watson D. The two-factor structure of sleep complaints and its relation to depression and anxiety. Journal of Abnormal Psychology. 2009;118:183–194. doi: 10.1037/a0013945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, deGruy FV, 3rd, Swindle R. A symptom checklist to screen for somatoform disorders in primary care. Psychosomatics. 1998;39:263–272. doi: 10.1016/S0033-3182(98)71343-X. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-15: Validity of a new measure for evaluating the severity of somatic symptoms. Psychosomatic Medicine. 2002;64:258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: An examination in a nationally representative sample. Pain. 2003;106:127–133. doi: 10.1016/s0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User's Guide. 7th. Los Angeles, CA: Muthen & Muthen; 2012. [Google Scholar]
- Naragon-Gainey K, Watson D, Markon KE. Differential relations of depression and social anxiety symptoms to the facets of extraversion/positive emotionality. Journal of Abnormal Psychology. 2009;118:299–310. doi: 10.1037/a0015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien EM, Waxenberg LB, Atchison JW, Gremillion HA, Staud RM, McCrae CS, et al. Negative mood mediates the effect of poor sleep on pain among chronic pain patients. Clinical Journal of Pain. 2010;26:310–319. doi: 10.1097/AJP.0b013e3181c328e9. [DOI] [PubMed] [Google Scholar]
- Ong AD, Exner-Cortens D, Riffin C, Steptoe A, Zautra A, Almeida DM. Linking stable and dynamic features of positive affect to sleep. Annals of Behavioral Medicine. 2013;46:52–61. doi: 10.1007/s12160-013-9484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polusny MA, Kehle SM, Nelson NW, Erbes CR, Arbisi PA, Thuras P. Longitudinal effects of mild traumatic brain injury and posttraumatic stress disorder comorbidity on postdeployment outcomes in national guard soldiers deployed to Iraq. Archives of General Psychiatry. 2011;68:79–89. doi: 10.1001/archgenpsychiatry.2010.172. [DOI] [PubMed] [Google Scholar]
- Quilty LC, Zhang KA, Bagby RM. The latent symptom structure of the Beck Depression Inventory-II in outpatients with major depression. Psychological Assessment. 2010;22:603–608. doi: 10.1037/a0019698. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. doi: 10.1037/1082-989X.7.2.147. [DOI] [PubMed] [Google Scholar]
- Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Medicine Reviews. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clinical Psychology Review. 2005;25:559–592. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Smith MT, Quartana PJ, Okonkwo RM, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: A conceptual model. Current Pain and Headache Reports. 2009;13:447–454. doi: 10.1007/s11916-009-0073-2. [DOI] [PubMed] [Google Scholar]
- Steptoe A, O'Donnell K, Marmot M, Wardle J. Positive affect, psychological well-being, and good sleep. Journal of Psychosomatic Research. 2008;64:409–415. doi: 10.1016/j.jpsychores.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Tang NK. Cognitive-behavioral therapy for sleep abnormalities of chronic pain patients. Current Rheumatology Reports. 2009;11:451–460. doi: 10.1007/s11926-009-0066-5. [DOI] [PubMed] [Google Scholar]
- Tang NK, Goodchild CE, Sanborn AN, Howard J, Salkovskis PM. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: A multilevel daily process study. Sleep. 2012;35:675–687A. doi: 10.5665/sleep.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Burden of Disease Collaborators. The state of US health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. Journal of Clinical Sleep Medicine. 2009;5:355–362. [PMC free article] [PubMed] [Google Scholar]
- Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychological Bulletin. 2009;135:731–748. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward LC. Comparison of factor structure models for the Beck Depression Inventory-II. Psychological Assessment. 2006;18:81–88. doi: 10.1037/1040-3590.18.1.81. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorders. Journal of Abnormal Psychology. 1988;97:346–353. doi: 10.1037//0021-843x.97.3.346. [DOI] [PubMed] [Google Scholar]
- Watson D, Gamez W, Simms LJ. Basic dimensions of temperament and their relation to anxiety and depression: A symptom-based perspective. Journal of Research in Personality. 2005;39:46–66. doi: 10.1016/j.jrp.2004.09.006. [DOI] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. [Accessed October 11, 2013];1993 annual meeting of the International Society for Traumatic Stress Studies, San Antonio, TX. 1993 from www.pdhealth.mil/library/downloads/pcl_sychometrics.doc.
- Whisman MA, Perez JE, Ramel W. Factor structure of the Beck Depression Inventory-Second Edition (BDI-II) in a student sample. Journal of Clinical Psychology. 2000;56:545–551. doi: 10.1002/(sici)1097-4679(200004)56:4<545::aid-jclp7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wilson KG, Eriksson MY, D'Eon JL, Mikail SF, Emery PC. Major depression and insomnia in chronic pain. Clinical Journal of Pain. 2002;18:77–83. doi: 10.1097/00002508-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Witthoft M, Hiller W, Loch N, Jasper F. The latent structure of medically unexplained symptoms and its relation to functional somatic syndromes. International Journal of Behavioral Medicine. 2013;20:172–183. doi: 10.1007/s12529-012-9237-2. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep--a prefrontal amygdala disconnect. Current Biology. 2007;17:R877–878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]


