Abstract
Objective
Depressed older adults with executive dysfunction (ED) may respond poorly to antidepressant treatment. ED is a multifaceted construct and different studies have measured different aspects of ED, making it unclear which aspects predict poor response. Meta-analytic methods were used to determine whether ED predicts poor antidepressant treatment response in late-life depression and to determine which domains of executive functioning are responsible for this relationship.
Methods
A Medline search was conducted to identify regimented treatment trials contrasting executive functioning between elderly responders and nonresponders; only regimented treatment trials for depressed outpatients aged 50 and older were included. Following the most recent PRISMA guidelines, 25 measures of executive functioning were extracted from eight studies. Six domains were identified: cognitive flexibility, planning and organization, response inhibition, selective attention, verbal fluency, and the Dementia Rating Scale Initiation/Perseveration composite score (DRS I/P). Hedge’s g was calculated for each measure of executive functioning. A three-level Bayesian hierarchical linear model (HLM) was used to estimate effect sizes for each domain of executive functioning.
Results
The effect of planning and organization was significantly different from zero (Bayesian HLM estimate of domain effect size: 0.91; 95% CI: 0.32–1.58), whereas cognitive flexibility, response inhibition, selective attention, verbal fluency, and the DRS I/P composite score were not.
Conclusion
The domain of planning and organization is meaningfully associated with poor antidepressant treatment response in late-life depression. These findings suggest that therapies that focus on planning and organization may provide effective augmentation strategies for antidepressant nonresponders with late-life depression.
Keywords: Executive dysfunction, antidepressant treatment, late-life depression, meta-analysis
INTRODUCTION
Depression is a common problem among older adults.1 Although antidepressant medication is the primary treatment for geriatric depression, response rates range from 25% to 60%.2 A number of studies have shown that deficits on measures of executive functioning predict poor response to antidepressant treatment in late-life depression.3–5 The construct of executive functioning is broad and is composed of numerous domains, including but not limited to response inhibition, cognitive flexibility, working memory, organization, and planning.6–9 Because studies that have examined the impact of executive dysfunction (ED) on antidepressant response have relied on different measures, it is unclear which aspects of ED predict poor response to antidepressant treatment.10 Identifying those features associated with poor response will enable us to focus on identifying the neurobiologic mechanisms by which these specific deficits take place and developing novel interventions that target these mechanisms.10
A meta-analysis examined the relationship between antidepressant response and neuropsychological test performance among depressed adults.11 This study showed that of seven measures of executive function, only the Dementia Rating Scale Initiation/Perseveration composite score (DRS I/P) predicted poor antidepressant treatment response and concluded that the findings did not provide strong support for the depression–ED model of late-life depression. The findings of this study, however, may be limited with respect to the impact of ED on antidepressant response in geriatric depression. First, the mean age of approximately half the studies in the meta-analysis was less than 50. Second, a number of geriatric depression studies were not included in the meta-analysis.5,12,13 Third, this study did not classify measures as belonging to specific domains of executive functioning. This is potentially important because it may give us insight into the neurobiologic substrates underlying the effects of ED on antidepressant response. Fourth, a number of studies included in this analysis were not regimented treatment trials. Finally, the authors chose to interpret only those effect sizes greater than 0.5 (moderate) as significant. This is potentially problematic because (as already noted) geriatric depression is common and antidepressant nonresponse frequent. Even a small statistical effect can have great clinical value. It makes sense to therefore investigate this problem more closely.
The purpose of this meta-analysis is to determine which components of ED predict poor antidepressant treatment response. We hope to improve on previous research by focusing exclusively on standardized trials of antidepressant medication among depressed older adults, examining the predictive utility of specific executive function domains and not restricting the significance of effect sizes to 0.5 when a small effect could be potentially important.
METHODS
We followed the most recent Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines14 for conducting and reporting the results of this systematic review.
Identification of Studies
A Medline search was conducted to identify regimented antidepressant treatment trials contrasting executive function between elderly responders and nonresponders. The index terms “executive dysfunction,” “executive function,” “executive control,” “working memory,” “verbal fluency,” “response inhibition,” “set switching,” “planning,” “prefrontal dysfunction,” “neuropsychological tests,” “cognitive function,” and “cognitive functioning” were combined using the “or” operator. In addition, the index terms “Depression,” “Depressive Disorder,” and “Depressive Disorder, Major” were combined using the “or” operator. This returned 2,498 results, which were limited to 1) English language articles, 2) age group 50 and older, and 3) publication types, including clinical trials, controlled clinical trials, comparative study, meta-analysis, multicenter study, randomized controlled trials, or review.
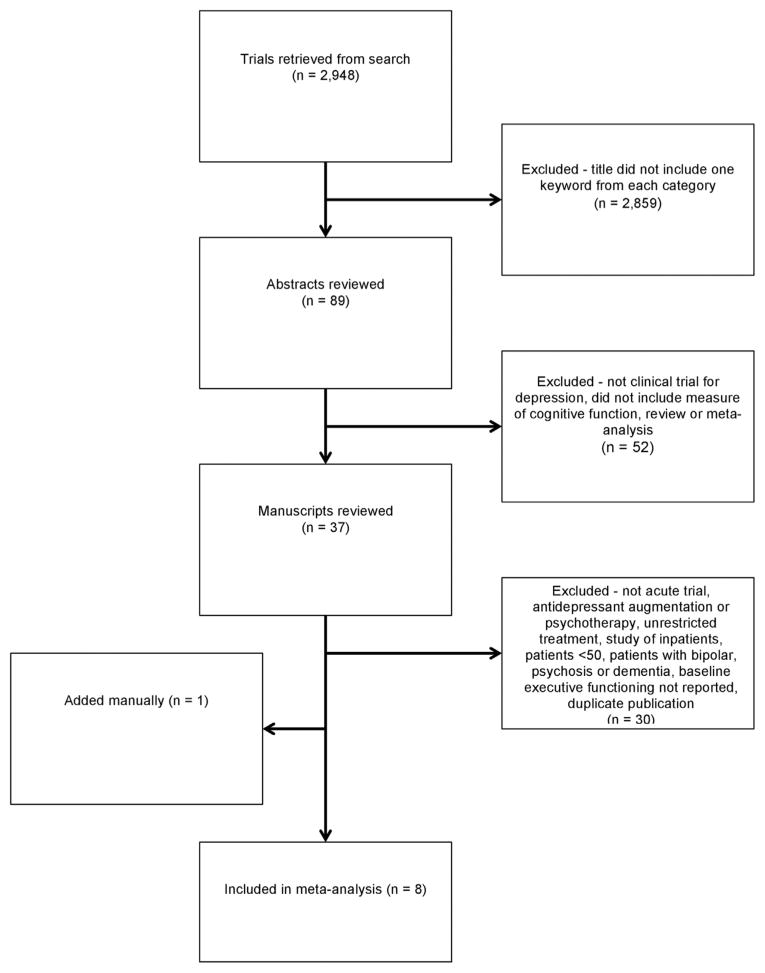
The first author (M.A.P.) conducted a review of these articles, sequentially progressing from title to abstract. A study was ruled out if the title did not contain at least one word from each of two categories. The first category contained the key words executive dysfunction, executive function, executive control, working memory, verbal fluency, response inhibition, set switching, planning, prefrontal dysfunction, neuropsychological test, cognitive function, cognitive functioning, neurocognitive, attention network, neuropsychological functioning, psychomotor, neuropsychological, Stroop, trails making, frontal dysfunction, digit span, clock drawing test, exit 25, N-back test, Wisconsin Card Sorting Test, Tower of London, Tower of Hanoi, Word Generation, Color/Word Test, Sorting, DRS-I/P, DRS, Dementia Rating Scale and Initiation/Perseveration. The second category contained the key words depression, major depressive disorder, depressive disorder, late-life depression, late-onset depression, vascular depression, and depressive symptoms. A study was kept if the abstract described an antidepressant treatment trial in which cognitive ability was a variable. This resulted in 40 studies, of which 37 were empirical studies and 3 were reviews or meta-analyses. The three meta-analyses and reviews were searched for additional references, but this search did not yield additional studies.
The remaining 37 articles were reviewed to determine whether they met inclusion criteria: The articles report outcome data from an antidepressant treatment trial for major depressive disorder in outpatient subjects older than 50 years. Ten studies were excluded because the study sample contained subjects younger than age 50.15–23 In addition, three of these publications included subjects with bipolar disorder.17,20,24 One study was excluded because it included subjects with vascular dementia and Alzheimer dementia.25 Four studies were excluded because they did not measure treatment response as a dependent variable.25–28 This resulted in 22 publications.
Two judges (M.A.P. and J.R.S.) reviewed the remaining 22 full paper texts to determine whether the manuscripts reported an acute treatment trial (≤12 weeks) and presented pretreatment scores on a measure of executive functioning separately for responders and nonresponders (or provided sufficient information for this to be derived from the data). Any differences between judges were resolved by discussion. Five studies were excluded because they were not an acute treatment trial,29–33 three studies because they included psychotherapy as a treatment modality,34–36 two studies because they did not include a specific test of ED,37,38 two studies because they did not include sufficient statistical information,39,40 two studies because of duplicate reporting,5,41 and one study because it included an inpatient sample.42 This resulted in seven studies that met our inclusion criteria.
To search for unpublished data, we examined the references of our final 40 articles, including reviews and meta-analyses. We also conducted an Internet search. One article that was not identified in our search was included.13 This article was supplied by one of our authors (J.R.S.) and was not identified in our original search because it had not yet been indexed in Medline. This ultimately resulted in the inclusion of eight articles in our analysis 4,12,13,27,56,65–67. Figure 1 provides a schematic representation of our study selection procedures.
FIGURE 1.
Flow chart of study selection.
Data Extraction
Publication information (author, year of publication), demographic characteristics of the included subjects (sample size, age, clinical characteristics), details of treatment condition (medication name, treatment format), measures of executive functioning used, and outcome data (baseline ED scores, response and remission rates) were extracted from each included trial by one author (M.A.P). Table 1 summarizes the methodologic features of these eight studies. Demographic information of participants in each study is shown in Table 2. Tests of executive functioning were categorized by domain by two authors based on a widely used reference for neuropsychological tests.9
TABLE 1.
Study Characteristics
| Trial Duration (wk) | Treatment Format | Treatment Type | Baseline Depression Scale | Definition of Remission | |
|---|---|---|---|---|---|
| Alexopoulos et al.4 | 8 | Standardized | Citalopram | HAM-D | 50% change in HDRS from baseline |
| Alexopoulos et al.27 | 8 | Standardized | Escitalopram | HAM-D | HDRS 10 or lower |
| Devanand et al.12 | 12 | Standardized | Sertraline | HAM-D | 50% change in HDRS from baseline |
| Kalayam & Alexopoulos65 | 6 | Standardized | Citalopram | HAM-D | HDRS 10 or lower |
| Morimoto et al.13 | 12 | Standardized | Escitalopram | HAM-D | HDRS 7 or lower |
| Potter et al.66 | 12 | Algorithm | SSRI, venlafaxine, bupropion, TCA, lithium | MADRS | MADRS 7 or lower |
| Sheline et al.67 | 12 | Standardized | Sertraline | MADRS | MADRS 7 or lower |
| Sneed et al.56 | 8 | Standardized | Citalopram | HAM-D | 50% change in HDRS from baseline |
Note: HDRS: Hamilton Depression Rating Scale; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; MADRS: Montgomery-Asberg Depression Rating Scale.
TABLE 2.
Sample Characteristics
| Complete Sample
|
Nonresponders
|
Responders
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Gender (% male) |
Age (SD) | Education (SD) |
Depression Severity at Baseline (SD) |
N | Gender (% male) |
Age (SD) | Education (SD) |
Depression Severity at Baseline (SD) |
N | Gender (% male) |
Age (SD) | Education (SD) |
Depression Severity at Baseline (SD) |
|
| Alexopoulos et al.4 | 112 | 29.62 (3.30) | 24.51 (4.72) | 44 | 75.66 (6.15) | 14.36 (3.10) | 25.32 (5.08) | 68 | 71.56 (6.25) | 15.26 (3.49) | 23.69 (4.35) | ||||
| Alexopoulos et al.27 | 12 | 20.6 (4.65) | 6 | 68.8 (6.3) | 22.0 (6.7) | 6 | 71.2 (5.0) | 19.2 (2.6) | |||||||
| Devanand et al.12 | 26 | 36 | 72.0 (10.2) | 12.0 (4.8) | 15.4 (4.5) | 9 | 82.3 (5.0) | 17 | 66.8 (9.4) | ||||||
| Kalayam & Alexopoulos65 | 22 | 14 (2.7) | 23.55 (6.2) | 9 | 74.9 (8.1) | 13.0 (2.8) | 25.4 (5.5) | 13 | 70.2 (7.4) | 15.0 (2.6) | 21.7 (6.9) | ||||
| Morimoto et al.13 | 70 | 15.9 (3.55) | 22.1 (3.9) | 35 | 70.4 (7.1) | 16.1 (3.6) | 22.4 (3.7) | 35 | 70.1 (5.8) | 15.7 (3.5) | 21.8 (4.1) | ||||
| Potter et al.66 | 110 | 41 | 73.78 (0.74) | 13.61 (0.85) | 24.65 (0.62) | 87 | 41.38 | 73.76 (0.80) | 14.00 (0.38) | 24.74 (0.67) | 23 | 39.13 | 73.87 (1.90) | 13.65 (0.94) | 24.35 (1.55) |
| Sheline et al.67 | 190 | 43.7 | 68.6 (7.3) | 14.4 (3.1) | 26.1 (4.4) | 118 | 44.1 | 69.2 (7.7) | 14.2 (2.9) | 26.6 (4.5) | 72 | 43.1 | 67.6 (6.7) | 14.7 (3.4) | 25.2 (4.1) |
| Sneed et al.56 | 174 | 42 | 79.57 (4.37) | 13.74 (3.31) | 24.31 (4.21) | 47 | 29 | ||||||||
Note: SD: standard deviation.
Data Analysis
A total of 25 individual measures extracted from eight different studies was included in this analysis as indicators of the six executive function domains (Table 3). Effect sizes (Hedge’s g) and standard errors were calculated for each domain using Comprehensive Meta-Analysis version 2.43 We used the open variant of the Bayesian inference using Gibbs Sampling software package (OpenBUGS)44,45 to estimate a three-level meta-analytic model46,47 in which observations are nested within executive function domains, which in turn are nested within study. Table 4 shows the key elements of the structure of this model:
TABLE 3.
Estimated Effect Sizes for Individual Measures (Hedge’s g [Standard Error])
| Cognitive Flexibility | DRS I/P | Planning and Organization | Response Inhibition | Verbal Fluency | Selective Attention | |
|---|---|---|---|---|---|---|
| Alexopoulos et al.4 | ||||||
| DRS I/P total | 0.50 (0.20) | |||||
| Stroop Color-Word | 0.49 (0.20) | |||||
| Alexopoulos et al.27 | ||||||
| Go/No-Go commission errors | −0.42 (0.54) | |||||
| WCST % perseverative errors | −0.23 (0.54) | |||||
| Kalayam & Alexopoulos65 | ||||||
| DRS I/P total | 1.06 (0.45) | |||||
| Stroop accuracy | 0.33 (0.42) | |||||
| Stroop reaction time | 0.64 (0.43) | |||||
| Morimoto et al.13 | ||||||
| DRS I/P complex verbal clusters | 0.79 (0.25) | |||||
| DRS I/P verbal perseverations | −0.15 (0.24) | |||||
| Potter et al.66 | ||||||
| Animal Naming total correct | 0.10 (0.23) | |||||
| Animal Naming perseverative errors | 0.24 (0.23) | |||||
| COWAT total correct | −0.09 (0.23) | |||||
| COWAT perseverative errors | 0.41 (0.24) | |||||
| Trails B | −0.22 (0.23) | |||||
| Sheline et al.67 | ||||||
| COWAT | 0.20 (0.16) | |||||
| Trails B | 0.35 (0.16) | |||||
| Stroop Color-Word | 0.14 (0.16) | |||||
| DRS I/P | 0.24 (0.16) | |||||
| WCST categories completed | 0.27 (0.16) | |||||
| Sneed, et al.56 | ||||||
| Stroop Color-Word | 0.27 (0.24) | |||||
| Devanand et al.12 | ||||||
| Category fluency | −0.37 (0.41) | |||||
| Animal Naming | 0.05 (0.41) | |||||
| Digit Symbol | 0.21 (0.41) | |||||
| Letter Cancellation time | −0.4 (0.41) | |||||
| Shape Cancellation time | 0.12 (0.41) | |||||
Notes: COWAT: Controlled Oral Word Association Test.
TABLE 4.
Estimated Effect Sizes for Domains Within Studies (Hedge’s g [Standard Error])
| Cognitive Flexibility | DRS I/P | Planning and Organization | Response Inhibition | Verbal Fluency | Selective Attention | |
|---|---|---|---|---|---|---|
| Alexopoulos et al.4 | 0.50 (0.20) | 0.49 (0.20) | ||||
| Alexopoulos et al.27 | −0.23 (0.54) | −0.45 (0.59) | ||||
| Kalayam & Alexopoulos65 | 1.06 (0.45) | 0.33 (0.42) 0.64 (0.43) |
||||
| Morimoto et al.13 | −0.15 (0.24) | 0.79 (0.25) | ||||
| Potter et al.66 | 0.24 (0.23) 0.41 (0.24) −0.22 (0.23) |
0.10 (0.24) −0.09 (0.23) |
||||
| Sheline et al.67 | 0.35 (0.16) 0.27 (0.16) |
0.24 (0.16) | 0.14 (0.16) | 0.20 (0.16) | ||
| Sneed et al.56 | 0.27 (0.24) | |||||
| Devanand et al.12 | −0.37 (0.41) 0.05 (0.41) |
0.21 (0.41) −0.4 (0.41) 0.12 (0.41) |
The design is an 8 (studies) × 6 (domains) factorial. One dimension (studies) is random, whereas the other (domain) is fixed. Thus, the model is a combination of a fixed and a random effects model and is therefore sometimes called a mixed effects model in meta-analysis.
Because some studies report multiple neuropsychological measures. These data are not independent; this dependence has been taken into account in the analysis.
Many cells in the design are empty, because not all studies measured all domains of executive function in the analysis. Therefore, care must be used in estimating the effects.
A large number of missing cells make it difficult to accurately estimate any potential interactions between studies and domains. The only cells contributing to such an estimate are cells that occur in a pattern of four cells, such that two studies measure the same two domains. We decided not to try to estimate this because of the spareness of the data.
Issues 1 through 3 can be dealt with by using a hierarchical linear model, with effect sizes of different domains nested within studies to take into account the dependence of the outcome data. As such, the statistical model for estimating domain effect sizes represented each observed effect size as a sum of three types of components: 1) an effect due to the specific study (where studies were considered random effects), 2) an effect due to the specific measure used, and 3) measurement error. Because some domains were measured in more studies than other domains and studies had different sample sizes, the accuracy with which we can estimate each effect (i.e., the standard errors) will be different.
The model can be written as
where i indexes the 25 observed effect sizes, g[ ] is the observed Hedge’s g, s[ ] is the standard error of g[ ], m[ ] is the true effect size, study[i] is the study in which the effect g[i] is found, and D, P, R, V, S, and C are dummy variables indicating whether the effect measures a particular domain. The parameter values in the vector b0 were assumed to have a normal distribution with mean mu0 and variance var0. All parameters were given vague prior distributions.
We used OpenBUGS, a Bayesian program, to estimate the parameters of this model, both because it can handle the complexities of the design and because Bayesian interpretation is conceptually (though not computationally) simple. A classical (frequentist) interpretation of the results is also possible for those who do not prefer Bayesian results; with vague prior distributions for the parameters, these results are nearly identical. Our interpretation will be made in classical terms, because we presume readers will be more familiar with these interpretations. The OpenBUGS code, data, and results for this procedure are reproduced in Appendix 1.
RESULTS
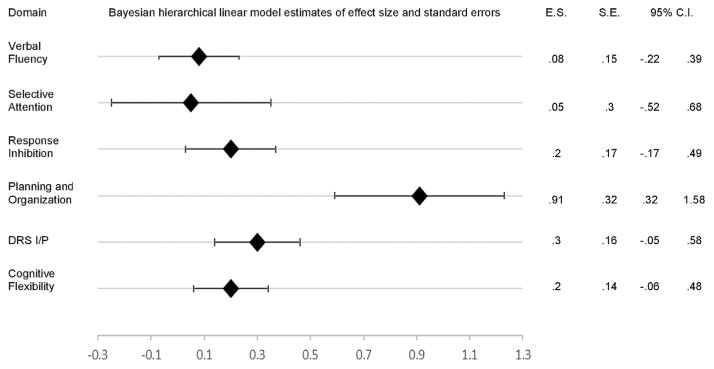
Eight studies met inclusion and exclusion criteria. All studies included in the current analysis measured at least one of six domains of executive function (response inhibition, verbal fluency, cognitive flexibility, selective attention, planning and organization, and the DRS I/P composite score). The Bayesian hierarchical linear model estimates of effect size for each domain is presented in Table 5, along with the standard error, 95% confidence interval, and z score for each estimate. Planning and organization was the only executive function domain that emerged with an estimated effect size significantly different from zero. The size of the estimated effect was large, and its confidence interval ranged from small to large (Figure 2). The estimated effect size for verbal fluency, cognitive flexibility, selective attention, response inhibition, and the DRS I/P composite score were not significantly different from zero (Figure 2).
TABLE 5.
Bayesian Hierarchical Linear Model Estimates of Domain Effect Sizes, Standard Errors, and Confidence Intervals
| Domain | Studies | Measures | Effect Sizes | Standard Error | 95% Confidence Interval | z |
|---|---|---|---|---|---|---|
| Cognitive flexibility | Alexopoulos et al.27, Morimoto et al.13, Potter et al.66, Sheline et al.67, Devanand et al.12 | WCST % perseverative errors, DRS I/P verbal perseverations, Animal Naming perseverative errors, COWAT perseverative errors, Trails B, WCST Categories completed | 0.20 | 0.14 | −0.06 to 0.48 | 1.46 |
| DRS I/P | Alexopoulos et al.4, Kalayam & Alexopoulos65, Sheline et al.67 | DRS I/P total | 0.30 | 0.16 | −0.05 to .58 | 1.87 |
| Planning and organization | Morimoto et al.13 | DRS I/P Complex verbal clusters | 0.91 | 0.32 | .32–1.58 | 3.08a |
| Response inhibition | Alexopoulos et al.4, Alexopoulos et al.27, Kalayam & Alexopoulos65, Sheline et al.67, Sneed et al.56 | Stroop Color-Word, Go/No-Go commission errors, Stroop accuracy, Stroop reaction time | 0.20 | 0.17 | −0.17 to 0.49 | 0.10 |
| Selective attention | Devanand et al.12 | Digit Symbol, Letter Cancellation time, Shape Cancellation time | 0.05 | 0.30 | −0.52 to 0.68 | 0.16 |
| Verbal fluency | Potter et al.66, Sheline et al.67, Devanand et al.12 | Animal Naming total correct, COWAT total correct, category fluency test, Animal Naming | 0.08 | 0.15 | −0.22 to 0.39 | 0.53 |
Notes: COWAT: Controlled Oral Word Association Test.
p <0.05.
FIGURE 2.
Forest plot of effect sizes (E.S.), standard errors (S.E.), and confidence intervals (95% C.I.).
DISCUSSION
The purpose of this meta-analysis was to determine which domains of executive function predict poor antidepressant treatment response. This created a special problem, because the data are nested within studies, studies and executive function domains are crossed, and not all studies measure all domains. To our knowledge, this report represents the first effort to take these features into account in an analysis of ED and poor antidepressant treatment response in late life.
Eight studies meeting inclusion and exclusion criteria were retrieved from the literature. From these eight studies, six domains of executive functioning were extracted (response inhibition, verbal fluency, cognitive flexibility, planning and organization, selective attention, and the DRS I/P composite score). Of these six executive function domains, only planning and organization was significantly associated with antidepressant treatment nonresponse. The estimated effect size was large, whereas its confidence interval ranged from small to large. The width of the confidence interval reflects that the estimate is based on only one study. The effect sizes for response inhibition, verbal fluency, cognitive flexibility, selective attention, and the DRS I/P composite score were small and not significantly different from zero. The current analysis does not provide evidence for an association between these domains and antidepressant treatment response in late life.
It is unclear why patients with poor planning and organization abilities may be less likely to respond to antidepressant medication than patients without planning and organization difficulties. One possibility is that older depressed adults with poor planning and organization abilities may be unable to benefit from the component of medication response that is attributable to patient expectancy. In recent years several studies have documented the influence of expectancy effects on medication response in antidepressant clinical trials, such that higher baseline expectancy has been shown to predict greater depressive symptom improvement.48–51 Expectancies involve the organization of cognitions and planning of behaviors related to the various possible outcomes of a future event.52 Patients with poor planning and organization abilities may therefore be unable to develop and maintain accurate expectancies about the possible outcomes of an antidepressant treatment, thereby lowering the treatment’s effectiveness.
The improvement of planning and organization skills in depressed older adults with ED may alleviate depressive symptoms and enhance response to antidepressant medication. Indeed, recent studies have demonstrated the effectiveness of problem-solving therapy in reducing depressive symptoms in older adults with ED.53,54 Problem-solving therapy is a behavioral intervention that trains patients to identify problems in daily life and provides a method for developing, selecting, and implementing solutions for these problems.55 The improvement of problem-solving skills may alleviate planning and organization deficits, thereby mitigating the behavioral disabilities that may underlie depression in older adults.
Contrary to our hypotheses, response inhibition and verbal fluency were not significantly associated with antidepressant treatment response. Our prediction that response inhibition would be associated with treatment response was based on findings that performance on the Stroop Color and Word Test has been found to predict nonresponse to antidepressant medication.4,5,36,56–58 Consistent with these results, other tests with a response inhibition component, such as the Attention Network Test,40 the Wisconsin Card Sorting Test (WCST),23,59 and the Go/No-Go Task,27 have been predictive of treatment response. Several of these studies, however, could not be included in our analysis because they did not meet inclusion criteria. Furthermore, although some measures such as the WCST contain a response inhibition component, they are primarily considered to assess other domains of executive function and were therefore not included in the response inhibition domain in our analysis.
We also predicted that verbal fluency would be associated with antidepressant treatment response. Studies have shown that the Controlled Oral Word Association Test, a measure of verbal fluency, is associated with remission.22,58 It has also been demonstrated that only the verbal fluency task of the DRS I/P subtest predicts remission.13 However, one study found that the use of semantic strategy explained the difference in performance between responders and nonresponders on this subtest.13 Although this requires replication, these findings appear to be consistent with the current results that planning and organization, and not verbal fluency, are predictive of treatment nonresponse, possibly suggesting a top-down processing effect in which impairment in planning and organization interferes with the generation of words in verbal fluency tasks.
The results of this review highlight the challenges associated with the use and interpretation of executive function measures in geriatric psychiatry.60 Cognitive abilities need to be well defined to allow reliable and valid neuropsychological measurement.61 However, executive function remains an ambiguous construct that lacks a clear definition.60,62 Measures of executive functioning may require the integration of several cognitive processes. For example, the WCST taps executive processes involving cognitive flexibility, problem solving, and response maintenance.63 The Trail Making Test part B enlists executive subcomponents such as processing speed and accuracy.64 As a result, impairment on measures such as the WCST and the Trail Making Test part B may be caused by deficits in several different areas of executive function. Performance on one measure within a domain may not be predictive of performance on another, making it difficult to categorize these measures into domains of executive function.
Limitations
The limitations of this study are balanced by its methodologic strengths. The available data created a methodologic problem because not all studies measured the same domains of executive functioning. As a result, there would inevitably be missing data in the crossing of studies by executive function domains. This required a three-level meta-analytic model to compute estimates for what the average effect size would be in each study, as if the study had measured all six domains, and estimates of what the average effect size would be for each domain, as if each study had measured that domain.
By including only regimented treatment trials, we were able to decrease the effects of confounding variables. This, however, also restricted the number of available studies. There were differences between studies in treatment duration, type of treatment, definition of treatment response, and type of measure used to quantify depression severity. Because of the number of studies included in this analysis, it was not possible to examine the effect of these variables.
CONCLUSION
Of the six domains of executive functioning (response inhibition, verbal fluency, cognitive flexibility, planning and organization, selective attention, and the DRS I/P composite score) assessed in this review, only planning and organization was significantly associated with treatment nonresponse. This suggests that patients with poor planning and organization abilities may be less likely to respond to antidepressant medication than patients without planning and organization difficulties. The improvement of planning and organization deficits in older depressed adults may mitigate the behavioral disabilities that underlie depression in these individuals. Therapies that focus on increasing planning and organization skills (e.g., problem-solving therapy or an individualized cognitive training protocol) may therefore provide effective augmentation strategies for treatment non-responders with late-life depression. More studies are needed to explain the relationship between planning and organization deficits and poor anti-depressant response in older adults.
Supplementary Material
Footnotes
Supplemental digital content is available for this article in the HTML and PDF versions of this article on the journal’s Web site (www.ajgponline.org).
References
- 1.Kessler RC, McGonagle KA, Nelson CB, et al. Sex and depression in the National Comorbidity Survey. II. Cohort effects. J Affect Disord. 1994;30:15–26. doi: 10.1016/0165-0327(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 2.Thase ME. Overview of antidepressant therapy. Manag Care. 2001;10:6–9. [PubMed] [Google Scholar]
- 3.Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119–1126. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulos GS, Kiosses DN, Heo M, et al. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Sneed J, Roose S, Keilp J, et al. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. Am J Geriatr Psychiatry. 2007;15:553–563. doi: 10.1097/JGP.0b013e3180302513. [DOI] [PubMed] [Google Scholar]
- 6.Miyake A, Friedman NP, Emerson MJ, et al. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 7.Burgess PW, Alderman N, Evans J, et al. The ecological validity of tests of executive function. J Int Neuropsychol Soc. 1998;4:547–558. doi: 10.1017/s1355617798466037. [DOI] [PubMed] [Google Scholar]
- 8.Stuss DT, Alexander MP, Hamer L, et al. The effects of focal anterior and posterior brain lesions on verbal fluency. J Int Neuropsychol Soc. 1998;4:265–278. [PubMed] [Google Scholar]
- 9.Lezak M, Howieson D, Loring D, et al. Neuropsychological Assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- 10.Pimontel MA, Culang-Reinlieb ME, Morimoto SS, et al. Executive dysfunction and treatment response in late-life depression. Int J Geriatr Psychiatry. 2012;27:893–899. doi: 10.1002/gps.2808. [DOI] [PubMed] [Google Scholar]
- 11.McLennan SN, Mathias JL. The depression-executive dysfunction (DED) syndrome and response to antidepressants: a meta-analytic review. Int J Geriatr Psychiatry. 2010;25:933–944. doi: 10.1002/gps.2431. [DOI] [PubMed] [Google Scholar]
- 12.Devanand D, Pelton GH, Marston K, et al. Sertraline treatment of elderly patients with depression and cognitive impairment. Int J Geriatr Psychiatry. 2003;18:123–130. doi: 10.1002/gps.802. [DOI] [PubMed] [Google Scholar]
- 13.Morimoto SS, Gunning FM, Murphy CF, et al. Executive function and short-term remission of geriatric depression: the role of semantic strategy. Am J Geriatr Psychiatry. 2010;19:115. doi: 10.1097/JGP.0b013e3181e751c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 15.Biringer E, Mykletun A, Sundet K, et al. A longitudinal analysis of neurocognitive function in unipolar depression. J Clin Exp Neuropsychol. 2007;29:879–891. doi: 10.1080/13803390601147686. [DOI] [PubMed] [Google Scholar]
- 16.Cook IA, Leuchter AF, Witte E, et al. Neurophysiologic predictors of treatment response to fluoxetine in major depression. Psychiatry Res. 1999;85:263–273. doi: 10.1016/s0165-1781(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 17.de Groot MH, Nolen WA, Huijsman AM, et al. Lateralized neuropsychological functioning in depressive patients before and after drug therapy. Biol Psychiatry. 1996;40:1282–1287. doi: 10.1016/0006-3223(95)00654-0. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher P, Robinson LJ, Gray JM, et al. Neurocognitive function following remission in major depressive disorder: potential objective marker of response? Aust N Z J Psychiatry. 2007;41:54–61. doi: 10.1080/00048670601057734. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Lin C, Chou K, et al. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage. 2010;1:347–356. doi: 10.1016/j.neuroimage.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Mandelli L, Serretti A, Colombo C, et al. Improvement of cognitive functioning in mood disorder patients with depressive symptomatic recovery during treatment: an exploratory analysis. Psychiatry Clin Neurosci. 2006;60:598–604. doi: 10.1111/j.1440-1819.2006.01564.x. [DOI] [PubMed] [Google Scholar]
- 21.Mayberg HS, Brannan SK, Mahurin RK, et al. Cingulate function in depression: a potential predictor of treatment response. Neuro-report. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 22.Taylor BP, Bruder GE, Stewart JW, et al. Psychomotor slowing as a predictor of fluoxetine nonresponse in depressed outpatients. Am J Psychiatry. 2006;163:73–78. doi: 10.1176/appi.ajp.163.1.73. [DOI] [PubMed] [Google Scholar]
- 23.Withall A, Harris LM, Cumming SR. The relationship between cognitive function and clinical and functional outcomes in major depressive disorder. Psychol Med. 2009;39:393–402. doi: 10.1017/S0033291708003620. [DOI] [PubMed] [Google Scholar]
- 24.Majer M, Ising M, Kunzel H, et al. Impaired divided attention predicts delayed response and risk to relapse in subjects with depressive disorders. Psychol Med. 2004;34:1453–1463. doi: 10.1017/s0033291704002697. [DOI] [PubMed] [Google Scholar]
- 25.Kiosses DN, Klimstra S, Murphy C, et al. Executive dysfunction and disability in elderly patients with major depression. Am J Geriatr Psychiatry. 2001;9:269–274. [PubMed] [Google Scholar]
- 26.Kohler S, Thomas AJ, Barnett NA, et al. The pattern and course of cognitive impairment in late-life depression. Psychol Med. 2010;40:591–602. doi: 10.1017/S0033291709990833. [DOI] [PubMed] [Google Scholar]
- 27.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, et al. Event-related potentials in an emotional go/no-go task and remission of geriatric depression. Neuroreport. 2007;18:217–221. doi: 10.1097/WNR.0b013e328013ceda. [DOI] [PubMed] [Google Scholar]
- 28.Doraiswamy PM, Krishnan KR, Oxman T, et al. Does antidepressant therapy improve cognition in elderly depressed patients? J Gerontol A Biol Sci Med Sci. 2003;58:M1137–M1144. doi: 10.1093/gerona/58.12.m1137. [DOI] [PubMed] [Google Scholar]
- 29.Murphy CF, Alexopoulos GS. Longitudinal association of initiation/perseveration and severity of geriatric depression. Am J Geriatr Psychiatry. 2004;12:50–56. [PubMed] [Google Scholar]
- 30.Story TJ, Potter GG, Attix DK, et al. Neurocognitive correlates of response to treatment in late-life depression. Am J Geriatr Psychiatry. 2008;16:752–759. doi: 10.1097/JGP.0b013e31817e739a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldwin RC, Gallagley A, Gourlay M, et al. Prognosis of late life depression: a three-year cohort study of outcome and potential predictors. Int J Geriatr Psychiatry. 2006;21:57–63. doi: 10.1002/gps.1424. [DOI] [PubMed] [Google Scholar]
- 32.Butters MA, Bhalla RK, Mulsant BH, et al. Executive functioning, illness course, and relapse/recurrence in continuation and maintenance treatment of late-life depression: is there a relationship? Am J Geriatr Psychiatry. 2004;12:387–394. doi: 10.1176/appi.ajgp.12.4.387. [DOI] [PubMed] [Google Scholar]
- 33.Marcos T, Portella MJ, Navarro V, et al. Neuropsychological prediction of recovery in late-onset major depression. Int J Geriatr Psychiatry. 2005;20:790–795. doi: 10.1002/gps.1363. [DOI] [PubMed] [Google Scholar]
- 34.Driscoll HC, Basinski J, Mulsant BH, et al. Late-onset major depression: clinical and treatment-response variability. Int J Geriatr Psychiatry. 2005;20:661–667. doi: 10.1002/gps.1334. [DOI] [PubMed] [Google Scholar]
- 35.Alexopoulos GS, Meyers BS, Young RC, et al. Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry. 2000;57:285–290. doi: 10.1001/archpsyc.57.3.285. [DOI] [PubMed] [Google Scholar]
- 36.Bogner HR, Bruce ML, Reynolds CF, 3rd, et al. The effects of memory, attention, and executive dysfunction on outcomes of depression in a primary care intervention trial: the PROSPECT study. Int J Geriatr Psychiatry. 2007;22:922–929. doi: 10.1002/gps.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sneed JR, Keilp JG, Brickman AM, et al. The specificity of neuropsychological impairment in predicting antidepressant non-response in the very old depressed. Int J Geriatr Psychiatry. 2008;23:319–323. doi: 10.1002/gps.1889. [DOI] [PubMed] [Google Scholar]
- 38.Saghafi R, Brown C, Butters MA, et al. Predicting 6-week treatment response to escitalopram pharmacotherapy in late-life major depressive disorder. Int J Geriatr Psychiatry. 2007;22:1141–1146. doi: 10.1002/gps.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson S, Baldwin RC, Jackson A, et al. Is subcortical disease associated with a poor response to antidepressants? Neurological, neuropsychological and neuroradiological findings in late-life depression. Psychol Med. 1998;28:1015–1026. doi: 10.1017/s003329179800693x. [DOI] [PubMed] [Google Scholar]
- 40.Murphy CF, Alexopoulos GS. Attention network dysfunction and treatment response of geriatric depression. J Clin Exp Neuropsychol. 2006;28:96–100. doi: 10.1080/13803390490918101. [DOI] [PubMed] [Google Scholar]
- 41.Alexopoulos GS, Kiosses DN, Murphy C, et al. Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology. 2004;29:2278–2284. doi: 10.1038/sj.npp.1300557. [DOI] [PubMed] [Google Scholar]
- 42.Kalayam B, Alexopoulos G. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry. 1999;56:713–718. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- 43.Borenstein M, Hedges LV, Higgins JP, et al. Introduction to Meta-Analysis. New York: John Wiley & Sons; 2011. [Google Scholar]
- 44.Lunn DJ, Thomas A, Best N, et al. WinBUGS-a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- 45.Spiegelhalter D, Thomas A, Best N, et al. WinBUGS Version 1.4 User Manual. Cambridge U.K: Medical Research Council Biostatistics Unit; 2000. [Google Scholar]
- 46.DuMouchel W. Hierarchical Bayes Linear Models for Meta-Analysis. North Carolina: National Institute of Statistical Sciences; 1994. [Google Scholar]
- 47.Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res. 2001;10:277–303. doi: 10.1177/096228020101000404. [DOI] [PubMed] [Google Scholar]
- 48.Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol. 2009;19:34–40. doi: 10.1016/j.euroneuro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Sinyor M, Levitt AJ, Cheung AH, et al. Does inclusion of a placebo arm influence response to active antidepressant treatment in randomized controlled trials? Results from pooled and meta-analyses. J Clin Psychiatry. 2010;71:270–279. doi: 10.4088/JCP.08r04516blu. [DOI] [PubMed] [Google Scholar]
- 50.Rutherford BR, Roose SP. A model of placebo response in anti-depressant clinical trials. Perspectives. 2013;170:723–733. doi: 10.1176/appi.ajp.2012.12040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rutherford BR, Wager TD, Roose SP. Expectancy and the treatment of depression: a review of experimental methodology and effects on patient outcome. Curr Psychiatry Rev. 2010;6:1. doi: 10.2174/157340010790596571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychol Bull. 2004;130:324. doi: 10.1037/0033-2909.130.2.324. [DOI] [PubMed] [Google Scholar]
- 53.Alexopoulos GS, Raue PJ, Kiosses DN, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction: effect on disability. Arch Gen Psychiatry. 2011;68:33–41. doi: 10.1001/archgenpsychiatry.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Areán PA, Raue P, Mackin RS, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction. Am J Psychiatry. 2010;167:1381–1398. doi: 10.1176/appi.ajp.2010.09091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Zurilla TJ, Nezu AM. Problem-Solving Therapy: A Social Competence Approach to Clinical Intervention. New York: Springer; 1999. [Google Scholar]
- 56.Sneed J, Culang M, Keilp J, et al. Antidepressant medication and executive dysfunction: a deleterious interaction in late-life depression. Am J Geriatr Psychiatry. 2010;18:128–135. doi: 10.1097/JGP.0b013e3181c796d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, et al. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165:238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- 58.Baldwin R, Jeffries S, Jackson A, et al. Treatment response in late-onset depression: relationship to neuropsychological, neuroradiological and vascular risk factors. Psychol Med. 2004;34:125–136. doi: 10.1017/s0033291703008870. [DOI] [PubMed] [Google Scholar]
- 59.Dunkin JJ, Leuchter AF, Cook IA, et al. Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord. 2000;60:13–23. doi: 10.1016/s0165-0327(99)00157-3. [DOI] [PubMed] [Google Scholar]
- 60.Jurado M, Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev. 2007;17:213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- 61.Bull R, Espy KA, Senn TE. A comparison of performance on the Towers of London and Hanoi in young children. J Child Psychol Psychiatry. 2004;45:743–754. doi: 10.1111/j.1469-7610.2004.00268.x. [DOI] [PubMed] [Google Scholar]
- 62.Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 63.Greve KW, Stickle TR, Love JM, et al. Latent structure of the Wisconsin Card Sorting Test: a confirmatory factor analytic study. Arch Clin Neuropsychol. 2005;20:355–364. doi: 10.1016/j.acn.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 64.Oosterman JM, Vogels RL, van Harten B, et al. Assessing mental flexibility: neuroanatomical and neuropsychological correlates of the Trail Making Test in elderly people. The Clinical Neuropsychologist. 2010;24:203–219. doi: 10.1080/13854040903482848. [DOI] [PubMed] [Google Scholar]
- 65.Kalayam B, Alexopoulos GS. A preliminary study of left frontal region error negativity and symptom improvement in geriatric depression. Am J Psychiatry. 2003;160:2054–2056. doi: 10.1176/appi.ajp.160.11.2054. [DOI] [PubMed] [Google Scholar]
- 66.Potter GG, Kittinger JD, Ryan Wagner H, et al. Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology. 2004;29:2266–2271. doi: 10.1038/sj.npp.1300551. [DOI] [PubMed] [Google Scholar]
- 67.Sheline YI, Pieper CF, Barch DM, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.