Abstract
Transrectal ultrasound (TRUS)-guided (12–14 core) systematic biopsy of the prostate is the recommended standard for patients with suspicion of prostate cancer (PCa). Advances in imaging have led to the application of magnetic resonance imaging (MRI) for the detection of PCa with subsequent development of software-based co-registration allowing for the integration of MRI with real-time TRUS during prostate biopsy. A number of fusion-guided methods and platforms are now commercially available with common elements in image and analysis and planning. Implementation of fusion-guided prostate biopsy has now been proven to improve the detection of clinically significant PCa in appropriately selected patients.
Keywords: prostate cancer, Transrectal ultrasound, magnetic resonance imaging, biopsy
Introduction
Transrectal ultrasound (TRUS)-guided random systematic biopsy is the diagnostic approach recommended to patients with a high suspicion for prostate cancer (PCa). This approach though systematic (based on geographic regions of the prostate), “blindly” samples the prostate without focus on any specific lesion. Standard biopsy’s inability to visualize and target prostate lesions results in sampling error. These errors lead to diagnostic uncertainty and additional biopsies, which can increase unnecessary morbidity and cost (1). Targeted prostate biopsy is an emerging technique that utilizes high-resolution multiparametric MRI (mpMRI) and ultrasound guidance to sample MR visible lesions. Though there are no compelling prospective studies suggesting that targeted biopsies alone can supplant systematic biopsies, the addition of systematic cores does capture only a limited additional number of intermediate and high-risk cancers (2). The combined approach should be considered as an additional level of safety to avoid under sampling the prostate and missing clinically significant prostate cancer (CSPCa).
Standard 12-core transrectal biopsy is a familiar, cost-effective, office-based procedure, but TRUS adds little imaging information that can improve PCa detection. MpMRI improves sensitivity, but adds significant up-front cost and lacks real-time imaging capabilities. An MR/US fusion-guided prostate biopsy allows clinicians to unify to the strengths of each modality resulting in an office-based biopsy platform that allows pre-procedure MR information to guide sampling in real-time (3).
Multiparametric MRI is comprised of T2-weighted (T2W), diffusion-weighted imaging (DWI) with apparent diffusion coefficient (ADC) maps, and dynamic contrast-enhanced (DCE) sequences. These provide detailed anatomic and functional imaging that improves sensitivity and the positive predictive value of detecting CSPCa. MpMRI-based suspicion for cancer correlates with biopsy pathology (4). As mpMRI is optimized towards higher-grade disease, it limits the detection of low-risk PCa potentially reducing over diagnosis/treatment of indolent cancers. MpMRI, a staging MRI, differs from the “screening” MRI (typically without an ERC) in that staging mpMRI studies are done with an ERC where improvements in signal-to-noise ratio translate to improved fidelity of the acquired images. The additional detail provided improves on the diagnosis of extracapsular extension or seminal vesicle invasion (5, 6). Though biparametric MRI has been suggested as a possible screening tool, the term “screening” for conventional mpMRI falsely implies the benefits of a screening test, which should select more patients, however it has been demonstrated that without a coil, some lesions go undetected thus decreasing sensitivity (7, 8). During the procedure, with MRI data available, operators can approach targeted biopsy in three major ways.
In-Bore MR-Guided Biopsy (MRGB) was the first method developed for the targeting of MRI positive prostate lesions. A pre-biopsy mpMRI is performed and identified lesions are subsequently targeted while the patient is positioned inside the MRI gantry. During the procedure, the patient is placed prone in the MRI scanner, and MR visible lesions are sampled [transrectal (most common), transperineal or transgluteal] under MR fluoroscopy guidance using core needles. Successive MR images are taken to confirm needle placement and biopsy locations are recorded (9). This method of targeted biopsy is highly precise. Hoeks et al in a cohort of 265 patients (with elevated PSA and prior negative TRUS biopsy), using in-bore MRGB, had a CDR of detected 41%, many (80%) of which were CSPCa (10). Despite its high precision, in-bore MRI targeted biopsy is not routinely used because it is costly, requires a lot of personnel time, specialized MRI compatible equipment, and discomfort from prone positioning. It may be particularly useful for patients in which TRUS may not be possible such as post-abdominoperineal resection (11).
Cognitive Fusion/Visual Registration
In this approach, the operator reviews the lesions on the pre-biopsy mpMRI and then tries to estimate the region/location where the lesion would be found on real-time TRUS imaging. Cognitive registration does not require significant capital investment or additional training with unfamiliar software or hardware. However, as accuracy is heavily operator dependent, it has a high propensity for errors at the distal apex or base (end-fire probes) or lateral edges (side-fire probe) of the prostate due to varying anatomic presentations on true axial MRI versus the fanned image-acquisition during TRUS (12). This can be partially overcome by careful attention to internal fiducials such as prostatic cysts, calcifications, or other relevant anatomy seen with both imaging modalities. In the PROFUS trial in which 172 patients, in the same biopsy session, underwent software MR/US fusion, cognitive fusion and systematic biopsy, cognitive fusion had similar CDRs (32% vs 27% PCa, p=0.14 and 20% vs 15% Gleason ≥7, p=0.05) compared to MR/US fusion (13). Haffner et al, in a cohort of 555, showed that cognitive fusion detected more PCa compared to TRUS biopsy [69% vs 59%, p=0.03 PCa and 67% vs 52%, p=0.001 CSPCa] (14). The increase in CDR compared to standard biopsy could in part be due to the use of MRI as a tool for selecting men at higher risk for having PCa with MR visible lesions. However, it falls short when compared to software-based MRI-US fusion biopsy.
Software-Based Registration Platforms
To minimize user error, maximize accuracy and perpetuate consistency of the MRI-US workflow, software-based fusion platforms incorporate complex algorithms to register and co-display MR and TRUS images during biopsy. Additionally, the needle location in 3D space can be tracked, mapped, recorded and stored for future reference (15). Positive cores may be recalled as targets and resampled, such as in patients on active surveillance (AS) (16, 17). While the software platforms address cognitive fusion shortcomings, users must become familiar with additional software and hardware.
Several software-based fusion platforms are commercially available and are the most commonly used in targeted biopsy. All fusion platforms have similar steps but differ in type of image registration (rigid versus elastic), method of needle tracking, presentation of fused MR-US images, software functionality (mapping and navigation), and route of biopsy.
Indications For Fusion Biopsy
As the adoption of fusion biopsy increases, the indications for its use are also expanding (18, 19). A number of studies have defined a role for fusion biopsy with additional benefit provided in:
Patients with continued suspicion for prostate cancer despite a negative systematic TRUS biopsy (20, 21). The conventional alternative in such cases is saturation biopsy sampling in excess of 20 cores, general anesthesia, and associated cost and morbidity. Vourganti et al showed in a prior negative biopsy cohort of 195 that fusion biopsy detected 21 high-grade while standard missed 11 high-grade cancers (22). In a similar cohort, Salami et al reported 20.9% of cases missed by standard and detected by fusion biopsy were clinically significant (23).
Patients on active surveillance. Fusion biopsy can be used to determine candidacy for AS via confirmatory biopsy, improve accuracy of re-sampling of those already on AS, and provide essential information regarding tumor volume (24–26). Hu et al in a cohort of 113, who qualified for active surveillance (based on standard 12-core biopsy), showed that software-based fusion biopsy resulted in a reclassification rate of 36% (27). Also, in a cohort of 152 patients who qualified for AS, 34 (22.4%) were reclassified (failed AS) on confirmatory fusion biopsy. The number needed to biopsy for one Gleason progression was 8.74 for standard 12-core repeat biopsy and 2.90 for fusion biopsy. Stable mpMRI findings were associated with Gleason score stability (28). These studies support the use of serial imaging and fusion biopsy in AS patients.
Image Analysis And Planning
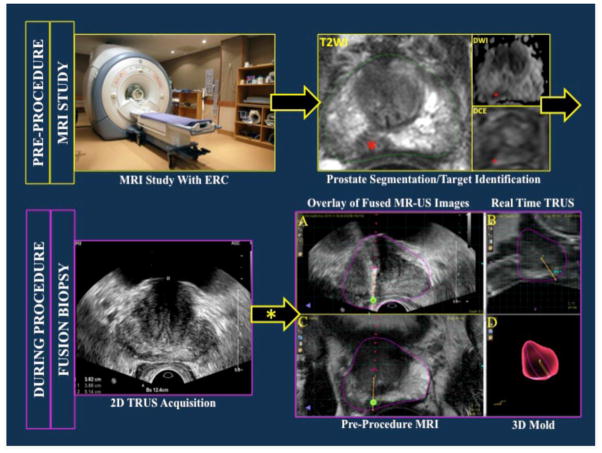
The steps required in the workflow of fusion biopsy are presented in Figure 1.
Figure 1.
Workflow of magnetic resonance imaging (MRI)/ultrasound (US) fusion-guided prostate biopsy. Red asterisks indicate a right medical apical lesion as seen on T2W=T2 weighted imaging (green contour shows MRI prostate segementation), DWI=diffusion weighted image and DCE=dynamic contrast enhanced image sequences. After 2D TRUS sweep of the prostate, the acquired images are used to create a 3D US reconstruction. This 3D rendering is co-registered (elastic/rigid registration) with the MRI dateset. MR-US co-registered images are co-displayed above (A) and beside (A,B) each other. 3D rendering of the prostate (with overlaid needle core location) generated after sampling and tracking. TRUS= transrectal ultrasound; 2D= 2-dimensional; 3D= 3-dimensional.
Segmentation
Using the T2W images the prostate outline is contoured semi-automatically and manually adjusted where needed. The MR images with the largest diameter of each lesion are utilized to define biopsy targets and approach angles. Once the prostate outline and targets are marked out on the T2W MRI, the data is transferred to the procedure workstation. Automation by computer-aided software may help in lesion identification but interpreter expertise is still required. (29). Some workstations allow for biopsy information to feedback and integrate with PACS enabling post biopsy image correlation as well as feedback to the radiologist that enhances their experience in prostate mpMRI interpretation.
Image Registration
Each pre-procedural MRI (along with segmentation and target data) selected for fusion biopsy needs to be overlaid “fused” to a 3D TRUS volume of the prostate constructed from a series of 2D TRUS images obtained via a “sweep” of the TRUS probe. Fusion of MR and US images is done using two possible software registration algorithms, rigid and elastic.
Rigid Registration aligns both images as they are captured with manipulation of each image set limited to rotation or translation (left/right or anterior/posterior correction). Rigid registration preserves the anatomy of the prostate and lesion location. If images are suboptimally aligned due to registration error, organ/patient movement, or organ deformation (by an inflated ERC), the operator can manually compensate with manual correction, alignment of indirect internal fiducials (fixed bony points, cysts, etc.), or adjust probe pressure (30). In cases where the TRUS prostate volume does not match the MR prostate segmentation, an elastic software algorithm can be used to force matching of the corresponding datasets.
Elastic Registration matches corresponding point landmarks (MR and US segmented volumes). This method of image registration may lead to anatomic distortion in an attempt to create the “perfect picture” thus sacrificing accuracy in registration of the internal architecture of the prostate in an effort to align surface contours.
Most fusion platforms are equipped with both elastic and rigid registration algorithms giving the operator the flexibility to fine-tune registration and optimize targeting. Reregistration during the procedure accounts for movement of the patient or TRUS probe pressure deformation of the prostate. Lesion target accuracy depends on how well the registration is done throughout the procedure.
Fused MR-US images can be presented to the operator in different ways depending on user preference or platform type. Some platforms have the fused images separated and displayed side-by-side or alternatively images can be overlaid atop each other.
Tracking Approaches
Tracking is the ability to, in real-time visualize the TRUS probe position and needle advancement in a 3D space throughout the procedure. This allows the operator to navigate to image slices of interest under complete visualization. Tracking can be achieved using a combination of different software and hardware, all of which may employ different technologies.
Electromagnetic (EM) Tracking: used by UroNav (In Vivo); Virtual Navigator (Esaote); Real-Time Virtual Sonography (Hitachi). This tracking approach applies Faraday’s Principle whereby current can be generated in a solenoid when placed in a continuously changing magnetic field. EM tracking has multiple additional interventional arenas applications including (31–33). In EM tracking, a small sensor (solenoid) attached to the TRUS probe, generates current due to a sequentially changing magnetic field (generated from a magnetic field generator). The current is relayed to the computer, which converts it into a 3D image display. This type of tracking allows the operator to freely manipulate the TRUS probe with multiple degrees of freedom (DoF), a process familiar to most urologists (34). The projection of real time 3D images onto fused MR-US images, guides the operator towards regions of interest (ROIs) as defined on pre-procedure MRI. The shortcoming here is that of human error due to unsteady hands at needle deployment, which can lead to registration errors.
Position-Encoded Joints in Smart Robotic Arms: used by Artemis (Eigen), BiopSee (Pi Medical), and BioJet (BK Ultrasound). The location of the TRUS probe in 3D space is tracked by direct attachment to the robotic arm or mechanical stepper. Angle sensors in the robotic arm joints, encode and automatically relay to the computer the position of the probe and needle in 3D space. The mechanical stepper similarly tracks and automatically records by utilizing position encoders in its arm joints (35, 36). The ultrasound images are obtained by manually rotating the probe along a fixed axis. There are 2 DoF allowed when manipulating the probe: in/out and rotation (37). Though the steady robotic arm addresses human error and improves on the positional accuracy, its software and unconventional fixed axis TRUS movement can add to the learning curve.
Image-Based Software Tracking: used by the Urostation (Koelis). This system relies on TRUS images alone (without any external tracking devices) to track the probe and biopsy needle position. Initially, a 3D anatomical TRUS reference image is created from 3 ultrasound volumes acquired from different positions. This panorama image is fused via elastic registration to the pre-procedure MRI data (38, 39). A 3D TRUS image is obtained, after spring-deployment and before needle retraction out of the prostate, to confirm/register needle position onto the 3D panoramic reference image as well as ensure adequate target sampling (34). Freehand probe movement is employed.
Mapping and Navigation
Mapping is like a GPS that tracks, records and locates (“pins”) the biopsy specimen onto the pre-biopsy MRI (“map”). The recorded information can be accessed and reviewed in the future when needed. Navigation is the real-time imaging feedback that guides needle location and advancing towards the ROI. A combination of tracking, mapping and navigation provide the operator superior visualization of ROI, accurate targeting and data archives for future use in follow up patients. For example, should a random sextant core turn out to be positive at a location where there was no MR lesion, the mapped needle core coordinate becomes a new target point in repeat fusion biopsy (17).
Biopsy Approach
Transrectal (TR) or transperineal (TP) are the two main biopsy approaches. In TR biopsy, access to the prostate is via the rectal mucosa and therefore increases the likelihood of introducing rectal flora into the bloodstream or urinary tract. The frequency of biopsy related infections varies amongst studies with reported infection rates ranging from 0–6.3%. There is however, evidence that TR patients are at an increased risk of biopsy–related urinary tract infections and sepsis compared to TP patients (40, 41). A study of 245 TP approach patients showed 0% rate of hospital re-admission for infection (42). The major culprit pathogens are the antibiotic (mostly fluoroquinolones) resistant bacteria. A recent meta-analysis of 2541 patients showed a higher prevalence of fluoroquinolone resistant bacteria in post biopsy rectal cultures (20.4%) than in pre-TRUS biopsy (12.8%) rectal cultures (43). Evaluating and developing efficient local prophylactic standard procedures may help reduce the rates of prostate biopsy related infections (44). TP biopsy may potentially be a reasonable alternative to patients with a history of sepsis or with significant concern for infectious complications. Though generally performed under sedation, TP biopsy under local anesthesia has been reported (45).
Major platforms
As earlier mentioned, there are 3 major fusion technologies available for prostate biopsy. It is important to note that many have and continue to evolve.
Electromagnetic Tracking
The UroNav platform (Invivo, USA): developed via a collaborative partnership between the National Institutes of Health (NIH) and Philips Medical/Invivo Corporation. This platform has undergone extensive testing and clinical trials since its approval by the FDA in 2006. The system is designed to integrate with various ultrasound vendors (Philips, GE, and BK) as well as interface with the commonly used PACS software thus reducing cost of system acquisition.
The workflow begins with a pre-procedure mpMRI study. The images (T2WI, DWI, DCE) obtained are pre-processed on a separate imaging workstation (DynaCad, Invivo) by the radiologist, who segments and defines target lesions. The MR data sets are sent via network connections between the imaging workstation and the UroNav device.
During the procedure, the patient is placed decubitus on the operating table. The field generator is affixed to the operating table and adjusted to sit above and close to the pelvis of the patient. An anesthetic gel is applied in the rectum to reduce pain during TRUS probe manipulation. A few minutes after the lidocaine jelly application, the operator gently inserts a 2D TRUS probe (with an attached solenoid sensor) and performs periprostatic nerve blocks after which an initial ultrasound “sweep” of the prostate is done. The “sweep” captures small slices of the prostate, which are automatically compounded to construct a 3D TRUS prostate volume that will be segmented and registered with the corresponding MR data set (46). Registered images are inspected and manually aligned as needed throughout the procedure (47).
The fused MR-US images are displayed simultaneously side-by-side as well as overlaid atop each other. The freehanded TRUS probe is guided using the UroNav navigation system to the ROI. Freehand TRUS probe movement allows for several DoF creating multiple angles of approach and visualization. The needle is constantly under visualization as it is advanced towards the target. In order to adequately sample the lesion, the needle is inserted with its tip as close as possible to the lesion prior to spring deployment with cores taken in the axial and sagittal planes (48). After the completion of the procedure, the data is then sent back to the imaging workstation to be reviewed and stored for future analysis. The UroNav system is accurate with registration/tracking error reported at 2–3 mm mm (49).
The UroNav system has evolved from its original design. Initially, only rigid registration and navigation were possible but today, elastic registration and mapping capabilities have been added, making it a more robust fusion platform. Most recently, a transperineal fusion platform has become available and is being prospectively evaluated. Fusion biopsy with UroNav has demonstrated significant benefit in prostate biopsy. In a landmark study by Siddiqui et al in which patients underwent both fusion biopsy and standard 12-core biopsy, the UroNav fusion platform detected 30% more high-risk cancers (Gleason score ≥4+3) than standard TRUS biopsy (2). In a phase III clinical trial, UroNav detected cancer in 72% of high-risk lesions versus 47% in low-risk lesions demonstrating improved detection of CSPCa (50).
Virtual Navigator [VNav] (Esaote, Italy) and Real-Time Virtual Sonography [RVS] (Hitachi, Japan). Like all other fusion platforms, a pre-procedure MRI study is performed, prostate MRI segmented, target points defined and data transferred to the procedure workstation. Both platforms enable real-time fusion imaging between ultrasound and a second reference imaging modality (PET/CT, MRI, 3D ultrasound) (51). These two platforms have been more commonly used in other interventional applications, including hepatobiliary interventions (52). The utility in prostate biopsy/treatment is sparse with very limited data available. Miyagawa et al, in a small cohort of 85 prior negative patients, reported an RVS CDR of 62%, higher than the reported 38–55% in other fusion platforms (53). VNav has also shown higher CDR than standard biopsy: 69% versus 59%, p=0.033 (54). VNav utilizes rigid registration and therefore is prone to associated errors as described previously (30). RVS utilizes both transrectal and transperineal routes of biopsy.
Mechanical Position Encoders
The Artemis (Eigen, Grass Valley, CA): Recruitment of patients started in 2009 soon after the commercial model of the Artemis was FDA cleared in 2007. The Artemis uses the independent software (Eigen ProFuse) workstation (55). Again, the workflow and software are similar to the other platforms. The major difference is TRUS probe manipulation. The Artemis does not require an external tracking system. Instead, the endfire TRUS probe held by a mechanical robotic arm (affixed to the operating table) is tracked in 3D space by angle sensing encoded joints. The pre-procedure MRI data (with targets) along with 12 preselected biopsy sites (generated by Artemis) are uploaded and aligned/fused semiautomatically to the initially constructed 3D ultrasound volume (56). In order to maximize accuracy, biopsy cores are obtained at 3 mm intervals based on a 1.2±1.1 mm tracking accuracy on repeat biopsy (57). While this addresses the issue of unstable human hands during needle deployment, it does however, limit the DoF of movement (along a fixed axis) of the TRUS probe.
The Artemis, like UroNav, has the ability to track and record biopsy sites, allowing the operator, to re-biopsy site of previous positive cores with a 1.2–3 mm accuracy. In a study of 106 active surveillance and 68 patients with a prior negative TRUS biopsy (with persistently increasing PSA) patients, the Artemis system was 3 times more likely to detect cancer than standard biopsy (21% vs 7%) (56).
The BiopSee platform (Pi Medical, Greece): In this platform, the TRUS probe is placed in a custom-made mechanical fixation device (the stepper) affixed to the operating table. TRUS probe movement is limited to 2 DoF: probe rotation and depth in/out of the rectum. Two built-in encoders track both movements and relay positional data to the computer workstation (35). Although limited in DoF, the 0.1 mm linear and 0.1° rotational resolution allows for reproducible high accuracy imaging of the entire prostate. Unlike most platforms, the BiopSee only utilizes a transperineal approach. The BiopSee hardware setup is similar to that used in brachytherapy; parts used in diagnosis can potentially be reused in therapy as well (58).
The software design is modular with each procedure step mapped in a separate software module. Pre-procedure MR images are segmented, lesions identified and target points defined. During biopsy, a series of 2D ultrasound images are obtained via a cranio-caudal sweep and used to construct a 3D ultrasound volume, which is then fused with pre-biopsy MRI data via rigid registration (59). Lastly, the needles are inserted under longitudinal guidance and biopsy cores obtained transperineally (58).
The BioJet platform is FDA approved and has its TRUS probe mounted to an angle-sensing encoded mechanical arm that exports information about the position of the probe to the workstation. This uses both the transperineal and transrectal biopsy approaches. Pre-procedure MRI data is fused with 3D TRUS construct using rigid registration. During transperineal biopsy, the biopsy template coordinates are shown on the computer monitor when the operator selects a given ROI (60). Side fire mode can be used to minimize probe deformation of the prostate. There is room for evolution of this platform as it only uses rigid registration currently (61). Shoji et al, with the first clinical application of this platform, detected cancer in 14 of 20 (70%) many of which were high grade with longer core lengths than standard biopsy (59). Also, BioJet CDRs have been shown to correlate with higher Prostate Imaging Reporting and Data Systems (PIRADS) scores (positive PCa: 4.0±1.3 vs negative PCa: 2.6±0.8) (61). Like most fusion platforms, BioJet early studies trend towards higher CDRs compared to standard biopsy.
Image-based tracking Platform
The Urostation platform (Koelis, France): Similar to other systems in the use of pre-procedure MRI, freehand probe movement, elastic and rigid registrations. It is currently the dominant platform utilized in Europe. The Urostation differs from the other platforms in several ways. It relies purely on 3D TRUS image tracking without any form of beam tracking external hardware.
Initially, three 3D TRUS volumes are acquired from different positions and compounded into a panoramic 3D TRUS reference volume of the prostate. An anatomical atlas is built by mapping the manually segmented pre-MRI slices onto the panoramic reference image. Image registration and fusion are elaborate processes that minimize registration errors due to probe deformation or unconscious patient movement thus improving accuracy. In other words, this platform is less sensitive to organ movement during procedure (39).
During biopsy, 2D real-time TRUS images are used to guide the operator but 3D TRUS is used when positional information about the targeted or collected sample is required (39). The biopsy needle is held in-situ after spring needle deployment for a minimum of 3 seconds (30, 34) until 3D TRUS image is taken retrospectively to locate the biopsy site. The locating TRUS image is registered onto the TRUS panorama reference image for confirmation and storage (62).
In a pre-clinical study, the Urostation showed high accuracy (24/27 MR ROIs: 84%) in targeting prostate phantom ROIs (62) and an even higher accuracy when used on patients (112/114 MR ROIs: 97%) (63).
Discussion and Conclusions
Multiparametric MRI and fusion-guided biopsy has demonstrated clinical benefit over systematic biopsy alone. The ability to co-register MRI to real-time TRUS images allows for accurate sampling of PCa leading to improved diagnosis, risk stratification, and treatment. The future of fusion biopsy technologies seems promising; significant investment in commercially available fusion platforms continues and adoption is expanding. The challenge we face is appropriate utilization in the patient population that will benefit the most (64, 65).
Clinical centers utilize different fusion platforms, varying selection criteria, as well as different MRI scoring systems (PIRADS, NIH suspicion score etc) making it difficult for head-to-head comparison of these platforms. A recent publication reported decrease in diagnostic accuracy of PIRADSv2 compared to 5 point Likert scale based on the ESUR guidelines from 2012 with each patient acting as their own control (66). Standardized reporting is essential, however interpretation is subjective and proposed scoring systems should reflect appropriate risk stratification with respect to the presence of CSPCa (67). Small differences in interpretation can alter outcomes of fusion biopsy devices and make comparing systems between institutions and historical data almost impossible. The authors advise centers to record granular sequence specific data to allow for future analyses (66).
The benefit trends (high overall CDRs with fewer cores, detection of more high risk than low risk disease, increase reclassification of patients on active surveillance, extended use in focal curative therapies etc) of fusion biopsy are consistent and supported by peer-reviewed evidence irrespective of the fusion platform used (2, 22, 34, 50, 68, 69). Rather than selecting the optimal technology or device, the challenge becomes ensuring responsible and judicious utilization by individuals with the appropriate expertise and training.
Footnotes
Conflict of Interest
Michael Kongnyuy and Arvin K. George each declare no potential conflicts of interest.
Ardeshir R. Rastinehad is a section editor for Current Urology Reports.
Peter A. Pinto reports a patent Method And System For Performing Biopsies issued to No: US 8,447,384 B2, and a patent System And Method For Planning And Performing a Repeat Interventional Procedure pending to File No: US 195-381Úpplication # 2015PF00912.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Michael Kongnyuy, Email: michael.kongnyuy@nih.gov.
Ardeshir R. Rastinehad, Email: art.rastinehad@mountsinai.org.
Peter A. Pinto, Email: pintop@mail.nih.gov.
References
- 1.Serefoglu EC, Altinova S, Ugras NS, Akincioglu E, Asil E, Balbay MD. How reliable is 12-core prostate biopsy procedure in the detection of prostate cancer? Can Urol Assoc J. 2013;7(5–6):E293–e8. doi: 10.5489/cuaj.11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2**.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. Jama. 2015;313(4):390–7. doi: 10.1001/jama.2014.17942. this is a landmark study that elucidates the role of targeted fusion biopsy in detection of prostate cancer. The study shows that targeted biopsy detects more clinically significant cancer and fewer clinically insignificant cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George AK, Pinto PA, Rais-Bahrami S. Multiparametric MRI in the PSA screening era. BioMed research international. 2014;2014:465816. doi: 10.1155/2014/465816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turkbey B, Mani H, Shah V, Rastinehad AR, Bernardo M, Pohida T, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186(5):1818–24. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raskolnikov D, George AK, Rais-Bahrami S, Turkbey B, Shakir NA, Okoro C, et al. Multiparametric magnetic resonance imaging and image-guided biopsy to detect seminal vesicle invasion by prostate cancer. Journal of endourology / Endourological Society. 2014;28(11):1283–9. doi: 10.1089/end.2014.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raskolnikov D, George AK, Rais-Bahrami S, Turkbey B, Siddiqui MM, Shakir NA, et al. The Role of Magnetic Resonance Image Guided Prostate Biopsy in Stratifying Men for Risk of Extracapsular Extension at Radical Prostatectomy. The Journal of urology. 2015;194(1):105–11. doi: 10.1016/j.juro.2015.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Fascelli M, Rais-Bahrami S, Sankineni S, Brown AM, George AK, Ho R, et al. Combined Biparametric Prostate MRI and Prostate Specific Antigen in the Detection of Prostate Cancer: a Validation Study in a Biopsy Naïve Patient Population. Urology. 2015 doi: 10.1016/j.urology.2015.09.035. is a review of how fusion biopsy can be used in active surveillance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turkbey B, Merino MJ, Gallardo EC, Shah V, Aras O, Bernardo M, et al. Comparison of endorectal coil and nonendorectal coil T2W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: correlation with whole-mount histopathology. Journal of magnetic resonance imaging : JMRI. 2014;39(6):1443–8. doi: 10.1002/jmri.24317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson NL, Emberton M, Moore CM. MRI-targeted prostate biopsy: a review of technique and results. Nat Rev Urol. 2013;10:589–597. doi: 10.1038/nrurol.2013.196. [DOI] [PubMed] [Google Scholar]
- 10.Hoeks CM, Schouten MG, Bomers JG, Hoogendoorn SP, Hulsbergen-van de Kaa CA, Hambrock T, et al. Three-Tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. European urology. 2012;62(5):902–9. doi: 10.1016/j.eururo.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 11.Kongnyuy M, Frye TP, George AK, et al. A Case of In-Bore Transperineal MRI-guided Prostate Biopsy of a Patient with Ileal Pouch-Anal Anastomosis. Case Reports in Urology. 2015 doi: 10.1155/2015/676930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwak JT, Hong CW, Pinto PA, Williams M, Xu S, Kruecker J, et al. Is visual registration equivalent to semiautomated registration in prostate biopsy? BioMed research international. 2015;2015:394742. doi: 10.1155/2015/394742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wysock JS, Rosenkrantz AB, Huang WC, Stifelman MD, Lepor H, Deng FM, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. European urology. 2014;66(2):343–51. doi: 10.1016/j.eururo.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 14.Haffner J, Lemaitre L, Puech P, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011;108:E171. doi: 10.1111/j.1464-410X.2011.10112.x. [DOI] [PubMed] [Google Scholar]
- 15.Raskolnikov D, Rais-Bahrami S, George AK, Turkbey B, Shakir NA, Okoro C, et al. The role of image guided biopsy targeting in patients with atypical small acinar proliferation. The Journal of urology. 2015;193(2):473–8. doi: 10.1016/j.juro.2014.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fascelli M, George AK, Frye T, Turkbey B, Choyke PL, Pinto PA. The role of MRI in active surveillance for prostate cancer. Current urology reports. 2015;16(6):42. doi: 10.1007/s11934-015-0507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonn GA, Filson CP, Chang E, Natarajan S, Margolis DJ, Macairan M, et al. Initial experience with electronic tracking of specific tumor sites in men undergoing active surveillance of prostate cancer. Urologic oncology. 2014;32(7):952–7. doi: 10.1016/j.urolonc.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller BG, Kaushal A, Sankineni S, Lita E, Hoang AN, George AK, et al. Multiparametric magnetic resonance imaging-transrectal ultrasound fusion-assisted biopsy for the diagnosis of local recurrence after radical prostatectomy. Urologic oncology. 2015;33(10):425, e1–6. doi: 10.1016/j.urolonc.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sankineni S, George AK, Brown AM, Rais-Bahrami S, Wood BJ, Merino MJ, et al. Posterior subcapsular prostate cancer: identification with mpMRI and MRI/TRUS fusion-guided biopsy. Abdominal imaging. 2015;40(7):2557–65. doi: 10.1007/s00261-015-0426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manfredi M, Costa Moretti TB, Emberton M, Villers A, Valerio M. MRI/TRUS fusion software-based targeted biopsy: the new standard of care? Minerva urologica e nefrologica = The Italian journal of urology and nephrology. 2015;67(3):233–46. [PubMed] [Google Scholar]
- 21.Valerio M, Donaldson I, Emberton M, Ehdaie B, Hadaschik BA, Marks LS, et al. Detection of Clinically Significant Prostate Cancer Using Magnetic Resonance Imaging-Ultrasound Fusion Targeted Biopsy: A Systematic Review. European urology. 2015;68(1):8–19. doi: 10.1016/j.eururo.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Vourganti S, Rastinehad A, Yerram NK, Nix J, Volkin D, Hoang A, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. The Journal of urology. 2012;188(6):2152–7. doi: 10.1016/j.juro.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Salami SS, Ben-Levi E, Yaskiv O, Ryniker L, Turkbey B, Kavoussi LR, et al. In patients with a previous negative prostate biopsy and a suspicious lesion on magnetic resonance imaging, is a 12-core biopsy still necessary in addition to a targeted biopsy? BJU international. 2015;115(4):562–70. doi: 10.1111/bju.12938. support use of fusion in prior negative patients. [DOI] [PubMed] [Google Scholar]
- 24.Eggener SE, Badani K, Barocas DA, Barrisford GW, Cheng JS, Chin AI, et al. Gleason 6 Prostate Cancer: Translating Biology into Population Health. The Journal of urology. 2015;194(3):626–34. doi: 10.1016/j.juro.2015.01.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okoro C, George AK, Siddiqui MM, Rais-Bahrami S, Walton-Diaz A, Shakir NA, et al. Magnetic Resonance Imaging/Transrectal Ultrasonography Fusion Prostate Biopsy Significantly Outperforms Systematic 12-Core Biopsy for Prediction of Total Magnetic Resonance Imaging Tumor Volume in Active Surveillance Patients. Journal of endourology / Endourological Society. 2015;29(10):1115–21. doi: 10.1089/end.2015.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George AK, Pinto PA. Editorial Comment Re: Multiparametric Magnetic Resonance Imaging Enhances Detection of Significant Tumor in Patients on Active Surveillance for Prostate Cancer. Urology. 2015;85(2):429. doi: 10.1016/j.urology.2014.09.060. [DOI] [PubMed] [Google Scholar]
- 27.Hu JC, Chang E, Natarajan S, Margolis DJ, Macairan M, Lieu P, et al. Targeted prostate biopsy in select men for active surveillance: do the Epstein criteria still apply? J Urol. 2014;192(2):385–90. doi: 10.1016/j.juro.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walton Diaz A, Shakir NA, George AK, Rais-Bahrami S, Turkbey B, Rothwax JT, et al. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urologic oncology. 2015;33(5):202, e1–7. doi: 10.1016/j.urolonc.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Burtt K, Turkbey B, Choyke P, Summers RM. Computer aided-diagnosis of prostate cancer on multiparametric MRI: a technical review of current research. BioMed research international. 2014;2014:789561. doi: 10.1155/2014/789561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logan JK, Rais-Bahrami S, Turkbey B, Gomella A, Amalou H, Choyke PL, et al. Current status of magnetic resonance imaging (MRI) and ultrasonography fusion software platforms for guidance of prostate biopsies. BJU Int. 2014;114(5):641–52. doi: 10.1111/bju.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewertsen C, Nielsen KR, Nielsen MB. Freehand biopsy guided by electromagnetic needle tracking: a phantom study. Ultraschall in der Medizin (Stuttgart, Germany : 1980) 2011;32(6):614–8. doi: 10.1055/s-0031-1281852. [DOI] [PubMed] [Google Scholar]
- 32.Ewertsen C, Saftoiu A, Gruionu LG, Karstrup S, Nielsen MB. Real-time image fusion involving diagnostic ultrasound. AJR American journal of roentgenology. 2013;200(3):W249–55. doi: 10.2214/AJR.12.8904. [DOI] [PubMed] [Google Scholar]
- 33.Hakime A, Deschamps F, De Carvalho EG, Barah A, Auperin A, De Baere T. Electromagnetic-tracked biopsy under ultrasound guidance: preliminary results. Cardiovascular and interventional radiology. 2012;35(4):898–905. doi: 10.1007/s00270-011-0278-8. [DOI] [PubMed] [Google Scholar]
- 34*.Sonn GA, Margolis DJ, Marks LS. Target detection: magnetic resonance imaging-ultrasound fusion-guided prostate biopsy. Urologic oncology. 2014;32(6):903–11. doi: 10.1016/j.urolonc.2013.08.006. is another paper supporting use of fusion biopsy in active surveillance patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadaschik BA, Kuru TH, Tulea C, Rieker P, Popeneciu IV, Simpfendorfer T, et al. A novel stereotactic prostate biopsy system integrating pre-interventional magnetic resonance imaging and live ultrasound fusion. The Journal of urology. 2011;186(6):2214–20. doi: 10.1016/j.juro.2011.07.102. [DOI] [PubMed] [Google Scholar]
- 36.Kuru TH, Roethke M, Popeneciu V, Teber D, Pahernik S, Zogal P, et al. Phantom study of a novel stereotactic prostate biopsy system integrating preinterventional magnetic resonance imaging and live ultrasonography fusion. Journal of endourology / Endourological Society. 2012;26(7):807–13. doi: 10.1089/end.2011.0609. [DOI] [PubMed] [Google Scholar]
- 37.Rothwax JT, George AK, Wood BJ, Pinto PA. Multiparametric MRI in biopsy guidance for prostate cancer: fusion-guided. BioMed research international. 2014;2014:439171. doi: 10.1155/2014/439171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baumann M, Mozer P, Daanen V, Troccaz J. Prostate biopsy tracking with deformation estimation. Medical image analysis. 2012;16(3):562–76. doi: 10.1016/j.media.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Martin S, Troccaz J, Daanenc V. Automated segmentation of the prostate in 3D MR images using a probabilistic atlas and a spatially constrained deformable model. Medical physics. 2010;37(4):1579–90. doi: 10.1118/1.3315367. [DOI] [PubMed] [Google Scholar]
- 40.Carignan A, Roussy JF, Lapointe V, Valiquette L, Sabbagh R, Pepin J. Increasing risk of infectious complications after transrectal ultrasound-guided prostate biopsies: time to reassess antimicrobial prophylaxis? European urology. 2012;62(3):453–9. doi: 10.1016/j.eururo.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 41.Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, et al. Systematic review of complications of prostate biopsy. European urology. 2013;64(6):876–92. doi: 10.1016/j.eururo.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 42.Grummet JP, Weerakoon M, Huang S, Lawrentschuk N, Frydenberg M, Moon DA, et al. Sepsis and ‘superbugs’: should we favour the transperineal over the transrectal approach for prostate biopsy? BJU international. 2014;114(3):384–8. doi: 10.1111/bju.12536. [DOI] [PubMed] [Google Scholar]
- 43.Roberts MJ, Williamson DA, Hadway P, Doi SA, Gardiner RA, Paterson DL. Baseline prevalence of antimicrobial resistance and subsequent infection following prostate biopsy using empirical or altered prophylaxis: A bias-adjusted meta-analysis. International journal of antimicrobial agents. 2014;43(4):301–9. doi: 10.1016/j.ijantimicag.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 44.Womble PR, Linsell SM, Gao Y, Ye Z, Montie JE, Gandhi TN, et al. A Statewide Intervention to Reduce Hospitalizations after Prostate Biopsy. The Journal of urology. 2015;194(2):403–9. doi: 10.1016/j.juro.2015.03.126. [DOI] [PubMed] [Google Scholar]
- 45.Kubo T, Kanemori K, Kusumoto R, Kawai T, Sueyoshi K, Naito T, et al. Simple and effective label-free capillary electrophoretic analysis of sugars by complexation using quinoline boronic acids. Analytical chemistry. 2015;87(10):5068–73. doi: 10.1021/acs.analchem.5b00998. [DOI] [PubMed] [Google Scholar]
- 46.Trobaugh JW, Trobaugh DJ, Richard WD. Three-dimensional imaging with stereotactic ultrasonography. Computerized medical imaging and graphics : the official journal of the Computerized Medical Imaging Society. 1994;18(5):315–23. doi: 10.1016/0895-6111(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 47.Singh AK, Kruecker J, Xu S, Glossop N, Guion P, Ullman K, et al. Initial clinical experience with real-time transrectal ultrasonography-magnetic resonance imaging fusion-guided prostate biopsy. BJU international. 2008;101(7):841–5. doi: 10.1111/j.1464-410X.2007.07348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong CW, Rais-Bahrami S, Walton-Diaz A, Shakir N, Su D, George AK, et al. Comparison of magnetic resonance imaging and ultrasound (MRI-US) fusion-guided prostate biopsies obtained from axial and sagittal approaches. BJU international. 2015;115(5):772–9. doi: 10.1111/bju.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu S, Kruecker J, Turkbey B, Glossop N, Singh AK, Choyke P, et al. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Computer aided surgery : official journal of the International Society for Computer Aided Surgery. 2008;13(5):255–64. doi: 10.1080/10929080802364645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rastinehad AR, Turkbey B, Salami SS, Yaskiv O, George AK, Fakhoury M, et al. Improving detection of clinically significant prostate cancer: magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy. The Journal of urology. 2014;191(6):1749–54. doi: 10.1016/j.juro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zogal P, Sakas G, Rösch W, Baltas D. Physics Contributions on: BiopSee®–transperineal stereotactic navigated prostate biopsy. J Contemp Brachytherapy. 2011;3(2):91–95. doi: 10.5114/jcb.2011.23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Mauro E, Solbiati M, De Beni S, Forzoni L, D’Onofrio S, Solbiati L. Virtual navigator real-time ultrasound fusion imaging with positron emission tomography for liver interventions. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2013;2013:1406–9. doi: 10.1109/EMBC.2013.6609773. [DOI] [PubMed] [Google Scholar]
- 53.Miyagawa T, Ishikawa S, Kimura T, Suetomi T, Tsutsumi M, Irie T, et al. Real-time Virtual Sonography for navigation during targeted prostate biopsy using magnetic resonance imaging data. International journal of urology : official journal of the Japanese Urological Association. 2010;17(10):855–60. doi: 10.1111/j.1442-2042.2010.02612.x. [DOI] [PubMed] [Google Scholar]
- 54.Puech P, Rouviere O, Renard-Penna R, Villers A, Devos P, Colombel M, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy--prospective multicenter study. Radiology. 2013;268(2):461–9. doi: 10.1148/radiol.13121501. [DOI] [PubMed] [Google Scholar]
- 55.Marks L, Young S, Natarajan S. MRI-ultrasound fusion for guidance of targeted prostate biopsy. Current opinion in urology. 2013;23(1):43–50. doi: 10.1097/MOU.0b013e32835ad3ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sonn GA, Natarajan S, Margolis DJ, MacAiran M, Lieu P, Huang J, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. The Journal of urology. 2013;189(1):86–91. doi: 10.1016/j.juro.2012.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Natarajan S, Marks LS, Margolis DJ, Huang J, Macairan ML, Lieu P, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urologic oncology. 2011;29(3):334–42. doi: 10.1016/j.urolonc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paoletti G, Silvani M, Confalone D, Lodigiani L, Manzoli L, D’Onofrio S, et al. Latest Advancements in Real-time Fusion Imaging for Prostate. ECR. 2015 Educational Exhibit. [Google Scholar]
- 59.Kuru TH, Roethke MC, Seidenader J, Simpfendorfer T, Boxler S, Alammar K, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. The Journal of urology. 2013;190(4):1380–6. doi: 10.1016/j.juro.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 60.Shoji S, Hiraiwa S, Endo J, Hashida K, Tomonaga T, Nakano M, et al. Manually controlled targeted prostate biopsy with real-time fusion imaging of multiparametric magnetic resonance imaging and transrectal ultrasound: an early experience. International journal of urology : official journal of the Japanese Urological Association. 2015;22(2):173–8. doi: 10.1111/iju.12643. [DOI] [PubMed] [Google Scholar]
- 61.Tewes S, Hueper K, Hartung D, Imkamp F, Herrmann TR, Weidemann J, et al. Targeted MRI/TRUS fusion-guided biopsy in men with previous prostate biopsies using a novel registration software and multiparametric MRI PI-RADS scores: first results. World journal of urology. 2015;33(11):1707–14. doi: 10.1007/s00345-015-1525-4. [DOI] [PubMed] [Google Scholar]
- 62.Ukimura O, Desai MM, Palmer S, Valencerina S, Gross M, Abreu AL, et al. 3-Dimensional elastic registration system of prostate biopsy location by real-time 3-dimensional transrectal ultrasound guidance with magnetic resonance/transrectal ultrasound image fusion. The Journal of urology. 2012;187(3):1080–6. doi: 10.1016/j.juro.2011.10.124. [DOI] [PubMed] [Google Scholar]
- 63.Rud E, Baco E, Eggesbo HB. MRI and ultrasound-guided prostate biopsy using soft image fusion. Anticancer research. 2012;32(8):3383–9. [PubMed] [Google Scholar]
- 64.Frye TP, Pinto PA, George AK. Optimizing Patient Population for MP-MRI and Fusion Biopsy for Prostate Cancer Detection. Current urology reports. 2015;16(7):50. doi: 10.1007/s11934-015-0521-y. [DOI] [PubMed] [Google Scholar]
- 65.Shakir NA, George AK, Siddiqui MM, Rothwax JT, Rais-Bahrami S, Stamatakis L, et al. Identification of threshold prostate specific antigen levels to optimize the detection of clinically significant prostate cancer by magnetic resonance imaging/ultrasound fusion guided biopsy. The Journal of urology. 2014;192(6):1642–8. doi: 10.1016/j.juro.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rastinehad AR, Waingankar N, Turkbey B, Yaskiv O, Sonstegard AM, Fakhoury M, et al. Comparison of Multiparametric MRI Scoring Systems and the Impact on Cancer Detection in Patients Undergoing MR US Fusion Guided Prostate Biopsies. PloS one. 2015;10(11):e0143404. doi: 10.1371/journal.pone.0143404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muller BG, Shih JH, Sankineni S, Marko J, Rais-Bahrami S, George AK, et al. Prostate Cancer: Interobserver Agreement and Accuracy with the Revised Prostate Imaging Reporting and Data System at Multiparametric MR Imaging. Radiology. 2015;277(3):741–50. doi: 10.1148/radiol.2015142818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mozer P, Roupret M, Le Cossec C, Granger B, Comperat E, de Gorski A, et al. First round of targeted biopsies using magnetic resonance imaging/ultrasonography fusion compared with conventional transrectal ultrasonography-guided biopsies for the diagnosis of localised prostate cancer. BJU international. 2015;115(1):50–7. doi: 10.1111/bju.12690. [DOI] [PubMed] [Google Scholar]
- 69.Sonn GA, Chang E, Natarajan S, Margolis DJ, Macairan M, Lieu P, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. European urology. 2014;65(4):809–15. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]