Effects of human immunodeficiency virus (HIV) infection on carotid artery structure may differ across the lifespan, with traditional determinants of cardiovascular disease burden playing a larger role and HIV a lesser role in older adults than in young adults and children.
Keywords: HIV infection, carotid artery intima-media thickness, aging, cardiovascular disease risk factors, biomarker
Abstract
Background. Age and human immunodeficiency virus (HIV) treatment may affect the association of HIV infection with atherosclerosis.
Methods. We used identical carotid artery B-mode ultrasonographic methods in 5 cohorts participating in the National Heart, Lung, and Blood Institute HIV-CVD Collaborative to measure intima-media thickness of the right far wall of the common carotid artery (CCA-IMT) and carotid artery bifurcation (BIF-IMT) between 2010 and 2013. Participants aged 6–75 years were either HIV infected or uninfected. Linear regression assessed associations of CCA-IMT and BIF-IMT with HIV infection and cardiovascular disease risk factors, within age and HIV treatment groups. Adjustment variables included sex, race/ethnicity, smoking, height, weight, and use of antihypertensive and lipid-lowering drugs.
Results. We studied 867 HIV-infected and 338 HIV-uninfected male and 696 HIV-infected and 246 HIV-uninfected female participants. Among both middle-aged (30–49 years) and older adults (50–75 years), HIV-infected participants had CCA-IMT and BIF-IMT values that were similar to or lower than those in HIV-uninfected participants. In contrast, among those aged 6–29 years, HIV infection was associated with higher CCA-IMT and BIF-IMT values. Among HIV-infected participants, associations of higher systolic blood pressure and lower high-density lipoprotein cholesterol with Carotid artery intima-media thickness strengthened with age.
Conclusions. The effects of HIV on carotid artery structure may differ across the lifespan, with traditional determinants of cardiovascular disease burden playing a larger role and HIV playing a lesser role in older adults than in young adults and children.
Individuals with human immunodeficiency virus (HIV) infection may have an increased risk of cardiovascular disease (CVD), even with universal initiation of antiretroviral therapy (ART). Factors potentially contributing to this increased risk include a preponderance of traditional risk factors, such as smoking [1], potential adverse effects of ART [2], and immune activation and inflammation [3, 4]. Identifying the leading modifiable CVD risk factors has become increasingly important with the growing number of older persons living with HIV [5].
Carotid artery intima-media thickness (cIMT) is a measure of subclinical atherosclerosis that predicts future CVD events in the general population [6]. Numerous studies have examined the association between HIV infection and cIMT, with inconsistent findings. Some studies have reported higher cIMT values in HIV-infected persons [7–9], whereas others have found associations only among certain subgroups, such as those taking protease inhibitors [10]. Reasons for inconsistencies may include limited sample size, varying approaches to assess cIMT, and the degree to which studies have been able to control for confounders. Importantly, although the prevalence and risk factors for atherosclerosis are strongly age-dependent, studies seldom enroll HIV-infected and uninfected participants across a wide age range. Findings from individual studies may therefore not be generalizable across all ages nor allow for comparisons across age groups, because CVD risk factors, such as blood pressure and lipid levels, tend to have different associations with future CVD risk as people age [11, 12]. Furthermore, over many years with HIV infection, the nature of risk factor-CVD associations may change owing to the cumulative effects of HIV-related immunologic and inflammatory processes and medication exposures [13]. Selective survival of HIV-infected patients diagnosed and/or treated earlier in the course of infection is an additional consideration that may influence study findings among older individuals.
Through a National Heart, Lung, and Blood Institute–funded research consortium called the HIV-CVD Collaborative, we analyzed cIMT measurements between 2010 and 2013 from >2000 HIV-infected and uninfected individuals. We assessed differences by HIV serostatus in cIMT at 2 carotid artery sites (common carotid artery and carotid artery bifurcation), both overall and in different age groups, to understand whether differences vary by age. We also examined cIMT differences by ART use, traditional CVD risk factors, including dyslipidemia and high blood pressure, and biomarkers of inflammation and coagulation previously associated with HIV infection and CVD.
METHODS
Study Cohorts and Participants
Participants included enrollees in 5 member cohort studies of the HIV-CVD Collaborative. A data coordinating center (University of Washington) facilitated protocol harmonization and data pooling. The 5 cohorts included a pediatric HIV study based at the University of Miami [14]; AIDS Clinical Trial Group (ACTG) substudy A5260s [15], in which all individuals included were HIV infected and naive to highly active ART (HAART); the Hawaii Aging with HIV-1 Cohort (HAHC) [16]; and 2 multisite cohorts: the Multicenter AIDS Cohort Study (MACS) [17, 18], and the Women's Interagency HIV Study (WIHS) [19, 20]. Eligibility for this analysis included confirmed HIV infection serostatus and cIMT measurements obtained between 2010 and 2013, to emphasize more recent standards of HIV care. A single scan per participant was examined. For A5260s, HAHC, and Miami, the scan examined in this analysis was the first of several performed; for MACS and WIHS, the scan examined was the most recent of a series over many years. Informed consent was obtained, and the original studies were institutional review board approved.
Measurements and Variables
A standard protocol for cIMT assessment was developed by the designated imaging center (University of Southern California). High-resolution B-mode carotid artery ultrasonographic (US) images were obtained at multiple locations in the right carotid artery. Primary outcomes were mean common carotid artery far wall intima-media thickness (CCA-IMT) and carotid artery bifurcation far wall intima-media thickness (BIF-IMT) as measured by automated computerized edge detection [21]. cIMT measurement in both artery segments occurred within regions that were plaque free [22]. Similar sonographer training, quality control, and acquisition procedures were used at all sites. Vascular risk factors, demographics, and HIV-related clinical variables were collected by each cohort at the time of US (Supplementary Material). Among a subset, serum biomarkers of inflammation and hemostasis were measured, including high-sensitivity C-reactive protein (hsCRP) (n = 1453), interleukin-6 (IL-6) (n = 1320), D-dimer (n = 1407), and fibrinogen (n = 1171). WIHS participants were excluded from all biomarker analyses, and A5260s participants were excluded from fibrinogen analyses, because measurements were not available at the time of US.
Statistical Methods
We compared crude and adjusted mean CCA-IMT and BIF-IMT between HIV-infected and HIV-uninfected individuals, both overall and in sex-specific analyses, and examined effect modification by age (6–29, 30–49, or 50–75 years), because this was a prespecified hypothesis. We also conducted analyses stratified by current HAART use and, alternatively, by HIV RNA suppression (defined as <80 copies/mL). Further analyses among the HIV-infected group examined additional risk factors for increased cIMT, including HIV-related variables, total cholesterol, high-density lipoprotein (HDL) cholesterol, systolic blood pressure (SBP), current smoking, hsCRP, IL-6, D-dimer, and fibrinogen. We examined effect modification by age in these associations. Statistical methods included robust linear regression models with Huber weights, with cIMT defined as a continuous outcome. Multivariable adjustment was performed throughout for age, race/ethnicity, smoking status, height, weight, and use of antihypertensive and lipid-lowering medications. Additional details are in the Supplementary Material.
RESULTS
Demographics and Risk Factors
Participants included 1205 male (867 HIV-infected and 338 HIV-uninfected) and 942 female (696 HIV-infected and 246 HIV-uninfected) participants from 5 cohorts (Supplementary Table 1). Within individual cohorts, numbers of male participants ranged from 85 (Miami) to 842 (MACS), and numbers of female participants ranged from 34 (A5260s and HAHC) to 777 (WIHS). Ages ranged from 6 to 75 years. Among HIV-infected participants, both male and female, the mean age was 44 years. Non-Hispanic whites were the largest race/ethnicity group among HIV-infected male participants, and non-Hispanic blacks were the largest among HIV-infected female participants. Two-thirds of participants were using ART, and more than half had suppressed HIV RNA levels at the time of the carotid artery scan.
Table 1 shows demographic characteristics and risk factor distributions further stratified by age (6–29, 30–49, or 50–75 years). Older participants had more CVD risk factors, including higher mean SBP and total cholesterol, and greater use of anti-hypertensive and lipid-lowering medications.
Table 1.
Characteristics of Human Immunodeficiency Virus (HIV)-Infected and HIV-Uninfected Participants in the National Heart, Lung, and Blood Institute HIV-Cardiovascular Disease Collaborative, by Age Group
| Characteristic | Age 6–29 y |
Age 30–49 y |
Age 50–75 y |
|||
|---|---|---|---|---|---|---|
| HIV Uninfected (n = 58) | HIV Infected (n = 221) | HIV Uninfected (n = 221) | HIV Infected (n = 738) | HIV Uninfected (n = 386) | HIV Infected (n = 730) | |
| Female sex, No. (%) | 24 (41) | 78 (35) | 138 (62) | 371 (50) | 84 (22) | 247 (34) |
| Black race, No. (%) | 42 (72) | 137 (62) | 108 (49) | 316 (43) | 114 (30) | 272 (37) |
| Hispanic ethnicity, No. (%) | 14 (24) | 42 (19) | 55 (25) | 208 (28) | 26 (7) | 99 (14) |
| Height, mean (SD), cm | 156 (17) | 167 (14) | 168 (10) | 168 (10) | 174 (10) | 172 (10) |
| Weight, mean (SD), kg | 59 (29) | 68 (20) | 89 (24) | 80 (20) | 85 (17) | 80 (17) |
| SBP, mean (SD), mm Hg | 105 (12) | 111 (12) | 122 (17) | 118 (15) | 129 (16) | 125 (16) |
| Total cholesterol, mean (SD), mg/dL | 165 (35) | 151 (33) | 184 (40) | 174 (37) | 187 (37) | 182 (41) |
| HDL, mean (SD), mg/dL | 57 (16) | 44 (15) | 56 (17) | 47 (16) | 55 (17) | 50 (17) |
| Current use of hypertension medication, No. (%) | 0 (0) | 1 (<1) | 50 (23) | 139 (19) | 141 (37) | 314 (43) |
| Current use of lipid-lowering medication, No. (%) | 0 (0) | 1 (<1) | 22 (10) | 87 (12) | 122 (32) | 268 (37) |
| Current smoker, No. (%) | 0 (0) | 44 (20) | 81 (37) | 270 (37) | 96 (25) | 202 (28) |
| HCV infection, No. (%) | 0 (0) | 1 (<1) | 17 (8) | 78 (11) | 43 (11) | 182 (25) |
| Current use of HAART, No. (%) | 107 (48) | 413 (56) | 573 (78) | |||
| Suppressed HIV RNA level, No. (%)a | 23 (10) | 377 (51) | 557 (76) | |||
| Current CD4+ T-cell count, mean (SD), cells/µL | 485 (364) | 499 (292) | 579 (292) | |||
| History of AIDS, No. (%) | 149 (67) | 227 (31) | 228 (31) | |||
| CCA-IMT, mean (SD), µm | 597 (70) | 607 (75) | 725 (96) | 709 (115) | 803 (133) | 801 (145) |
| BIF-IMT, mean (SD), µm | 654 (104) | 671 (83) | 825 (141) | 802 (143) | 928 (165) | 900 (161) |
Abbreviations: BIF-IMT, carotid artery bifurcation intima-media thickness; CCA-IMT, common carotid artery intima-media thickness; HAART, highly active antiretroviral therapy; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; SBP, systolic blood pressure; SD, standard deviation.
a Defined as <80 copies/mL.
Pooled cIMT Estimates by HIV Serostatus and Sex
Compared with uninfected controls, HIV-infected participants had lower mean CCA-IMT (732 vs 756 µm) and lower mean BIF-IMT (825 vs 868 µm) in unadjusted analyses (Table 2). In multivariable-adjusted analyses, HIV-infected participants had a trend suggesting lower adjusted CCA-IMT (−8 µm; 95% confidence interval [CI], −17 to .75 µm) and significantly lower adjusted BIF-IMT (−16 µm; 95% CI, −27 to −4 µm) than uninfected controls. When we analyzed cIMT separately by sex, the HIV-infected group had lower cIMT values than the HIV-infected group among both men and women, although differences were significant only for BIF-IMT among men.
Table 2.
Association Between Human Immunodeficiency Virus (HIV) Serostatus, Current Highly Active Antiretroviral Therapy Use, HIV RNA Level, and Carotid Artery Intima-Media, Overall and by Sexa
| Outcome and Group | Male and Female Participants Combined |
Male Participants Only |
Female Participants Only |
|||
|---|---|---|---|---|---|---|
| cIMT, Mean (SD), µm | β (95% CI), µm | cIMT, Mean (SD), µm | β (95% CI), µm | cIMT, Mean (SD), µm | β (95% CI), µm | |
| CCA-IMT | ||||||
| HIV uninfected | 756 (132) | Reference | 767 (137) | Reference | 741 (123) | Reference |
| HIV infected, stratified by HAART use | 732 (140) | −8 (−17 to 1) | 734 (150) | −6 (−19 to 7) | 729 (126) | −8 (−20 to 4) |
| Receiving HAART | 753 (142) | 2 (−7 to 11) | 777 (153) | 8 (−6 to 21) | 726 (123) | −3 (−16 to 10) |
| Not receiving HAART | 696 (129) | −26 (−37 to −15) | 670 (121) | −33 (−50 to −17) | 734 (132) | −18 (−33 to −3) |
| HIV infected, stratified by HIV RNA level | ||||||
| Nonviremic (HIV RNA <80 copies/mL) | 764 (141) | −4 (−13 to 6) | 785 (151) | 4 (−10 to 18) | 739 (123) | −9 (−23 to 4) |
| Viremic (HIV RNA ≥80 copies/mL) | 693 (128) | −16 (−27 to −5) | 674 (125) | −23 (−40 to −6) | 717 (129) | −9 (−23 to 5) |
| BIF-IMT | ||||||
| HIV uninfected | 868 (174) | Reference | 897 (175) | Reference | 818 (160) | Reference |
| HIV infected, stratified by HAART use | 825 (164) | −16 (−27 to −4) | 840 (171) | −19 (−35 to −3) | 815 (361) | −3 (−20 to 15) |
| Receiving HAART | 846 (162) | −7 (−19 to 6) | 879 (163) | −11 (−27 to −6) | 779 (148) | 3 (−15 to 21) |
| Not receiving HAART | 785 (160) | −33 (−47 to −18) | 772 (162) | −40 (−61 to −19) | 807 (153) | −13 (−34 to −8) |
| HIV infected, stratified by HIV RNA level | ||||||
| Nonviremic (HIV RNA <80 copies/mL) | 861 (163) | −13 (−26 to −.4) | 890 (164) | −14 (−32 to 3) | 817 (151) | −3 (−23 to 16) |
| Viremic (HIV RNA ≥80 copies/mL) | 778 (152) | −21 (−36 to −6) | 775 (157) | −29 (−50 to −8) | 783 (146) | −5 (−25 to 15) |
Abbreviations: BIF-IMT, carotid artery bifurcation intima-media thickness; CCA-IMT, common carotid artery intima-media thickness; CI, confidence interval; cIMT, carotid artery intima-media thickness; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; SD, standard deviation.
a Mean cIMT values are unadjusted. β represents difference in cIMT, adjusted for age, race/ethnicity, smoking, antihypertensive medication use, lipid medication use, height, and weight.
We repeated analyses after stratifying HIV-infected individuals based on current HAART use. HIV-infected individuals not using HAART were more likely to have lower CD4+ T-cell counts (median, 351 and 433 cells/µL for men and women, respectively) than those using HAART (median, 580 and 544 cells/µL, respectively). Compared with HAART users, nonusers were much less likely to have suppressed HIV RNA levels (87% vs 7% for men and 68% vs 37% for women). Stratified analyses suggested that the lower cIMT values observed in HIV-infected participants was driven primarily by lower values in those not using HAART (Table 2). After adjustment for cardiometabolic risk factors, CCA-IMT in HIV-infected individuals not using HAART was 26 µm lower than in HIV-uninfected individuals (95% CI, −37 to −15 µm). In contrast, CCA-IMT in HIV-infected individuals using HAART did not differ significantly from that in uninfected individuals (adjusted difference, 2 µm; 95% CI, −7 to 11 µm). Similar associations were observed for BIF-IMT. These findings persisted in separate analyses of male and female participants.
Stratifying the HIV-infected population by presence of HIV viremia rather than by HAART use suggested that the findings of lower cIMT values relative to the HIV-uninfected group were limited to viremic individuals (Table 2). We also examined cumulative HAART use, current CD4+ T-cell count and history of AIDS with respect to CCA-IMT and BIF-IMT, none of which showed consistent associations (Supplementary Table 2). Leave-one-out analysis showed that differences between HIV-infected and HIV-uninfected groups persisted when excluding the HAHC, MACS, or WIHS cohort (Supplementary Figure 1). However, excluding either the A5260s cohort (all HAART naive) or the pediatric Miami cohort resulted in attenuation of the inverse association between HIV serostatus and cIMT.
Pooled cIMT Estimates by HIV Serostatus and Age
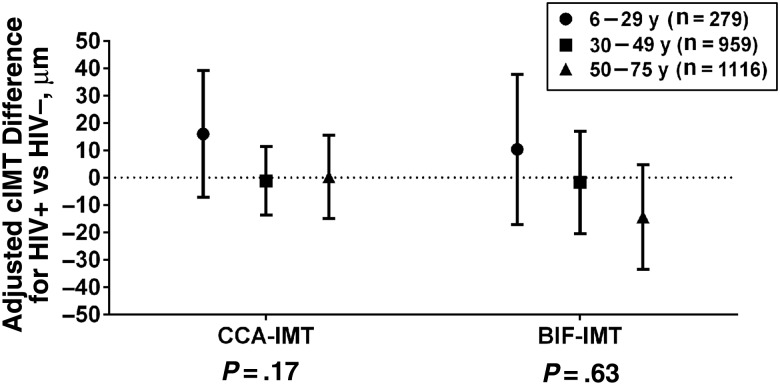
Age was positively correlated with cIMT among both HIV-infected individuals (r = 0.52 and 0.51 for CCA-IMT and BIF-IMT, respectively) and HIV-uninfected individuals (r = 0.51 and 0.52). In unadjusted models, we observed that age modified the association between HIV serostatus and CCA-IMT (interaction P = .03) but not BIF-IMT (interaction P = .57). Neither interaction was statistically significant after multivariable adjustment (Figure 1).
Figure 1.
Association between human immunodeficiency virus (HIV) serostatus and carotid artery intima-media thickness (cIMT) by age group, adjusted for sex, race/ethnicity, smoking, height, weight, and antihypertensive and lipid medication use. P values represent interaction between age group and HIV serostatus. Abbreviations: BIF-IMT, carotid artery bifurcation intima-media thickness; CCA-IMT, common carotid artery intima-media thickness.
Multivariable analyses were conducted within age groups. Among 1116 individuals in the oldest group (50–75 years), BIF-IMT values were lower in the HIV-infected than in the HIV-uninfected group (β, −14 µm; 95% CI, −34 to 5 µm; P = .14) (Figure 1). This difference was not apparent for CCA-IMT (β, 0.3 µm; 95% CI, −15 to 16 µm). Among 959 individuals aged 30–49 years, both CCA-IMT and BIF-IMT were similar in HIV-infected and HIV-uninfected individuals. In contrast, in the 6–29-year age group (n = 279), adjusted CCA-IMT and BIF-IMT suggested higher values in the HIV-infected than in the HIV-uninfected groups, although estimates had wide CIs. The adjusted β for CCA-IMT associated with HIV in the 6–29-year group was 16 µm (95% CI, −7 to 39 µm; P = .18). In sensitivity analyses that limited the youngest age group to those infected with HIV through perinatal transmission, the increase in CCA-IMT among the youngest HIV-infected individuals was strengthened (β, 21 µm; 95% CI, −3 to 46 µm; P = .08).
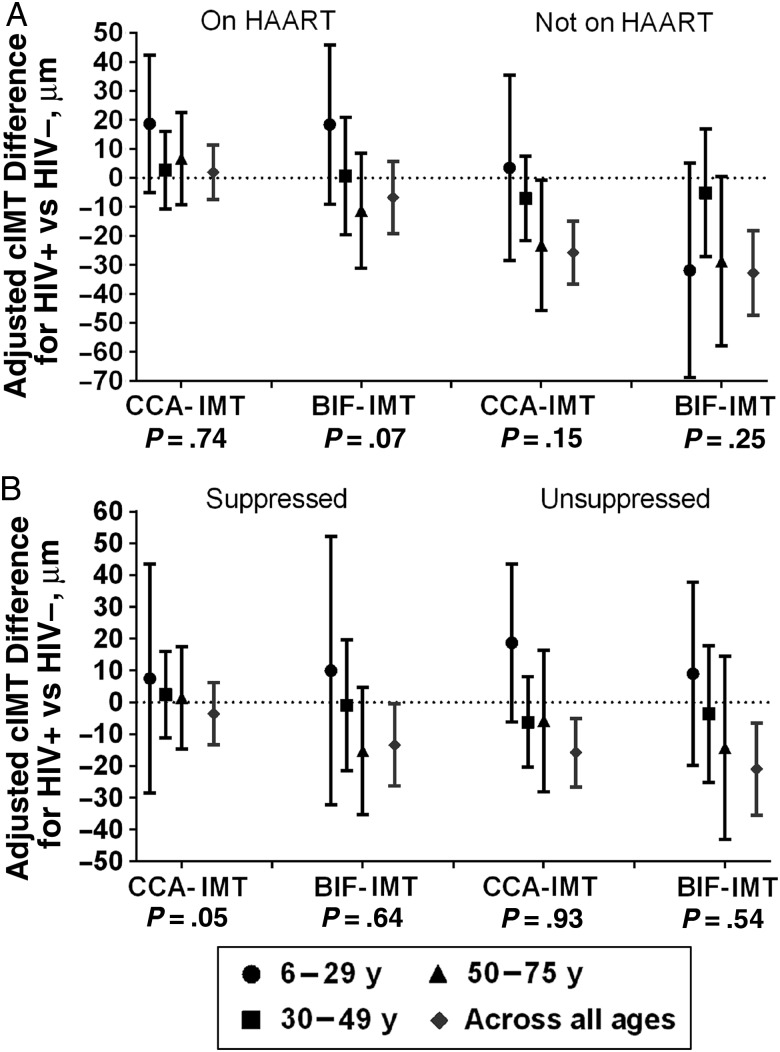
Because both HAART use and age modified the associations between HIV serostatus and cIMT, we cross-classified the study population by both characteristics simultaneously. Figure 2A shows age-specific results with the HIV-infected group stratified by HAART use. In the youngest age group (6–29 years), we found that HIV-infected participants using HAART had higher values for both CCA-IMT and BIF-IMT than HIV-uninfected comparison subjects. In contrast, among the oldest HIV-infected participants who were not using HAART (aged 50–75), both CCA-IMT and BIF-IMT were lower than in HIV-uninfected controls. Aside from these 2 subgroups, other subgroups of HIV-infected patients defined by HAART use and age did not have consistently higher or lower cIMT values than controls. In sensitivity analyses, unsuppressed HIV-infected participants had associations with CCA-IMT similar to those among those not receiving HAART (Figure 2B). In addition, a history of AIDS was associated with higher cIMT values in the 6–29-year age group but not in older groups (interaction with age, P < .001 for CCA-IMT and P = .003 for BIF-IMT) (Supplementary Table 3).
Figure 2.
Association between human immunodeficiency virus (HIV) serostatus and carotid artery intima-media thickness (cIMT), within subgroups defined by age and current use of highly active antiretroviral therapy (HAART) (A) or current virologic suppression (B), adjusted for sex, race/ethnicity, smoking, height, weight, and antihypertensive and lipid medication use. P values for interaction between age group and HIV serostatus are provided beneath the x-axes. Abbreviations: BIF-IMT, carotid artery bifurcation intima-media thickness; CCA-IMT, common carotid artery intima-media thickness.
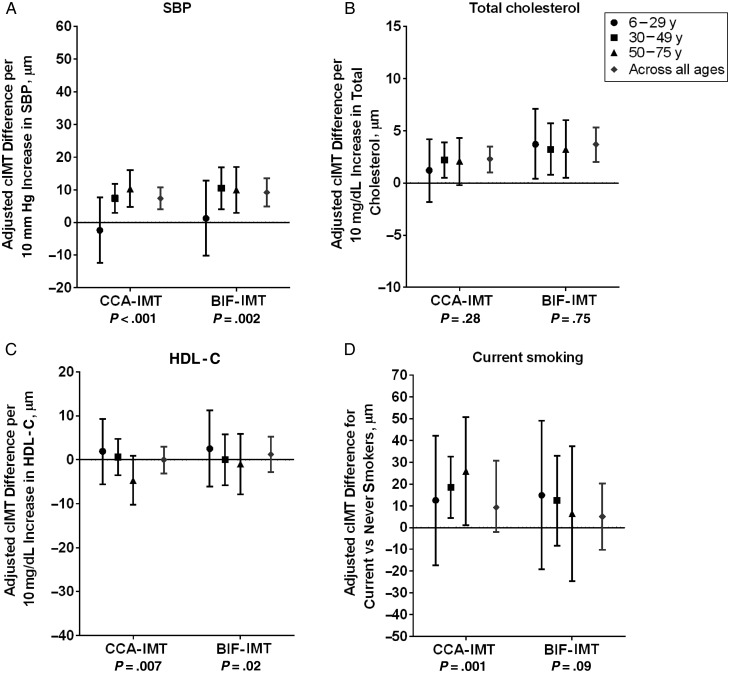
Associations Between Traditional and Nontraditional CVD Risk Factors and cIMT by Age Among HIV-infected Participants
Among HIV-infected individuals, higher SBP was significantly associated with both higher CCA-IMT and higher BIF-IMT values (Figure 3A). The effects of SBP on cIMT were limited to the middle-aged to older adult population and were absent in the 6–29-year age group (interaction with age, P < .001 for CCA-IMT and P = .002 for BIF-IMT). Higher total cholesterol levels were associated with greater CCA-IMT and BIF-IMT, with no variation by age in the strength of associations (Figure 3B). Neither HDL cholesterol nor current smoking had an overall significant association with cIMT, but for both variables we observed significant effect modification by age. The oldest HIV-infected individuals had the strongest evidence of a favorable effect of high HDL cholesterol (Figure 3C) and an adverse effect of smoking (Figure 3D) on cIMT.
Figure 3.
Association between traditional cardiovascular risk factors and carotid artery intima-media thickness (cIMT) in human immunodeficiency virus–infected participants by age group, adjusted for sex, race/ethnicity, height, weight, and antihypertensive and lipid medication use. Risk factors include systolic blood pressure (SBP) (A), total cholesterol (B), high-density lipoprotein cholesterol (HDL-C) (C), and current smoking (D). P values for interaction between age group and risk factors are provided beneath the x-axes. Abbreviations: BIF-IMT, carotid artery bifurcation intima-media thickness; CCA-IMT, common carotid artery intima-media thickness.
HIV-infected individuals had higher levels of hsCRP, D-dimer, IL-6, and fibrinogen than uninfected individuals, with results for hsCRP and IL-6 achieving statistical significance (P = .01 and P = .04, respectively, Supplementary Table 4). Supplementary Figure 2A–2D show associations of cIMT with levels of hsCRP, D-dimer, IL-6, and fibrinogen among HIV-infected participants by current HAART use, both overall and by age group. Inflammation markers did not have a consistent predicted association with higher cIMT values in any age or HAART treatment group (Supplementary Figure 3). Additional details of associations between inflammation markers and cIMT are in the Supplementary Material.
DISCUSSION
In our pooled analysis of >2000 HIV-infected and uninfected individuals studied with an identically standardized arterial US protocol, the association of HIV with carotid artery wall thickness was age dependent. HIV-infected children and young adults, compared with uninfected controls, had greater carotid artery wall thickness after controlling for demographic and cardiovascular risk-related variables. Among individuals aged 30–49 years, HIV infection had no association with cIMT, and in the oldest age group (50–75 years) HIV infection seemed to be associated with lower cIMT values than in HIV-uninfected controls. The effect of traditional CVD risk factors on cIMT also differed between younger and older HIV-infected persons. Known correlates of higher cIMT values, including higher SBP, lower HDL cholesterol, and smoking were strongly associated with cIMT in older HIV-infected individuals, but had weak associations with cIMT among younger individuals. Collectively, this suggests that whereas HIV infection itself may play an important role in increased carotid artery thickening among adolescents and young adults, among middle-aged and older HIV-infected individuals the cumulative effects of traditional CVD risk factors may outweigh HIV-specific mechanisms of atherosclerotic risk.
Although prior studies have addressed the association of HIV infection with cIMT, to our knowledge ours has the largest sample size of HIV-infected individuals who all received a common, standardized protocol across multiple contributing studies, with a wide range of ages and confounder control based on the same covariates. We also obtained measurements of both the common carotid artery and the carotid artery bifurcation to provide confirmatory evidence of associations across 2 carotid artery segments. The participating cohorts make up a wide cross-section of the HIV-infected population in the United States, with substantial diversity in age, distribution of CVD risk factors, and ART history, allowing for a broader generalizability of our inferences than what can be inferred from single-site studies [15]. Finally, all studies were performed within the last 6 years, providing information on the role of contemporary HIV treatment in subclinical atherosclerosis.
Our study has some limitations. First, only cross-sectional data were available, and therefore causal associations cannot be established. Future studies examining age should assess longitudinal data to better distinguish age and time effects. Also, the children contributing to this study originated from a single study site. However, data on children with HIV in the United States, are rare, and therefore their inclusion here is an important contribution. Our use of cIMT as a marker of subclinical atherosclerosis implies that greater thickness leads to clinical CVD; although this has been demonstrated in non–HIV-infected populations [6], it has not been confirmed in the HIV-infected population. Although we controlled for major known confounders to the relationship of age and HIV with atherosclerosis, residual confounding may explain some of the remaining differences. Finally, carotid artery wall thickening describes only one aspect of arterial injury. Other studies have found that though cIMT may not differ by HIV status, atherosclerotic plaque, which may have different underlying mechanisms than those leading to wall thickening [23], may be increased in individuals with HIV infection [19].
We identified an HIV-infected group with lower cIMT values than controls, characterized by being relatively older (>50 years) and untreated with HAART and having high circulating HIV RNA levels and low CD4+ T-cell counts. We speculate that these individuals may represent a survivor cohort that may have reduced predisposition to atherosclerotic disease because of favorable health behaviors or other characteristics that have enabled their long-term survival with HIV infection. The time of seroconversion is unknown for most participants, unfortunately, making it difficult to test this supposition. Another potential explanation may be related to effects of chronic disease processes on atherosclerosis that have been known for >50 years. Autopsy studies have found that patients with cancer have fewer coronary artery lesions [24, 25]. The etiology of this effect remains unknown but may be related to cachexia or tumor necrosis factor α levels [26]. Furthermore, lower cIMT values, despite overall increased risk of atherosclerotic CVD, has been observed among individuals with rheumatoid arthritis [27], which like HIV infection may be associated with decades of exposure to inflammation. Thus, our finding of lower cIMT values among older, untreated HIV-infected participants may represent a previously unrecognized scenario whereby long-standing HIV infection may reduce arterial wall thickness. Although this scenario is not likely to become commonplace with the current standard of universal ART use, additional studies may be able to shed further light on this potential mechanism suggested by our preliminary findings.
We explored well-known associations between traditional CVD risk factors and cIMT to examine whether these associations are also present across a wide age range of HIV-infected individuals. Our study confirms known CVD risk factor associations, most prominently with respect to SBP and total cholesterol [8, 15, 28, 29]. Moreover, we found that the associations of SBP and smoking with cIMT increase with age, which has been suggested in other HIV-infected populations [30]. These findings are pertinent in considering potential interventions to reduce CVD in the aging HIV-infected population; [5] important factors should include the high prevalence of smoking and increasing prevalence of hypertension [1, 31].
Prior research suggests that the association between HIV infection, HAART, and levels of inflammation and hemostasis biomarkers is complex [32–35]. In our analysis, we found higher levels of each of the markers we examined among HIV-infected participants as a whole as compared with HIV-uninfected individuals, although differences were relatively small and only statistically significant for hsCRP and IL-6. However, we found little evidence that these biomarkers explained the associations between HIV serostatus and cIMT. Our biomarker findings should be interpreted cautiously because we were limited to a single measure of vascular disease, lacked information on major health events, and were not able to include other markers of inflammation or immune activation that have been associated with CVD among HIV-infected individuals [36–39].
In summary, results from our large pooled study suggest that the effects of HIV infection on carotid artery thickening may differ by age. The cumulative effects of traditional determinants of CVD burden, such as blood pressure, lipids, and smoking, seem to play a larger role in older adults than in young adults or children. Longitudinal studies that can examine the influences of HIV and traditional CVD risk factors across the lifespan will be important to further elucidate these mechanisms.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Financial support. This work was supported by the National Institutes of Health (NIH; grant R21-HL-120394 with additional funding from grants R01-HL-083760, R01-HL-095140, R01-HL-126543, R01-HL-132794, U01-AI-035004, U01-AI-031834, U01-AI-034993, U01-AI-034994, U01-AI-034989, U01-AI-042590, U01-HD-032632, UL1-TR-000004, U01-AI-35042, P30-AI-051519, R01-HL-095132, R01-HL-095129, U01-AI-35039, U01-AI-35040, U01-AI-35041, UM1-AI-35043, UL1-TR-001079, R01-HL-095127, R01-HL-095135, R01-HL-095136). Partial funding for laboratory and imaging work and study coordination was provided by the University of Washington's cardiovascular disease and Metabolic Complications of human immunodeficiency virus/AIDS Data Coordinating Center through the NIH (grant R01-HL-095126).
Potential conflicts of interest. J. H. S. reports intellectual property/royalties from the Wisconsin Alumni Research Foundation for a patent related to carotid US and “vascular age,” a research grant from Gilead to the University of Wisconsin for being a core US reading laboratory for an antiretroviral therapy study, and service on a data safety monitoring board for Eli Lilly. K. A. reports advisory board membership for Bristol-Myers Squibb. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mdodo R, Frazier EL, Dube SR et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015; 162:335–44. [DOI] [PubMed] [Google Scholar]
- 2.Friis-Moller N, Reiss P, Sabin CA et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–35. [DOI] [PubMed] [Google Scholar]
- 3.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 2009; 51:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramanian S, Tawakol A, Burdo TH et al. Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks JT, Buchacz K, Gebo KA, Mermin J. HIV infection and older Americans: the public health perspective. Am J Public Health 2012; 102:1516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodis HN, Mack WJ, LaBree L et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med 1998; 128:262–9. [DOI] [PubMed] [Google Scholar]
- 7.Grunfeld C, Delaney JA, Wanke C et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS 2009; 23:1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsue PY, Ordovas K, Lee T et al. Carotid intima-media thickness among human immunodeficiency virus-infected patients without coronary calcium. Am J Cardiol 2012; 109:742–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sainz T, Alvarez-Fuente M, Navarro ML et al. Subclinical atherosclerosis and markers of immune activation in HIV-infected children and adolescents: the CaroVIH Study. J Acquir Immune Defic Syndr 2014; 65:42–9. [DOI] [PubMed] [Google Scholar]
- 10.Johnsen S, Dolan SE, Fitch KV et al. Carotid intimal medial thickness in human immunodeficiency virus-infected women: effects of protease inhibitor use, cardiac risk factors, and the metabolic syndrome. J Clin Endocrinol Metab 2006; 91:4916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hakala SM, Tilvis RS. Determinants and significance of declining blood pressure in old age. A prospective birth cohort study. Eur Heart J 1998; 19:1872–8. [DOI] [PubMed] [Google Scholar]
- 12.Manolio TA, Cushman M, Gottdiener JS, Dobs A, Kuller LH, Kronmal RA. Predictors of falling cholesterol levels in older adults: the Cardiovascular Health Study. Ann Epidemiol 2004; 14:325–31. [DOI] [PubMed] [Google Scholar]
- 13.Stein JH, Hsue PY. Inflammation, immune activation, and CVD risk in individuals with HIV infection. JAMA 2012; 308:405–6. [DOI] [PubMed] [Google Scholar]
- 14.Miller TL, Somarriba G, Orav EJ et al. Biomarkers of vascular dysfunction in children infected with human immunodeficiency virus-1. J Acquir Immune Defic Syndr 2010; 55:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein JH, Brown TT, Ribaudo HJ et al. Ultrasonographic measures of cardiovascular disease risk in antiretroviral treatment-naive individuals with HIV infection. AIDS 2013; 27:929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valcour V, Shikuma C, Shiramizu B et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology 2004; 63:822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126:310–8. [DOI] [PubMed] [Google Scholar]
- 18.Post WS, Budoff M, Kingsley L et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 2014; 160:458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanna DB, Post WS, Deal JA et al. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis 2015; 61:640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barkan SE, Melnick SL, Preston-Martin S et al. The Women's Interagency HIV Study. Epidemiology 1998; 9:117–25. [PubMed] [Google Scholar]
- 21.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis 2001; 154:185–93. [DOI] [PubMed] [Google Scholar]
- 22.Touboul PJ, Hennerici MG, Meairs S et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). Cerebrovasc Dis 2012; 34:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging 2014; 7:1025–38. [DOI] [PubMed] [Google Scholar]
- 24.Wanscher O, Clemmesen J, Nielsen A. Negative correlation between atherosclerosis and carcinoma. Br J Cancer 1951; 5:172–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juhl S. Cancer and atherosclerosis: negative correlation. Acta Pathol Microbiol Scand 1955; 37:167–81. [PubMed] [Google Scholar]
- 26.Walsmith J, Roubenoff R. Cachexia in rheumatoid arthritis. Int J Cardiol 2002; 85:89–99. [DOI] [PubMed] [Google Scholar]
- 27.Roman MJ, Moeller E, Davis A et al. Preclinical carotid atherosclerosis in patients with rheumatoid arthritis. Ann Intern Med 2006; 144:249–56. [DOI] [PubMed] [Google Scholar]
- 28.Delaney JA, Scherzer R, Biggs ML et al. Associations of antiretroviral drug use and HIV-specific risk factors with carotid intima-media thickness. AIDS 2010; 24:2201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volpe GE, Tang AM, Polak JF, Mangili A, Skinner SC, Wanke CA. Progression of carotid intima-media thickness and coronary artery calcium over 6 years in an HIV-infected cohort. J Acquir Immune Defic Syndr 2013; 64:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitch KV, Looby SE, Rope A et al. Effects of aging and smoking on carotid intima-media thickness in HIV-infection. AIDS 2013; 27:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong C, Gange SJ, Horberg MA et al. Age-related morbidities among HIV-infected adults from 2000–2010 [abstract 1053]. Conference on Retroviruses and Opportunistic Infections Seattle, WA, 2015. Available at: http://www.croiconference.org/sessions/age-related-morbidities-among-hiv-infected-adults-2000-2010. Accessed 6 May 2016. [Google Scholar]
- 32.Madden E, Lee G, Kotler DP et al. Association of antiretroviral therapy with fibrinogen levels in HIV-infection. AIDS 2008; 22:707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neuhaus J, Jacobs DR Jr, Baker JV et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010; 201:1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palella FJ Jr, Gange SJ, Benning L et al. Inflammatory biomarkers and abacavir use in the Women's Interagency HIV Study and the Multicenter AIDS Cohort Study. AIDS 2010; 24:1657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada NI, Jacobson LP, Margolick JB et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012; 206:1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaked I, Hanna DB, Gleissner C et al. Macrophage inflammatory markers are associated with subclinical carotid artery disease in women with human immunodeficiency virus or hepatitis C virus infection. Arterioscler Thromb Vasc Biol 2014; 34:1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKibben RA, Margolick JB, Grinspoon S et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis 2015; 211:1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplan RC, Sinclair E, Landay AL et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis 2011; 203:452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.