In this cross-sectional substudy of a large randomized controlled clinical trial, monotherapy with protease inhibitor showed no excess risk of cognitive impairment compared to standard combination antiretroviral therapy in stable human immunodeficiency virus-positive patients. We observed no difference between arms on neuroimaging markers either.
Keywords: neurocognitive function, HIV, neuroimaging, monotherapy, protease inhibitor
Abstract
Background. To determine whether treatment with ritonavir-boosted protease inhibitor (PI) monotherapy is associated with detrimental effects on neurocognitive function or brain imaging markers compared to standard antiretroviral therapy (ART).
Methods. Neuropsychological assessment and brain magnetic resonance imaging were performed at the last study visit in a subset of participants randomized to PI monotherapy (PI-mono group) or ongoing triple ART (OT group) in the PIVOT trial. We calculated a global z-score (NPZ-7) from the average of the individual test z-scores and the proportion of participants with symptomatic neurocognitive impairment (score >1 standard deviation below normative means in ≥2 cognitive domains and neurocognitive symptoms). In a subgroup, white matter hyperintensities, bicaudate index, global cortical (GCA) and medial temporal lobe atrophy scores and single voxel (basal ganglia) N-acetylaspartate (NAA)/Choline, NAA/Creatine and myo-inositol/Creatine ratios were measured.
Results. 146 participants (75 PI-mono) had neurocognitive testing (median time after randomization 3.8 years), of whom 78 were imaged. We found no difference between arms in NPZ-7 score (median −0.4 (interquartile range [IQR] = −0.7; 0.1) vs −0.3 (IQR = −0.7; 0.3) for the PI-mono and OT groups respectively, P = .28), the proportion with symptomatic neurocognitive impairment (13% and 18% in the PI-mono and OT groups respectively; P = .41), or any of the neuroimaging variables (P > .05). Symptomatic neurocognitive impairment was associated with higher GCA score (OR = 6.2 per additional score; 95% confidence interval, 1.7–22.3 P = .005) but no other imaging variables.
Conclusions. Based on a comprehensive neuropsychological assessment and brain imaging, PI monotherapy does not increase the risk of neurocognitive impairment in stable human immunodeficiency virus-positive patients.
(See the Editorial Commentary by Carvalhal on pages 265–7.)
Neurocognitive impairment (NCI) is frequently reported in patients infected with human immunodeficiency virus (HIV), with prevalence figures ranging between 40% and 60%, even after prolonged and effective viral suppression with combination antiretroviral therapy (cART) [1–4]. It has been suggested that different treatment strategies may have differential effect on viral replication in the central nervous system (CNS); therefore, some regimens may be less effective in preventing the development or progression of NCI [5–7].
Ritonavir-boosted protease inhibitor (PI) monotherapy has been explored as a simplification strategy in effectively suppressed, ART-experienced patients in a number of randomized controlled trials (RCT) [8]. Given that PI monotherapy includes only 1 active drug compared to 3 in standard cART regimens, the possibility of persistent viral replication within the CNS that could lead to progression of neurological complications, including NCI, has been expressed as a concern with this approach [9]. Although the PIVOT trial found no evidence of accelerated neurocognitive function decline in participants on PI monotherapy compared to cART over 3–5 years of follow-up, this was based on a testing with simple battery of neuropsychological tests designed to be suitable for repeated use in large numbers of participants [10].
The aim of this substudy was to look in more detail for evidence of neurocognitive impairment or neuroimaging abnormalities in patients taking PI monotherapy compared to patients on standard cART using a more comprehensive neuropsychological testing battery and brain magnetic resonance imaging/spectroscopy (MRI/MRS).
METHODS
PIVOT was a noninferiority, randomized parallel-group trial (ISRCTN-04857074), conducted in 43 sites in the United Kingdom between 2008 and 2013, where 587 effectively suppressed (viral load [VL] <50 copies/mL) HIV-positive adults on cART (2 nucleoside reverse transcriptase inhibitors [NRTIs] and 1 non-NRTI [NNRTI] or PI) were randomly assigned 1:1 to maintain ongoing triple therapy (OT) or switched to PI-mono. All licensed PIs were allowed, but ritonavir-boosted darunavir (DRV/r) 800 mg/100 mg once daily or ritonavir-boosted lopinavir (LPV/r) 400 mg/100 mg twice daily were recommended. The primary outcome was loss of future drug options, defined as new intermediate/high level resistance to drugs in contemporary use to which the patient's virus was considered to be sensitive at trial entry. Neurocognitive function was assessed in all study participants with a brief neuropsychological testing battery at baseline, week 12, and annually thereafter until the end of the study period.
This substudy was done at 5 of the larger sites of the PIVOT trial and offered to all participants attending the final PIVOT study visit. In addition to the standard PIVOT neurocognitive testing battery (comprising the Hopkins Verbal Learning Test-Revised [11], color trail tests 1 and 2 [CTT-1 and CTT-2] [12] and the grooved pegboard test [GPT] [13]), participants underwent additional tests: Rey complex figure test [14], Stroop color and word test (SCWT) [15], finger tapping test (FTT) [16], and the WAIS-III digit symbol-coding test [17] to ascertain function on the attention/psychomotor speed, executive functioning, fine motor skills and verbal and nonverbal learning and memory cognitive domains.
The presence of cognitive symptoms was assessed from responses to the relevant questions (attention, concentration, memory, problem solving or decision making within the past 4 weeks) on the MOS-HIV questionnaire performed at the last study visit and participants were considered symptomatic if responded “a good bit of the time” or more often to any of the questions [18]. In addition, participants were asked about alcohol consumption (AUDIT questionnaire [19]), recreational drug use, and self-reported anxiety / depression (responses to the relevant question on EQ-5D [20]).
Neuroimaging Investigations
Multimodal MRI/MRS of the brain was performed using a 3 Tesla Philips Achieva System (Best, the Netherlands) at the Institute of Neurology, UCL in a single scan session. Seven predefined measures were obtained from the scans: (1) total volume of white matter hyperintensities, calculated from hand-drawn regions of interest on a high-resolution 3D fluid-attenuated inversion recovery sequence [21]; (2) N-acetylaspartate to choline ratio (NAA/Cho); (3) N-acetylaspartate to creatine ratio (NAA/Cr); and (4) Myo-inositol to creatine ratio of the left basal ganglia, as found using the MRS data of a single voxel [22]. Finally, (5) global cortical atrophy score (GCA) [23], (6) medial temporal lobe atrophy score [23], and the (7) Bicaudate index, defined as the ratio of width of both lateral ventricles at the level of the caudate nucleus to the distance between outer tables of skull at the same level [24]. Scans were analyzed by individuals who were blinded to the participants' treatment allocation.
Sample Size Calculation
The hypothesis was that PI monotherapy is not inferior to cART on the NPZ-7 score. We assumed no difference in mean NPZ-7 between the two randomization arms, and that a difference of ≤0.4 between arms could be regarded as noninferior. A sample size was calculated to give 80% power to exclude a difference of greater than 0.4 between the arms (2-sided α = 0.05) (assuming a standard deviation of 0.8, based on the variation of the NPZ-5 score in the main PIVOT trial at baseline) [25]. The sample size for MRI scans was limited due to feasibility and funding constraints.
Statistical Analysis
Raw scores for each cognitive test were transformed to z-scores using the manufacturers' normative data adjusted for age (all tests) and years of education (CTT, SCWT). For the GPT and FTT, the z-scores for the dominant and non-dominant hands were averaged. The SCWT was scored as average of word, color, and color-word subtests. Cognitive domain z-scores were calculated by averaging the scores of the relevant tests when appropriate. NPZ-7 scores were then calculated by averaging all 7 cognitive domains. For all individual test z-scores and the NPZ-7, values below zero denote below-average neurocognitive function compared to the reference population. For the purposes of group comparisons, we defined neurocognitive impairment as a z-score <−1 of the normative mean in at least two cognitive domains (similar to the Frascati definition of NCI) [26]. Symptomatic neurocognitive impairment was defined as neurocognitive impairment with reported symptoms (an answer of “a good bit of the time” or worse to any of the four components of question 10 (assessing problem solving and decision-making, memory, attention, and concentration) of the MOS-HIV QoL questionnaire) [27]. A sensitivity analysis was performed assuming verbal and nonverbal learning and verbal and nonverbal memory are expression of the same cognitive domains resulting in a sub-study NPZ-5 with 2 tests for each domain.
Primary analyses were according to intention to treat. Additional sensitivity analyses were performed based on actual treatment taken. Proportions were compared using χ2 or Fisher exact tests as appropriate. Continuous test scores were compared using t-test or Mann–Whitney rank tests. Multivariable logistic, ordered logistic and linear regression models were used to examine associations with neurocognitive impairment and the following variables: gender, age, ethnicity, years of education, nadir and current CD4+ T-cell count, time known HIV-positive, current and past smoking, and use of recreational drugs and alcohol. Correlations between individual test z-scores within each cognitive domain were explored using Pearson coefficient. Moderate correlation was defined based on coefficients ranging from 0.4 to 0.6 whereas coefficients >0.6 were considered evidence of strong correlations.
The main PIVOT protocol and this substudy protocol were approved by the Cambridgeshire 4 Research Ethics Committee and all relevant R&D offices. All participants provided written informed consent.
RESULTS
Study Population
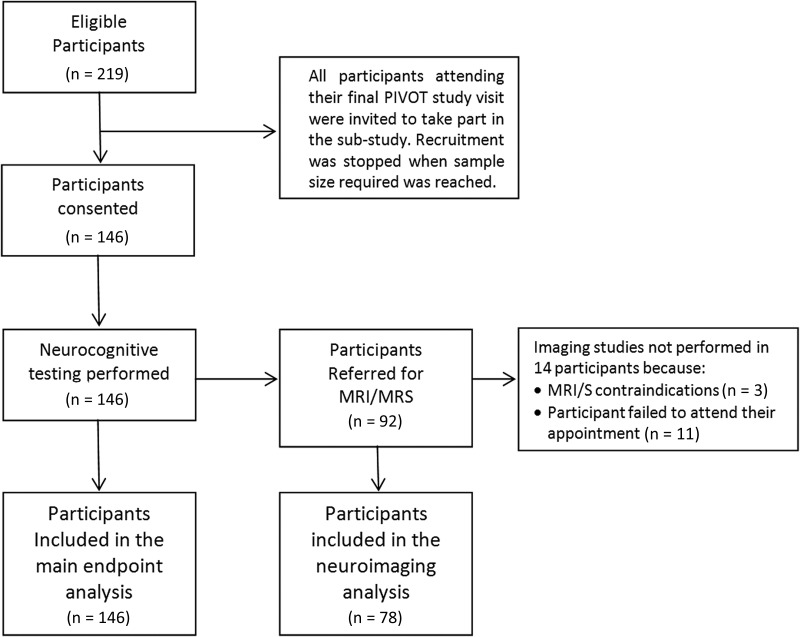
Of 219 PIVOT participants who attended their final trial visit (median 3.8 years from randomization) at the 5 participating sites, 146 (67%) (75 PI-mono, 71 OT) were enrolled in the substudy (Figure 1). Enrolled participants were older and more commonly had VL suppression (<50 copies/mL) than those who were not enrolled at the sites (Supplementary Table 1).
Figure 1.
Participant distribution. Abbreviations: MRI/MRS, magnetic resonance imaging/spectroscopy.
Substudy participants were mainly white men, with a median of 15 years formal education, and substantial rates of self-reported anxiety / depression (32%), smoking (24%), risky alcohol consumption (36%), and current recreational drug use (31%) (Table 1, Supplementary Table 2a and 2b). Participants in the PI-mono group were older (mean 49 vs 46 years; P = .022), and fewer were cigarette smokers (15% vs 34%; P = .01) compared to those in the OT group (Table 1). At the time of the substudy visit, 49 (65%) of those in the PI-mono group were taking PI-monotherapy (41 DRV/r, 6 LPV/r, 2 atazanavir (ATV/r)) and 4 in the PI-group and 12 in the OT group were taking efavirenz.
Table 1.
Characteristics of Substudy Participants by Randomization Arm
| PI-mono (N = 75) | OT (N = 71) | P Value | Overall (N = 146) | |
|---|---|---|---|---|
| Age, Mean (SD) | 49 (9) | 46 (8) | .02 | 48 (9) |
| Sex, N (%) | ||||
| Male | 63 (84.0) | 63 (88.7) | .41 | 126 (86.3) |
| Ethnicity, N (%) | .41 | |||
| White | 58 (77.3) | 61 (85.9) | 119 (81.5) | |
| Black | 14 (18.7) | 8 (11.3) | 22 (15.1) | |
| Other | 3 (4.0) | 2 (2.8) | 5 (3.4) | |
| Years of formal education, Medi (IQR) | 15 (12–18) | 15 (13–18) | .53 | 15 (12–18) |
| CD4 cell count nadir, Median (IQR) | 170 (90–250) | 191 (100–269) | .41 | 180 (90–260) |
| CD4 cell count at entry, Median (IQR) | 621 (467–760) | 650 (540–830) | .27 | 640 (483–785) |
| HIV-RNA <50 copies/mL, N (%) | 72 (96.0) | 67 (95.7) | 1.00 | 139 (95.9) |
| Risky alcohol consumption, N (%)a | 22 (29.3) | 30 (42.9) | .09 | 52 (35.9) |
| Recreational drugs use, N (%) | .54 | |||
| In the past | 26 (35.1) | 30 (44.1) | 56 (39.4) | |
| Currently | 25 (33.8) | 19 (27.9) | 44 (31.0) | |
| Smokers at substudy visit, N (%) | 11 (14.7) | 24 (33.8) | .01 | 35 (24.0) |
| Depression/anxietyb | .63 | |||
| Moderate | 24 (34.8) | 20 (29.4) | 44 (32.1) | |
| Severe | 2 (2.9) | 4 (5.9) | 6 (4.4) | |
| Neurocognitive symptoms c | 17 (23.0) | 16 (22.5) | .95 | 17 (23.0) |
| ART exposure at substudy visit | <.001 | |||
| 2NRTI + 1NNRTI | 9 (12.0) | 22 (31.0) | 31 (21.2) | |
| 2NRTI + 1PI | 15 (20.0) | 40 (56.3) | 55 (37.7) | |
| PI monotherapy | 49 (65.3) | 5 (7.0) | 54 (37.0) | |
| Other ART combination | 2 (2.7) | 3 (4.2) | 5 (3.4) | |
| Off ART | 0 (0.0) | 1 (1.4) | 1 (0.7) |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; OT, ongoing triple therapy; PI, protease inhibitor; SD, standard deviation.
a Based on AUDIT questionnaire score.
b Based on EQ-5D Health Status questionnaire.
c Based on MOS-HIV QoL questionnaire.
Neurocognitive Function
We found no difference between the groups in the median z-score on any individual test or cognitive domain (Table 2). There was also no difference between study groups in summary NPZ-7 score (−0.4 in the PI-mono group vs −0.3 in the OT group; P = .25; difference 0.14 (95% CI, −.10 to .39); noninferiority criterion formally met). Furthermore, we found no difference between groups when average z-score on the PIVOT short battery (NPZ-5; P = .31) or the additional 4 substudy tests (P = .20) were compared separately. There also was no relationship between randomized arm and NPZ-7 score in multivariable regression analyses; the only independent associations with the lower NPZ-7 score were older age and black ethnicity (Table 3). The results were unchanged in sensitivity analyses based on treatment taken at the time of the substudy, or using substudy NPZ-5 as outcome instead of NPZ-7.
Table 2.
Neurocognitive Function and Impairment by Study Arm
| PI-mono | OT | P Valuea | Overall | |
|---|---|---|---|---|
| Attention/concentration | ||||
| Color trails test- Part 1b | 0.3 (−0.3, 0.9) | 0.5 (−0.2, 0.9) | .86 | 0.4 (0.2, 0.9) |
| Symbol-digit test | −0.7 (−1.0, 0.3) | 0.0 (−0.7, 0.7) | .10 | −0.3 (−1.0, 0.3) |
| Executive functioning | ||||
| Color trails test- Part 2b | 0.8 (0.2, 1.3) | 0.9 (0.5, 1.3) | .84 | 0.9 (0.3, 1.3) |
| Stroop color-word test | −0.7 (−1.6, 0.2) | −0.3 (−1.0, 0.4) | .16 | −0.5 (−1.2, 0.3) |
| Fine motor skills | ||||
| Grooved pegboard test: both handsb | −0.1 (−0.8, 0.6) | 0.1 (−0.9, 0.6) | .25 | 0.0 (−0.9, 0.6) |
| Finger tapping: both hands | −1.8 (−2.5, −0.8) | −1.6 (−2.2, −0.8) | .86 | −1.8 (−2.5, −0.8) |
| Verbal learning | ||||
| Hopkins verbal learning test (Revised)b | −0.4 (−1.2, 0.1) | −0.1 (−1.0, 0.7) | .45 | −0.3 (−1.2, 0.4) |
| Verbal memory | ||||
| Hopkins verbal learning test (Revised)b | 0.0 (−1.1, 0.8) | −0.1 (−1.0, 0.9) | .33 | −0.1 (−1.0, 0.9) |
| Nonverbal learning | ||||
| Rey complex figure test | −0.4 (−0.8, 0.3) | −0.2 (−1.4, 0.7) | .69 | −0.4 (−1.2, 0.6) |
| Nonverbal memory | ||||
| Rey complex figure test | −0.4 (−1.1, 0.2) | −0.5 (−1.5, 0.7) | .98 | −0.4 (−1.3, 0.5) |
| Summary z-scores | ||||
| PIVOT summary Z-score (NPZ-5)b | 0.1 (−0.5, 0.6) | 0.2 (−0.3, 0.6) | .31 | 0.1 (−0.4, 0.6) |
| Substudy summary Z-score (NPZ-7) | −0.4 (−0.7, 0.1) | −0.3 (−0.7, 0.3) | .25 | −0.3 (−0.7, 0.2) |
| Substudy summary Z-score (sNPZ-5)c | −0.3 (−0.8, 0.0) | −0.3 (−0.7, 0.3) | .23 | −0.3 (−0.7, 0.2) |
| Neurocognitive impairmentd, N (%) | ||||
| Symptomatic | 10 (13.3) | 13 (18.3) | .41 | 23 (15.8) |
| Overall | 34 (45.3) | 35 (49.3) | .63 | 69 (47.3) |
Results presented as z-scores median interquartile range unless otherwise stated.
Abbreviations: OT, ongoing triple therapy; PI, protease inhibitor.
a P-values from t-test or χ2-test.
b PIVOT neuropsychological testing battery.
c Substudy testing battery: Considering verbal and nonverbal learning and verbal and nonverbal memory measures of the same domains.
d Neurocognitive impairment: defined as z-score <−1 in ≥2 cognitive domains.
Table 3.
Factors Associated with Global Neurocognitive Score, Defined as the Average z-Score Across Seven Cognitive Domains: Linear Regression Models
| Coef. | 95% CI | P Value | |
|---|---|---|---|
| PI-mono | −0.00 | −.24, .23 | .989 |
| Age (per 10 additional years) | −0.16 | −.31, −.02 | .024 |
| Female gender | −0.19 | −.65, .28 | .421 |
| Ethnicity (black) | −0.71 | −1.17, −.25 | .003 |
| Education (per additional year) | 0.02 | −.02, .06 | .409 |
| Risky alcohol consumptiona | 0.20 | −.04, .45 | .102 |
| Recreational drugs use | |||
| In the past | −0.14 | −.45, .17 | .372 |
| Current use | 0.08 | −.25, .41 | .649 |
| Smoking | |||
| In the past | 0.04 | −.23, .32 | .751 |
| Current use | −0.05 | −.37, .27 | .758 |
Bolded text indicates the significant result.
Abbreviations: CI, confidence interval; PI, protease inhibitor.
a AUDIT questionnaire score: Hazardous or harmful consumption and likely dependency.
Moderate correlation was observed between tests measuring the same cognitive domain, except in the case of tests measuring fine motor skills. Similarly, there was strong correlation between tests measuring verbal learning and memory and nonverbal learning and memory (Supplementary Table 3).
Considering the whole substudy population, there were no differences between the groups in the proportion of participants with overall neurocognitive impairment (45% in the PI-mono vs 49% in the OT group; P = .63) or in the proportion of participants with symptomatic neurocognitive impairment (13% in the PI-mono vs 18% in the OT group; P = .41; Table 2). There also was no association between study arm and overall neurocognitive impairment or symptomatic neurocognitive impairment using logistic regression adjusting for age, ethnicity, education and nadir CD4 count. The only significant association with overall neurocognitive impairment was with black ethnicity (OR = 5.5; 95% CI, 1.8–16.2; P = .002); there were no significant associations found with symptomatic neurocognitive impairment.
Neuroimaging Markers
Brain MRI/MRS was performed in 78 of the 146 substudy participants (53%, 39 on each study arm). There were no differences between participants with and without neuroimaging in any of the measured variables (Supplementary Table 4). We found no differences between arms in any of the neuroimaging measures (Table 4). No associations were found between any of the neuroimaging measures and test-specific, domain or global (NPZ-7) z-scores (data not shown) or the presence of overall neurocognitive impairment (Table 5).
Table 4.
Neuroimaging Markers by Study Arm
| PI-mono (n = 39) | OT (n = 39) | P Value | |
|---|---|---|---|
| WMH | |||
| WMH present, N (%) | 22 (56.4) | 25 (64.1) | .64 |
| Volume (mm3), median (IQR) | 76 (0–667) | 101 (0–347) | .91 |
| Atrophy measures | |||
| GCA score, median (IQR) | 1 (0–2) | 1 (1–1) | .60 |
| TLA score, median (IQR) | 1 (1–2) | 1 (1–1) | .77 |
| Bicaudate index, median (IQR) | 0.11 (0.10–0.13) | 0.10 (0.09–0.12) | .07 |
| Single Voxel MRS | |||
| NAA/Cho, median (IQR) | 4.2 (3.9–4.8) | 4.4 (4.0–4.7) | .37 |
| NAA/Cr, median (IQR) | 1.0 (0.9 −1.0) | 1.0 (0.9–1.0) | .53 |
| mI/Cr, median (IQR) | 0.6 (0.5–0.7) | 0.5 (0.5–0.6) | .56 |
Abbreviations: GCA, global cortical atrophy score; IQR, interquartile range; mI/Cr, Myo-inositol to creatine ratio; MRS, magnetic resonance spectroscopy; NAA/Cho, N-acetyl aspartate to choline ratio; NAA/Cr, N-acetyl aspartate to creatine ratio; OT, ongoing triple therapy; PI, protease inhibitor; TLA, Medial temporal lobe atrophy score; WMH, White matter hyperintensities.
Table 5.
Association Between Imaging Measurements and Neurocognitive Impairment, Defined as a z-score <−1 in at Least Two Out of Seven Cognitive Domains: Logistic Regression Modelsa
| Overall Neurocognitive Impairment |
Symptomatic Neurocognitive Impairment |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| White matter hyperintensities | ||||||
| Lesion volume (log10)a | 1.0 | .9, 1.1 | .857 | 1.1 | 1.0, 1.2 | .266 |
| Atrophy scores | ||||||
| GCA | 1.2 | .6, 2.7 | .580 | 6.2 | 1.7, 22.3 | .005 |
| TLA | 1.3 | .6, 2.7 | .515 | 1.8 | .8, 4.4 | .173 |
| Bicaudate index | 1.1 | .6, 2.2 | .737 | 2.0 | .9, 4.4 | .100 |
| Single voxel MRS | ||||||
| NAA/Choa | 1.2 | .6, 2.6 | .590 | 1.9 | .8, 4.5 | .140 |
| NAA/Cra | 1.7 | .8, 3.4 | .164 | 1.9 | .9, 4.3 | .116 |
| mI/Cra | 1.0 | .5, 1.8 | .953 | 0.8 | .4, 1.5 | .454 |
Bolded text indicates the significant result.
Abbreviations: CI, confidence interval; GCA, Global cortical atrophy score; mI/Cr, Myo-inositol to creatine ratio; MRS, magnetic resonance spectroscopy; NAA/Cho, N-acetyl aspartate to choline ratio; NAA/Cr, N-acetyl aspartate to creatine ratio; OR, odds ratio; TLA, Medial temporal lobe atrophy score.
a Adjusted for study arm allocation, age (per additional year), ethnicity (black vs other), education (per additional year on formal education) and nadir CD4 count (per 100c more).
We also did not find any association between neuroimaging measures and the presence of symptomatic neurocognitive impairment, apart from GCA where we found a higher risk with increasing scores (OR 6.2; 95% CI, 1.7–22.3; P = .005) (Table 5). However, further analyses suggested that this was driven by the significant association of GCA scores with neurocognitive symptoms irrespective of neurocognitive test performance.
DISCUSSION
Our findings support the results of the main PIVOT trial that found no difference in neurocognitive function between PI monotherapy and triple therapy arms over 3–5 years of follow-up (also consistent with 48 week changes in another PI monotherapy trial) [10, 28]. The findings of this substudy strengthen the earlier conclusions by extending the neurocognitive assessment to include a more comprehensive neuropsychological testing battery than the brief one used for longitudinal assessment in these trials, which would be expected to be more sensitive in detecting between-group differences if they existed. In particular, the substudy battery included 2 different tests to measure some of the cognitive domains explored (ie, attention-concentration, executive functioning, and fine motor skills), which is recommended [26]. In addition, we added tests to explore nonverbal learning and memory, which are domains less likely to be affected by unmeasured cultural factors [25]. We also collected additional information on comorbid conditions such as alcohol or recreational drugs use and mood disorders to allow analyses to be adjusted for these important factors.
The consistent results in this sub-study between the expanded battery and the short battery (used in the main trial) also lend confidence to the validity of neurocognitive testing results reported for the main trial. Extensive and detailed longitudinal investigation of neurocognitive function in large, multicenter, strategy, RCT would have been very difficult to implement and extremely onerous. Therefore, more pragmatic approaches, as the one we implemented in PIVOT, are probably more appropriate.
The proportion of substudy participants that met the definition of impairment used for this study was high (45%). However, the proportion of participants meeting the criteria for symptomatic neurocognitive impairment, a more relevant clinical endpoint [29, 30], was much lower (15.8%). The absence of between-group differences on the neurocognitive tests, whether based on a composite z-score or on a threshold classification, as well as the absence of differences on neuroimaging investigations lends support to the conclusion from the main PIVOT trial (and other studies) that there is no added risk from PI monotherapy [28, 31].
Different neuroimaging techniques have been used to identify markers of HIV-associated neurocognitive impairment, including MRS, and the effect of different treatment options on these markers has been explored in treatment naïve patients starting cART [32]. However, information on effectively suppressed patients is lacking, and in particular there are very limited data comparing neuroimaging markers between patients on PI monotherapy and cART [33].
Consistent with our results, associations between imaging markers of cerebral atrophy and neurocognitive impairment have been previously reported in both naive and ART-experienced patients [34–36]. However, in our study, the association between an atrophy measurement, higher GCA score, and cognitive function was limited to those with symptomatic impairment. Conversely, cortical atrophy has also been described in patients with long-term HIV disease with normal cognitive function [37], whereas symptomatic neurocognitive impairment has been associated with longer duration of both HIV disease and exposure to cART [38]. Our participants were very ART-experienced patients and free from virological failure (at baseline), but cognitive symptoms were not infrequent (23%). However, we found no association between any HIV or ART-related variables and neurocognitive function, symptoms or neuroimaging measurements.
Our study has some limitations. The substudy recruited a subset of randomized participants (67% of those at the participating sites). Although there is the possibility of selection bias, the overall similarity of those recruited to the overall trial population at the sites (minor differences in age and proportion with VL suppression below 50 copies/mL) suggests this is unlikely to have had an important effect. Furthermore, the fact that neurocognitive results in this substudy are consistent with the results of the main trial (no differences between treatment arms) also strengthens confidence in their validity. Participants in clinical trials generally tend to be highly selected and this may impact generalizability. This may be the case in this trial and substudy, because in PIVOT all participants were free of previous episodes of virological failure suggesting high level of adherence to their ART and the prevalence of comorbid conditions likely to affect cognition, such hepatitis C coinfection or CNS opportunistic infections was very low. However, the study population was very homogeneous and therefore ideal to assess cognitive function in effectively suppressed patients with no major comorbidities. The criteria used to define neurocognitive symptoms and the definition of neurocognitive impairment we used were somewhat arbitrary, but that we considered appropriate for the population studied based on the available data collected in the main trial. In addition, a similar threshold approach is commonly used [39–41]. Alternative thresholds might give different overall proportions of patients classified as neurocognitively impaired, but this is of less relevance for this study which focuses on the comparison of treatment groups. The conclusions were the same, whether the analyses were based on this threshold approach or on composite z scores. The cross-sectional study design is also a limitation for the neuroimaging component since we have no baseline imaging and could not assess any difference between arms in change over time.
In summary, using a comprehensive neuropsychological testing battery, this analysis confirms previous observations made using brief testing batteries showing no excess risk of cognitive impairment in patients on PI-mono compared to standard cART [10, 28]. The absence of differences between arms on detailed MRI/MRS analysis also supports the earlier conclusion that PI-monotherapy does not carry a substantive risk of CNS damage and should give confidence to patients and physicians who wish to use this therapeutic option for long-term management of HIV infection.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank all the patients and staff from all the centres participating in the PIVOT neurocognitive substudy.
Disclaimer. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Health Technology Assessment (HTA) programme, National Institute for Health Research (NIHR), National Health System or the Department of Health.
Financial support. This analysis was funded by an unconditional scientific grant from Janssen Cilag Ltd. PIVOT was funded by the NIHR-HTA programme (project number 06/403/90).
Potential conflicts of interest. A. A.-P. and W. S. have received research grants from Janssen-Cilag Ltd, the National Institute of Health Research HTA programme and European and Developing Countries Clinical Trials Partnership programme (money paid to the institution). H. R. J. has received research grants from the UK Department of Health and Wellcome Trust Health Innovation Challenge Fund, Stroke Association Grant, The Stroke Association/British Heart Foundation Joint Program Grant in Stroke, Samantha Dickson Brain Tumour Research Trust and the Biomedical Research Centre Neuroscience Programme. L. H. has received research grants from ViiV Healthcare, BHIVA, NIHR-HTA and UCL Medical School Charitable Trust. He also has received honoraria from Janssen. A. C. has received travel, accommodation and conference fees reimbursed to attend human immunodeficiency virus related conferences, from Gilead Sciences, Janssen and Bristol Myers Squibb. M. J. has served as a consultant, has receives research funding and has received payment for manuscript preparation. A. W. has received honoraria or research grants, or been a consultant or investigator, in clinical trials sponsored by Abbott/AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline (GSK), Janssen Cilag, Roche, Pfizer and ViiV Healthcare. X. G. has received research grants from Chiesi Pharmaceutici and royalties from Chiesi Pharmaceutici and Cambridge University Press. N. I. P. reports grants from NIHR-HTA programme, grants, personal fees and nonfinancial support from AbbVie, personal fees and nonfinancial support from Merck, grants, personal fees and nonfinancial support from Janssen, personal fees and nonfinancial support from Roche, grants and non-financial support from GSK, nonfinancial support from Gilead. All other authors have no potential conflicts of interest to declare. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
The PIVOT Neurocognitive sub-study Team are:
Participating Sites: Elton John Centre, Brighton: Martin Fisher, Amanda Clarke, Wendy Hadley, David Stacey. Royal Free Hospital, London: Margaret Johnson, Pat Byrne. Mortimer Market Centre, London: Lewis Haddow, Ian Williams, Nahum De Esteban, Pierre Pellegrino, Alejandro Arenas-Pinto, Rita Trombin. Royal Berkshire Hospital, Reading: Fabian Chen, Ruth Wilson, Elizabeth Green, John Masterson. St Mary's Hospital, London: Alan Winston, Scott Mullaney.
MRC Clinical Trials Unit at UCL: Alejandro Arenas-Pinto, Nicholas Paton, Wolfgang Stöhr, Karen Scott, David Dunn, Karen Sanders, Janet Cairns.
UCL Institute of Neurology: Hans Rolf Jager, Claudia Godi, Steffi Thust, Bhavana Solanky, Marios Yiannakas, Xavier Golay.
PIVOT Trial Steering Committee: Andrew Freedman (Chair), Ben Cromarty, Danielle Mercey, Sarah Fidler, Estee Torok, Abdel Babiker, Brian Gazzard, Chloe Orkin, Nicholas Paton.
PIVOT Data Monitoring Committee: Tim Peto (Chair), David Lalloo, Andrew Phillips and Robert James.
Contributor Information
Collaborators: for the PIVOT Neurocognitive sub-study Team, Martin Fisher, Amanda Clarke, Wendy Hadley, David Stacey, Margaret Johnson, Pat Byrne, Lewis Haddow, Ian Williams, Nahum De Esteban, Pierre Pellegrino, Alejandro Arenas-Pinto, Rita Trombin, Fabian Chen, Ruth Wilson, Elizabeth Green, John Masterson, Alan Winston, Scott Mullaney, Alejandro Arenas-Pinto, Nicholas Paton, Wolfgang Stöhr, Karen Scott, David Dunn, Karen Sanders, Janet Cairns, Rolf Jager, Claudia Godi, Steffi Thust, Bhavana Solanky, Marios Yiannakas, Xavier Golay, Andrew Freedman, Ben Cromarty, Danielle Mercey, Sarah Fidler, Estee Torok, Abdel Babiker, Brian Gazzard, Chloe Orkin, Nicholas Paton, Tim Peto, David Lalloo, Andrew Phillips, and Robert James
References
- 1.Heaton RK, Clifford DB, Franklin DR Jr et al. . HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCutchan JA, Marquie-Beck JA, Fitzsimons CA et al. . Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 2012; 78:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnet F, Amieva H, Marquant F et al. . Cognitive disorders in HIV-infected patients: are they HIV-related? ANRS CO3 Aquitaine Cohort, Bordeaux, France, 2007-2009. AIDS 2013; 27:391–400. [DOI] [PubMed] [Google Scholar]
- 4.Simioni S, Cavassini M, Annoni JM et al. . Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010; 24:1243–50. [DOI] [PubMed] [Google Scholar]
- 5.Letendre S, Marquie-Beck J, Capparelli E et al. . Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008; 65:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tozzi V, Balestra P, Bellagamba R et al. . Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr 2007; 45:174–82. [DOI] [PubMed] [Google Scholar]
- 7.Tozzi V, Balestra P, Salvatori MF et al. . Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. J Acquir Immune Defic Syndr 2009; 52:56–63. [DOI] [PubMed] [Google Scholar]
- 8.Mathis S, Khanlari B, Pulido F et al. . Effectiveness of protease inhibitor monotherapy versus combination antiretroviral maintenance therapy: a meta-analysis. PLoS One 2011; 6:e22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powderly W, Hill A, Moecklinghoff C. Is there a higher risk of CNS adverse events for PI monotherapy versus triple therapy? A review of results from randomized clinical trials. HIV Clin Trials 2014; 15:79–86. [DOI] [PubMed] [Google Scholar]
- 10.Paton NI, Stohr W, Arenas-Pinto A et al. . Protease inhibitor monotherapy for long-term management of HIV infection: a randomised, controlled, open-label, non-inferiority trial. Lancet HIV 2015; 2:e417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandt J, Benedict RHB. Hopkins Verbal Learning Test-Revised Professional Manual. 3rd ed Lutz, FL, USA: PAR Psychological Assessment Resources, Inc., 2001. [Google Scholar]
- 12.D'Elia L, Satz P, Uchiyama CL, White T. Color Trails Test: Professional Manual. In: Psychological Assessment Resources I, ed. Lutz, FL: Psychological Assessment Resources, Inc, 1996. [Google Scholar]
- 13.Grooved Pegboard User's Manual. In: Europe LIC, ed. Loughborough, UK: Lafayette Instrument Co. Europe, 2003. [Google Scholar]
- 14.Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial: Professional Manual. In: Inc., PAR, ed. Lutz, FL, USA: Psychpological Assessment Resources Inc., 1995. [Google Scholar]
- 15.Golden CJ, Freshwater SM. The Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. In: Co. S, ed. Wood Dale, IL, USA: Stoelting Co., 2002. [Google Scholar]
- 16.Reitan RM. Manual for the administration of neuropsychological test batteries for adults and children. Tucson, AZ: Neuropsychology Laboratory, 1979. [Google Scholar]
- 17.Tulsky D, Zhu J, Ledbetter M. WAIS-III and WMS-III Technical Manual. In: Corporation TP, ed. USA: The Psychological Corporation, 2002. [Google Scholar]
- 18.Wu AW, Rubin HR, Mathews WC et al. . A health status questionnaire using 30 items from the Medical Outcomes Study.: Preliminary validation in persons with early HIV infection. Med Care 1991; 29:786–98. [DOI] [PubMed] [Google Scholar]
- 19.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. In: WHO, ed. World Health Organization Department of Mental Health and Substance Dependence. 2nd ed Geneva, Switzerland: World Health Organization, 2001. [Google Scholar]
- 20.EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990; 16:199–208. [DOI] [PubMed] [Google Scholar]
- 21.De Coene B, Hajnal JV, Gatehouse P et al. . MR of the brain using fluid-attenuated inversion recovery (FLAIR) pulse sequences. Am J Neuroradiol 1992; 13:1555–64. [PMC free article] [PubMed] [Google Scholar]
- 22.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci 1987; 508:333–48. [DOI] [PubMed] [Google Scholar]
- 23.Wattjes MP, Henneman WJP, Flier WM et al. . Diagnostic Imaging of Patients in a Memory Clinic: Comparison of MR Imaging and 64–Detector Row CT. Radiology 2009; 253:174–83. [DOI] [PubMed] [Google Scholar]
- 24.Butzkueven H, Kolbe SC, Jolley DJ et al. . Validation of linear cerebral atrophy markers in multiple sclerosis. J Clin Neurosci 2008; 15:130–7. [DOI] [PubMed] [Google Scholar]
- 25.Winston A, Arenas-Pinto A, Stohr W et al. . Neurocognitive Function in HIV Infected Patients on Antiretroviral Therapy. PLoS One 2013; 8:e61949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antinori A, Arendt G, Becker JT et al. . Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu AW, Hays RD, Kelly S, Malitz F, Bozzette SA. Applications of the Medical Outcomes Study health-related quality of life measures in HIV/AIDS. Qual Life Res 1997; 6:531–54. [DOI] [PubMed] [Google Scholar]
- 28.Antinori A, Clarke A, Svedhem-Johansson V et al. . Week 48 efficacy and central nervous system analysis of darunavir/ritonavir monotherapy versus darunavir/ritonavir with two nucleoside analogues. AIDS 2015; 29:1811–20. [DOI] [PubMed] [Google Scholar]
- 29.Torti C, Foca E, Cesana BM, Lescure FX. Asymptomatic neurocognitive disorders in patients infected by HIV: fact or fiction? BMC Med 2011; 9:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer AC, Boscardin WJ, Kwasa JK, Price RW. Is it time to rethink how neuropsychological tests are used to diagnose mild forms of HIV-associated neurocognitive disorders? Impact of false-positive rates on prevalence and power. Neuroepidemiology 2013; 41:208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Valero I, Gonzalez-Baeza A, Estebanez M et al. . Neurocognitive impairment in patients treated with protease inhibitor monotherapy or triple drug antiretroviral therapy. PLoS One 2013; 8:e69493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ances BM, Hammoud DA. Neuroimaging of HIV-associated neurocognitive disorders (HAND). Curr Opin HIV AIDS 2014; 9:545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valero IP, Baeza AG, Hernandez-Tamames JA, Monge S, Arnalich F, Arribas JR. Cerebral volumes, neuronal integrity and brain inflammation measured by MRI in patients receiving PI monotherapy or triple therapy. J Int AIDS Soc 2014; 17(4 Suppl 3):19578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinbrink F, Evers S, Buerke B et al. . Cognitive impairment in HIV infection is associated with MRI and CSF pattern of neurodegeneration. Eur J Neurol 2013; 20:420–8. [DOI] [PubMed] [Google Scholar]
- 35.Heaps JM, Sithinamsuwan P, Paul R et al. . Association between brain volumes and HAND in cART-naive HIV+ individuals from Thailand. J Neurovirol 2015; 21:105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfefferbaum A, Rosenbloom MJ, Sassoon SA et al. . Regional brain structural dysmorphology in human immunodeficiency virus infection: effects of acquired immune deficiency syndrome, alcoholism, and age. Biol Psychiatry 2012; 72:361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen RA, Harezlak J, Schifitto G et al. . Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol 2010; 16:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vassallo M, Durant J, Lebrun-Frenay C et al. . Virologically suppressed patients with asymptomatic and symptomatic HIV-associated neurocognitive disorders do not display the same pattern of immune activation. HIV Med 2015; 16:431–40. [DOI] [PubMed] [Google Scholar]
- 39.Gisslen M, Price RW, Nilsson S. The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis 2011; 11:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su T, Schouten J, Geurtsen GJ et al. . Multivariate normative comparison, a novel method for more reliably detecting cognitive impairment in HIV infection. AIDS 2015; 29:547–57. [DOI] [PubMed] [Google Scholar]
- 41.Arenas-Pinto A, Winston A, Stohr W et al. . Neurocognitive function in HIV-infected patients: comparison of two methods to define impairment. PLoS One 2014; 9:e103498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.