Human metapneumovirus infections among hematopoietic cell transplantation recipients correlate with infections in the community, and the risk of progression to lower respiratory tract disease is significantly increased in patients with low lymphocyte count or steroid dose of ≥1 mg/kg before diagnosis.
Keywords: human metapneumovirus, hematopoietic cell transplantation, lower respiratory tract disease, progression
Abstract
Background. Human metapneumovirus (HMPV) is a newly identified pulmonary pathogen that can cause fatal lower respiratory tract disease (LRD) in hematopoietic cell transplantation (HCT) recipients. Little is known about progression rates from upper respiratory tract infection (URI) to LRD and risk factors associated with progression.
Methods. A total of 118 HCT recipients receiving transplantation between 2004 and 2014 who had HMPV detected in nasopharyngeal, bronchoalveolar lavage, or lung biopsy samples by real-time reverse transcription polymerase chain reaction were retrospectively analyzed.
Results. More than 90% of the cases were identified between December and May. Among the 118 HCT patients, 88 and 30 had URI alone and LRD, respectively. Among 30 patients with LRD, 17 patients progressed from URI to LRD after a median of 7 days (range, 2–63 days). The probability of progression to LRD within 40 days after URI was 16%. In Cox regression analysis, steroid use ≥1 mg/kg prior to URI diagnosis (hazard ratio [HR], 5.10; P = .004), low lymphocyte count (HR, 3.43; P = .011), and early onset of HMPV infection after HCT (before day 30 after HCT; HR, 3.54; P = .013) were associated with higher progression to LRD. The median viral load in nasal wash samples was 1.1 × 106 copies/mL (range, 3.3 × 102–1.7 × 109) with no correlation between the viral load and progression.
Conclusions. Progression from URI to LRD occurred in up to 60% of HCT recipients with risk factors such as systemic corticosteroid use or low lymphocyte counts. Further studies are needed to define the role of viral load in the pathogenesis of progressive disease.
Human metapneumovirus (HMPV) is a newly recognized respiratory pathogen first identified in 2001 [1] and is structurally similar to respiratory syncytial virus (RSV) [2]. Serological epidemiology shows a 90% exposure by adulthood [3]. HMPV infection occurs in approximately 5% of hematopoietic cell transplantation (HCT) recipients [4, 5], and can cause severe or sometimes fatal lower respiratory tract disease (LRD) [6–9]. The mortality rate after HMPV LRD in patients with hematological malignancy or HCT recipients ranges from 10% to 40% [9–12]. To date, most studies in HCT recipients are very small and have focused on the LRD characteristics and outcome [7, 8, 10, 12]. Minimal data exist on the timing of, proportion of, and risk factors for progression from upper respiratory tract infection (URI) to LRD. These data are critical for the design of clinical trials that aim to prevent progression from URI to LRD. The purpose of this study was to examine the seasonality of HMPV infections in HCT recipients and to identify patient, transplantation, and viral risk factors for progression to LRD.
METHODS
Study Design
This retrospective cohort study includes patients who underwent transplantation between March 2004 and April 2014 at the Fred Hutchinson Cancer Research Center (FHCRC) and had documented HMPV infection during pretransplant conditioning or after transplantation. Only an individual's first episode of HMPV infection was analyzed. Patients' demographic data and transplantation information were retrieved from the FHCRC database, and other data related to the clinical course of HMPV infections were collected by medical record reviews. The data about HMPV infection in the community were based on the University of Washington Molecular Virology Laboratory data, which include data from Seattle Children's Hospital and from samples that were sent to the laboratory from regional providers (http://depts.washington.edu/rspvirus/respiratory.htm). The study was approved by the FHCRC Institutional Review Board.
Laboratory Testing
Nasopharyngeal samples were collected when HCT recipients had URI symptoms. We also collected a bronchoalveolar lavage (BAL) sample when patients had lower respiratory tract symptoms and a radiographic abnormality. Follow-up nasopharyngeal samples were obtained in a subset of patients. We initially applied direct fluorescent antibody testing and individual reverse transcription polymerase chain reaction (RT-PCR) assay to detect HMPV and started multiplex PCR testing in 2008 [13, 14]. Samples were considered positive if the PCR amplification plot crossed the threshold at <40 cycles. Viral load was determined by quantitative RT-PCR using nasopharyngeal samples at diagnosis of URI [15].
Definitions
URI was defined as HMPV detection in a nasopharyngeal sample with URI symptoms, such as rhinorrhea, stuffy nose, or sore throat. LRD was defined as detection of HMPV in a BAL or lung biopsy sample with new pulmonary infiltrates detected on radiographic studies; in analogy to a previous article on parainfluenza virus LRD, this manifestation was termed “proven LRD” [16]. “Possible LRD” was defined as viral detection in a nasopharyngeal sample and new pulmonary infiltrates without bronchoscopic examination, and these cases were included as URI in this study. Patients diagnosed with LRD ≥2 days after URI diagnosis were considered to have progression to LRD. Duration of viral shedding was defined as days between the first and the last date of HMPV detection.
A copathogen was defined as a significant pathogen when detected in a concurrent respiratory sample or in a blood sample obtained within 2 days of diagnosis of HMPV infection. Coagulase-negative staphylococcal bacteremia and cytomegalovirus viremia or antigenemia were not considered significant copathogens. Peak steroid dose before diagnosis was recorded from the period within 2 weeks [16].
Statistical Analysis
Patients' demographic characteristics were compared among disease categories using χ2 or Fisher exact test for categorical variables and Wilcoxon rank-sum test for continuous variables (as appropriate). The probability of progression to LRD among patients who presented with URI was estimated by cumulative incidence curves, treating death as a competing risk. Cox proportional hazards models were used to evaluate unadjusted and adjusted hazard ratios for progression to LRD. Logistic regression models were used to evaluate cross-sectional association between each risk factor and occurrence of LRD among all patients (including patients who presented with LRD). Risk factors for duration of shedding were assessed using linear regression on the ranks of days of shedding by day 100. Two-sided P values <.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.3 for Windows (SAS Institute, Cary, North Carolina).
RESULTS
Patient Characteristics and Seasonality
HMPV was detected in 118 HCT recipients; 88 (75%) and 30 (25%) had URI alone and LRD, respectively. Characteristics of each HMPV infection group are shown in Table 1. The median time to HMPV detection after HCT was 278.5 days (range, −5 to 2773 days), and the median viral load in nasal wash samples was 1.1 × 106 copies/mL (range, 3.3 × 102–1.7 × 109 copies/mL). The median day of engraftment was 16 days (range, 0–39 days), and only 1 patient who did not have engraftment prior to death had LRD, which was documented 63 days after HCT. Earlier transplantation year, HMPV infection sooner after HCT, copathogens, and low monocyte counts were observed more frequently in LRD cases than URI-only cases. There were 21 “possible LRD” cases, of which 8 (38%) and 13 (62%) received their transplantation before and after 2009, respectively. Other respiratory viral copathogens such as rhinovirus (n = 13 [11%]), coronaviruses (n = 11 [9%]), or parainfluenza virus (n = 5 [4%]) were occasionally detected in the nasopharyngeal or BAL samples. Nonviral copathogens in BAL included Pseudomonas aeruginosa (n = 1 [1%]) and Aspergillus fumigatus (n = 2 [2%]). Four patients had bacteremia due to Stenotrophomonas maltophilia, Acinetobacter baumannii, Enterococcus faecium, or Streptococcus pneumoniae at diagnosis.
Table 1.
Characteristics of All Patients With Human Metapneumovirus Infection (N = 118)
| Characteristic | Total (N = 118) | URI Alone (n = 88) | LRD (n = 30) | P Value |
|---|---|---|---|---|
| Sex | .53 | |||
| Male | 63 (53) | 45 (51) | 18 (60) | |
| Female | 55 (47) | 43 (49) | 12 (40) | |
| Age at HCT, y | .62 | |||
| ≤20 | 19 (16) | 16 (18) | 3 (10) | |
| 21–60 | 81 (69) | 59 (67) | 22 (73) | |
| >60 | 18 (15) | 13 (15) | 5 (17) | |
| Disease risk at HCT | .12 | |||
| Standard | 78 (66) | 62 (70) | 16 (53) | |
| High | 40 (34) | 26 (30) | 14 (47) | |
| Transplantation year | .011 | |||
| 2004–2009 | 58 (49) | 37 (42) | 21 (70) | |
| 2010–2014 | 60 (51) | 51 (58) | 9 (30) | |
| Transplantation number | .19 | |||
| First | 94 (80) | 73 (83) | 21 (70) | |
| Second | 22 (18) | 14 (16) | 8 (27) | |
| Third | 2 (2) | 1 (1) | 1 (3) | |
| Stem cell source | .27 | |||
| Bone marrow | 22 (18) | 14 (16) | 8 (27) | |
| Peripheral blood stem cell | 81 (69) | 61 (69) | 20 (67) | |
| Cord blood | 15 (13) | 13 (15) | 2 (6) | |
| Donor type | 1.00 | |||
| Autologous | 25 (21) | 19 (22) | 6 (20) | |
| Related | 30 (26) | 22 (25) | 8 (27) | |
| Unrelated | 63 (53) | 47 (53) | 16 (53) | |
| Conditioning regimen | .23 | |||
| MA including high-dose TBI (≥12 Gy) | 22 (19) | 18 (20) | 4 (13) | |
| MA ± low-dose TBI (≤2 Gy) | 53 (45) | 42 (48) | 11 (37) | |
| Reduced intensity | 43 (36) | 28 (32) | 15 (50) | |
| GVHD prophylaxis | .64 | |||
| CNI + MTX | 38 (41) | 30 (43) | 8 (33) | |
| CNI + MMF | 49 (53) | 35 (51) | 14 (59) | |
| Others | 6 (6) | 4 (6) | 2 (8) | |
| Days between HCT and infection | .027 | |||
| ≤30 | 18 (15) | 9 (10) | 9 (30) | |
| 31–365 | 52 (44) | 39 (44) | 13 (43) | |
| >365 | 48 (41) | 40 (46) | 8 (27) | |
| Quantitative viral load at diagnosis, median (range) | 1.1 × 106 (3.3 × 102–1.7 × 109) | 2.6 × 106 (3.3 × 102–1.7 × 109) | 4.2 × 105 (5.4 × 102–1.3 × 108) | .15 |
| Copathogen | .038 | |||
| None | 82 (69) | 66 (75) | 16 (53) | |
| Any pathogen | 36 (31) | 22 (25) | 14 (47) | |
| %FEV1/FVC pre HMPV LRD | .58 | |||
| ≥70 | 76 (78) | 57 (80) | 19 (73) | |
| <70 | 21 (22) | 14 (20) | 7 (27) | |
| %TLC pre HMPV LRD | 1.00 | |||
| ≥80 | 77 (85) | 55 (85) | 22 (85) | |
| <80 | 14 (15) | 10 (15) | 4 (15) | |
| White blood cell count at diagnosis | .15 | |||
| >1000 × 106/L | 96 (90) | 73 (92) | 23 (82) | |
| ≤1000 × 106/L | 11 (10) | 6 (8) | 5 (18) | |
| Neutrophil count at diagnosis | .14 | |||
| >1000 × 106/L | 89 (84) | 68 (87) | 21 (75) | |
| ≤1000 × 106/L | 17 (16) | 10 (13) | 7 (25) | |
| Lymphocyte count at diagnosis | .06 | |||
| >300 × 106/L | 83 (78) | 65 (83) | 18 (64) | |
| ≤300 × 106/L | 23 (22) | 13 (17) | 10 (36) | |
| Monocyte count at diagnosis | .025 | |||
| >300 × 106/L | 65 (61) | 53 (68) | 12 (43) | |
| ≤300 × 106/L | 41 (39) | 25 (32) | 16 (57) | |
| Steroid dose before diagnosis | .10 | |||
| None | 59 (50) | 44 (50) | 15 (52) | |
| <1 mg/kg | 51 (44) | 41 (47) | 10 (34) | |
| ≥1 mg/kg | 7 (6) | 3 (3) | 4 (14) | |
| Intravenous immunoglobulin | .015 | |||
| No | 115 (97) | 88 (100) | 27 (90) | |
| Yes | 3 (3) | 0 (0) | 3 (10) | |
| Ribavirin | <.001 | |||
| No | 101 (86) | 85 (97) | 16 (53) | |
| Yes | 17 (14) | 3 (3) | 14 (47) |
Data are presented as No. (%) unless otherwise specified. All variables in Table 1 were used for the univariate analyses.
Abbreviations: CNI, calcineurin inhibitor; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GVHD, graft-vs-host disease; HCT, hematopoietic cell transplantation; HMPV, human metapneumovirus; LRD, lower respiratory tract disease; MA, myeloablative; MMF, mycophenolate mofetil; MTX, methotrexate; TBI, total body irradiation; %TLC, percentage of predicted total lung capacity; URI, upper respiratory tract infection.
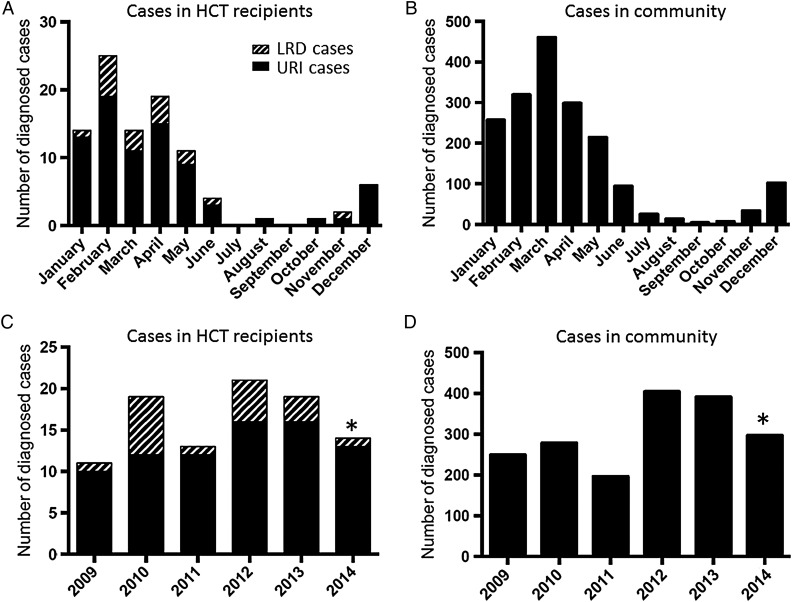
The number of HMPV infections by month between July 2008 and June 2014 is shown in Figure 1A. Most of the infections (93%) were identified between December and May of each calendar year. The incidence of HMPV infections in HCT recipients was similar to that in the community based on University of Washington reference laboratory data (Figure 1B). The numbers of HMPV cases by year are shown in Figure 1C and 1D. When HMPV infections were frequently detected, LRD occurred more frequently (Figure 1A and 1C).
Figure 1.
Monthly distribution of human metapneumovirus (HMPV) infection. A, Number of cases with HMPV infection by month, July 2008–June 2014, at our transplantation center. B, Number of cases with HMPV infection by month, July 2008–June 2014, diagnosed by the University of Washington (UW) reference laboratory, which tests samples from both Seattle and regional hospitals and healthcare providers. C, Number of cases with HMPV infection by year, January 2009–June 2014, at our center. *Number of cases in 2014 was obtained between January and June. D, Number of cases with HMPV infection by year, January 2009–June 2014, diagnosed by the UW reference laboratory. *Number of cases in 2014 was obtained between January and June. Abbreviations: HCT, hematopoietic cell transplantation; LRD, lower respiratory tract disease; URI, upper respiratory tract infection.
Progression From URI to LRD
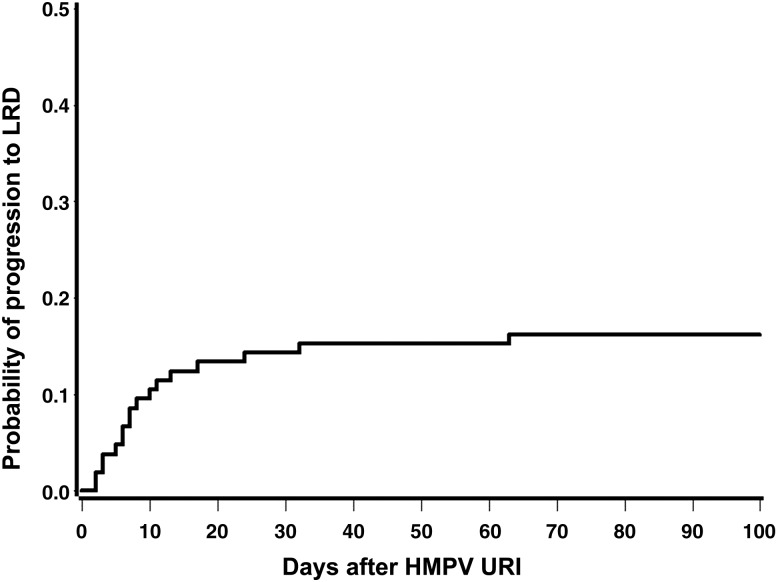
Among 30 patients with HMPV LRD, 17 (57%) had detected HMPV URI before diagnosis of LRD and progressed to LRD in a median of 7 days (range, 2–63 days) after URI. In total, among 105 patients with URI, the probability of progression to LRD within 40 days was 16% (Figure 2). Approximately 75% (14/17) of the patients with URI who progressed to LRD did so within 2 weeks after URI.
Figure 2.
Probability of progression to lower respiratory tract disease (LRD) after human metapneumovirus (HMPV) upper respiratory tract infection (URI) diagnosis.
Risk Factors for Progression to LRD
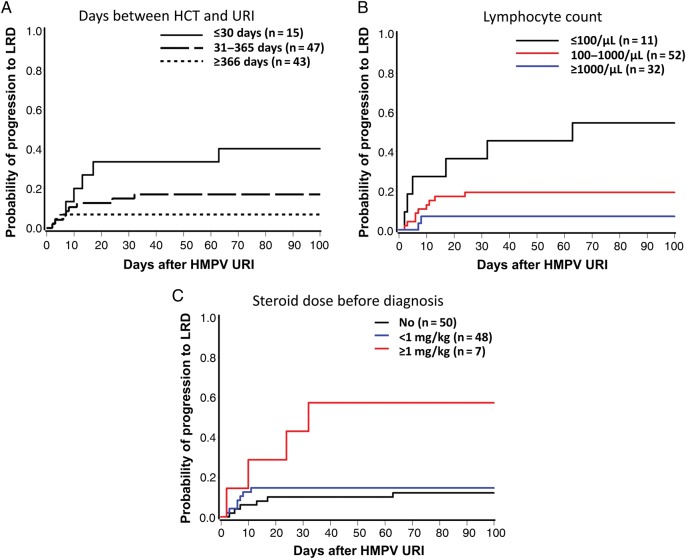
In the univariate Cox model, the factors shown in Table 1 were used to identify risk factors for progression to LRD among patients who presented with URI. Early HMPV infection after HCT, low lymphocyte count, and steroid use of ≥1 mg/kg before diagnosis were significantly associated with disease progression (Table 2), of which steroid dose remained significant in one bivariate model (Table 3). Probabilities of progression to LRD by these factors are shown in Figure 3. Approximately 60% of HCT recipients with low lymphocyte count or steroid use of ≥1 mg/kg before virologic diagnosis subsequently progressed to LRD.
Table 2.
Risk Factors for Progression From Upper Respiratory Tract Infection to Lower Respiratory Tract Disease (n = 105)
| Risk Factor | Univariate Analysis |
||
|---|---|---|---|
| HR | 95% CI | P Value | |
| Transplantation yeara | 0.90 | .73–1.10 | .31 |
| Days between HCT and infection | |||
| ≤30 | 1.00 | ||
| >30 | 3.54 | 1.31–9.60 | .013 |
| Copathogen | |||
| None | 1.00 | ||
| Any pathogen | 2.42 | .93–6.27 | .07 |
| White blood cell count at diagnosis | |||
| >1000 × 106/L | 1.00 | ||
| ≤1000 × 106/L | 2.71 | .88–8.33 | .08 |
| Lymphocyte count at diagnosis | |||
| >300 × 106/L | 1.00 | ||
| ≤300 × 106/L | 3.43 | 1.32–8.90 | .011 |
| Monocyte count at diagnosis | |||
| >300 × 106/L | 1.00 | ||
| ≤300 × 106/L | 2.31 | .89–6.00 | .08 |
| Steroid dose before diagnosis | |||
| None | 1.00 | ||
| <1 mg/kg | 1.27 | .43–3.77 | .67 |
| ≥1 mg/kg | 5.74 | 1.62–20.40 | .007 |
| Viral load | |||
| Low | 1.00 | ||
| High | 0.47 | .17–1.28 | .14 |
All variables in Table 1 were used for the univariable analysis. Only variables with P < .1 are shown in this table. Transplantation year and viral load were shown regardless of P values.
Abbreviations: CI, confidence interval; HCT, hematopoietic cell transplantation; HR, hazard ratio.
a This variable was analyzed as continuous.
Table 3.
Bivariate Analysis of Risk Factors for Progression From Upper Respiratory Tract Infection to Lower Respiratory Tract Disease (n = 105)
| Variables | Model 1 |
Model 2 |
Model 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Days between HCT and infection | |||||||||
| >30 | 1 | 1 | |||||||
| ≤30 | 1.89 | .60–5.99 | .28 | 2.58 | .87–7.64 | .09 | |||
| Lymphocyte count at diagnosis | |||||||||
| >300/µL | 1 | 1 | |||||||
| ≤300/µL | 2.60 | .86–7.85 | .09 | 2.60 | .90–7.49 | .08 | |||
| Steroid dose before diagnosis | |||||||||
| <1 mg/kg | 1 | 1 | |||||||
| ≥1 mg/kg | 3.44 | 1.01–11.70 | .048 | 2.72 | .78–9.45 | .12 | |||
Abbreviations: CI, confidence interval; HCT, hematopoietic cell transplantation; HR, hazard ratio.
Figure 3.
Incidence of progression to lower respiratory tract disease (LRD) after diagnosis of human metapneumovirus (HMPV) upper respiratory tract infection (URI). A, Cumulative incidence of progression to LRD by days between hematopoietic cell transplantation (HCT) and diagnosis of URI (global P = .01, log-rank test). B, Cumulative incidence of progression to LRD by lymphocyte count (global P = .0007). C, Cumulative incidence of progression to LRD by steroid dose before diagnosis of URI (global P = .006).
In univariate logistic regression models among all patients, factors associated with the development of LRD were early onset of HMPV infection after HCT (odds ratio [OR], 3.70; 95% confidence interval [CI], 1.33–11.1; P = .010), low lymphocyte count (OR, 2.78; 95% CI, 1.05–7.37; P = .040), earlier transplantation year (OR, 1.22; 95% CI, 1.02–1.46; P = .028), presence of copathogens (OR, 2.63; 95% CI, 1.11–6.23; P = .029), and low monocyte count (OR, 3.00; 95% CI, 1.23–7.30; P = .016); use of steroids (≥1 mg/kg) approached statistical significance (OR, 4.53; 95% CI, .95–21.62; P = .06). In a multivariable logistic model that included transplantation year, low lymphocyte count, and high steroid use before diagnosis, earlier transplantation year (adjusted OR [aOR], 1.33; 95% CI, 1.09–1.63; P = .005) and low lymphocyte count (aOR, 3.38; 95% CI, 1.07–10.67; P = .038) remained statistically significant. When early onset after transplantation was included in the model, lymphocytopenia was no longer significant, whereas early onset remained significant (aOR, 3.30; 95% CI, 1.01–10.83; P = .048).
Viral Shedding and Viral Load in Nasopharyngeal Samples
Thirty-nine patients had continuous HMPV detection for up to 182 days. Only 2 patients who died before day 100 had prolonged shedding: 1 patient who died at day 20 had 20 days of shedding, and another who died at day 31 had 17 days of shedding. In a bivariate analysis of risk factors for duration of shedding that included the same 3 variables as in Table 3, steroid use of ≥1 mg/kg was associated with longer shedding (P = .030).
Among 101 patients with detected viruses in nasal wash samples, we compared disease status by viral load. There was no evidence of a correlation between disease status and viral load in the nasal wash samples at presentation (data not shown).
DISCUSSION
This study demonstrated that low lymphocyte count and steroid use of ≥1 mg/kg at the time of URI diagnosis are associated with an increased risk of progression to LRD with progression rates approaching 60% in patients in the highest-risk categories. The viral load in nasopharyngeal samples at the time that HMPV URI was diagnosed did not appear to predict the risk of progression to LRD.
HMPV is a relatively newly discovered respiratory pathogen and the effect of HMPV infections on HCT recipients is poorly understood. Numerous studies of respiratory disease due to RSV, a genetically similar respiratory virus, in transplantation recipients have been reported with progression rates to LRD and mortality after LRD ranging from 20% to 50% and 15% to 50%, respectively [17–21]. Due to the high incidence of RSV-related LRD and subsequent high mortality from RSV infection, RSV infections after HCT are considered a very important complication. Mortality following HMPV is comparably as high as that seen with RSV, at rates of approximately 40% [10]. HMPV is difficult to diagnose with culture methods, but several multiplex PCR platforms are now available that permit rapid, sensitive, and specific detection of HMPV.
The progression rate to LRD may be affected by the underlying conditions of the HCT recipient. We identified steroid use of ≥1 mg/kg before diagnosis, low lymphocyte count, and onset of HMPV infection during the first month after HCT as important risk factors for progression. As shown in Figure 3, 60% of the patients with steroid use of ≥1 mg/kg or with low lymphocyte count had progression. These 2 characteristics are generally associated with high mortality after LRD by respiratory virus [10, 16, 20]. The logistic regression model provided additional insights. Lymphopenia was important but lost significance when the timing after transplantation was included. This suggests that other host defense mechanisms in the early time period after transplantation, such as monocytes, are also important. With only 30 events of LRD, we were unable to fit a model including all factors that are potentially important.
Disease progression following infection with RSV, a virus that is genetically similar to HMPV, has been related to patient characteristics including neutropenia, lymphocytopenia, unrelated donor status, conditioning including total body irradiation, fungal coinfections, and smoking history [17]. In our study, however, none of these factors except lymphocytopenia was associated with progression. Although we did not collect data on smoking history, we evaluated pulmonary function prior to HMPV infection, and no association between pulmonary dysfunction and progression of HMPV infections was detected.
To improve patient outcome of HMPV infection, strategies need to be developed to prevent progression to LRD. Our study identified high-risk clinical situations during which early intervention strategies could be studied. To date, there is no proven effective treatment for HMPV infection [22, 23]. Several small reports support the use of ribavirin with or without intravenous immunoglobulin (IVIG) for HMPV infection [24–27]. A larger study, however, showed no protective effect of ribavirin for HMPV LRD [10]. All studies were nonrandomized. In our study, a maintenance dose of IVIG was not associated with a lower progression rate (data not shown). We were unable to analyze the effect of ribavirin or high-dose IVIG because the number of patients who received it before progression was low. Based on our finding, reduction of steroid doses if patients receive high-dose steroid may be a possible strategy. However, whether such an approach would be effective cannot be determined from this study.
Other important findings in this study are that steroid use of ≥1 mg/kg before diagnosis was associated with longer viral shedding and that viral load in a nasopharyngeal sample was not correlated with progression to LRD. As for the effect of viral load, we may have failed to show the relationship due to issues related to host immune function, which we did not quantify in this study. We generally perform nasopharyngeal testing when patients have URI symptoms. Although URI symptoms are based on host immune response, the level of the immune response in HCT recipients may not be correlated to viral load. Another possible reason is that progression to pneumonia may not be related to viral load in the upper respiratory tract. Previous studies of parainfluenza virus or pathogens detected in patients with idiopathic pneumonia syndrome after HCT failed to show an association of viral load in a BAL sample with the level of lung injury or outcome, which is consistent with the finding in the current study [16, 28]. Although we did not demonstrate a correlation between viral load and progression to LRD, it is possible that larger studies with standardized BAL viral load measurements that control for dilution may improve our understanding of the role of viral load in disease progression.
This study has several limitations. Although this cohort represents the largest number of HCT recipients infected with HMPV who received a quantitative virological workup of both the upper and lower respiratory tract samples, the sample size was too small to definitively assess all the patient characteristics we studied and the role of individual copathogens. We also did not have enough events for multivariable analyses. Nevertheless, we were able to perform several different bivariate Cox models. Another limitation is the long observation period, during which various transplantation procedures have changed. Low lymphocyte count or steroid dose before diagnosis are important factors for progression to LRD. Reduced intensity conditioning regimens that are related to mild lymphocytopenia are frequently used. Our center has recently adopted lower dose of steroids for the initial treatment of acute graft-vs-host disease [29], which may have affected the incidence of LRD in recent years. Although we included possible factors affecting the progression rate in the analyses, we may have missed some important factors that changed during the study period. The main methods to detect HMPV also changed because of the long observation period. However, there was no apparent bias in the way samples were collected for testing. We performed bronchoscopy when patients had lower respiratory tract symptoms and radiographic abnormalities. Nevertheless, the final decision was left to the attending physician. Therefore, an aggressive workup was declined in some “possible LRD” cases, especially in cases late after HCT, which may have resulted in underreporting of progression to LRD in some cases. The virological analyses were also limited because of inconsistent surveillance of viral shedding and incomplete ascertainment of viral load by dilution factor of the nasal wash samples, although a previous study suggested that the dilution effect is rather limited [13].
In conclusion, our findings show that HMPV infections in HCT recipients occurred primarily in the winter and spring. The progression rate from URI to LRD approached 60% in patients who acquired HMPV infection and had low lymphocyte count or use of ≥1 mg/kg of steroids prior to URI. These data provide the necessary foundation to design clinical trials to study the efficacy of new antiviral compounds, which are urgently needed. Further studies are needed to define the role of viral load in the pathogenesis of progressive disease.
Notes
Acknowledgments. We thank Terry Stevens-Ayers for laboratory assistance, Angela P. Campbell for collection of laboratory data, and Brad Edmison and Lisa Chung for assistance with creating figures.
Financial support. This work was supported by the National Institutes of Health (grant number K24HL093294). S. S. is a recipient of the Joel Meyers Endowment Scholarship.
Potential conflicts of interest. M. B. has served as a consultant to Humabs Biomed and has received research funding from Ansun Biopharma. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.van den Hoogen BG, de Jong JC, Groen J et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med 2001; 7:719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schildgen V, van den Hoogen B, Fouchier R et al. Human metapneumovirus: lessons learned over the first decade. Clin Microbiol Rev 2011; 24:734–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebihara T, Endo R, Ishiguro N, Nakayama T, Sawada H, Kikuta H. Early reinfection with human metapneumovirus in an infant. J Clin Microbiol 2004; 42:5944–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milano F, Campbell AP, Guthrie KA et al. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood 2010; 115:2088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliveira R, Machado A, Tateno A, Boas LV, Pannuti C, Machado C. Frequency of human metapneumovirus infection in hematopoietic SCT recipients during 3 consecutive years. Bone Marrow Transplant 2008; 42:265–9. [DOI] [PubMed] [Google Scholar]
- 6.Englund JA, Boeckh M, Kuypers J et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med 2006; 144:344–9. [DOI] [PubMed] [Google Scholar]
- 7.Cane PA, van den Hoogen BG, Chakrabarti S, Fegan CD, Osterhaus AD. Human metapneumovirus in a haematopoietic stem cell transplant recipient with fatal lower respiratory tract disease. Bone Marrow Transplant 2003; 31:309–10. [DOI] [PubMed] [Google Scholar]
- 8.Dokos C, Masjosthusmann K, Rellensmann G et al. Fatal human metapneumovirus infection following allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 2013; 15:E97–101. [DOI] [PubMed] [Google Scholar]
- 9.Hoellein A, Hecker J, Hoffmann D et al. Serious outbreak of human metapneumovirus in patients with hematologic malignancies. Leuk Lymphoma 2016; 57:623–7. [DOI] [PubMed] [Google Scholar]
- 10.Renaud C, Xie H, Seo S et al. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol Blood Marrow Transplant 2013; 19:1220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams JV, Martino R, Rabella N et al. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J Infect Dis 2005; 192:1061–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egli A, Bucher C, Dumoulin A et al. Human metapneumovirus infection after allogeneic hematopoietic stem cell transplantation. Infection 2012; 40:677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peck AJ, Englund JA, Kuypers J et al. Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood 2007; 110:1681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuypers J, Wright N, Corey L, Morrow R. Detection and quantification of human metapneumovirus in pediatric specimens by real-time RT-PCR. J Clin Virol 2005; 33:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuypers J, Campbell AP, Cent A, Corey L, Boeckh M. Comparison of conventional and molecular detection of respiratory viruses in hematopoietic cell transplant recipients. Transpl Infect Dis 2009; 11:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo S, Xie H, Campbell AP et al. Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome. Clin Infect Dis 2014; 58:1357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YJ, Guthrie KA, Waghmare A et al. Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. J Infect Dis 2014; 209:1195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah DP, Ghantoji SS, Shah JN et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother 2013; 68:1872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo S, Campbell AP, Xie H et al. Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: significance of stem cell source and oxygen requirement. Biol Blood Marrow Transplant 2013; 19:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waghmare A, Campbell AP, Xie H et al. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin Infect Dis 2013; 57:1731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renaud C, Campbell AP. Changing epidemiology of respiratory viral infections in hematopoietic cell transplant recipients and solid organ transplant recipients. Curr Opin Infect Dis 2011; 24:333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Principi N, Esposito S. Paediatric human metapneumovirus infection: epidemiology, prevention and therapy. J Clin Virol 2014; 59:141–7. [DOI] [PubMed] [Google Scholar]
- 23.Wen SC, Williams JV. New approaches for immunization and therapy against human metapneumovirus. Clin Vaccine Immunol 2015; 22:858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonney D, Razali H, Turner A, Will A. Successful treatment of human metapneumovirus pneumonia using combination therapy with intravenous ribavirin and immune globulin. Br J Haematol 2009; 145:667–9. [DOI] [PubMed] [Google Scholar]
- 25.Kamble RT, Bollard C, Demmler G, LaSala PR, Carrum G. Human metapneumovirus infection in a hematopoietic transplant recipient. Bone Marrow Transplant 2007; 40:699–700. [DOI] [PubMed] [Google Scholar]
- 26.Raza K, Ismailjee SB, Crespo M et al. Successful outcome of human metapneumovirus (hMPV) pneumonia in a lung transplant recipient treated with intravenous ribavirin. J Heart Lung Transplant 2007; 26:862–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safdar A. Immune modulatory activity of ribavirin for serious human metapneumovirus disease: early i.v. therapy may improve outcomes in immunosuppressed SCT recipients. Bone Marrow Transplant 2008; 41:707–8. [DOI] [PubMed] [Google Scholar]
- 28.Seo S, Renaud C, Kuypers JM et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 2015; 125:3789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mielcarek M, Furlong T, Storer BE et al. Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Haematologica 2015; 100:842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]