Our population-based cohort study found that the incidence of herpes zoster has increased over the last 6 decades. This temporal increase is unlikely to be due to the introduction of varicella vaccination, antiviral therapy, or immunosuppressive medications.
Keywords: herpes zoster, shingles, epidemiology
Abstract
Background. Temporal increases in the incidence of herpes zoster (HZ) have been reported but studies have examined short study periods, and the cause of the increase remains unknown. We examined the long-term trend of HZ.
Methods. A population-based cohort study was conducted in Olmsted County, Minnesota, using data from 1945–1960 and 1980–2007. Medical records review of possible cases was performed to confirm incident cases of HZ, the patient's immune status, and prescribing of antivirals for HZ. We examined the relative change in the temporal trend in the incidence rates before and after the introduction of the varicella vaccination program.
Results. Of the 8017 patients with HZ, 58.7% were females and 6.6% were immunocompromised. The age- and sex-adjusted incidence rate of HZ increased from 0.76 per 1000 person-years (PY) (95% confidence interval [CI], .63–.89) in 1945–1949 to 3.15 per 1000 PY (95% CI, 3.04–3.26) in 2000–2007. The rate of increase across the time period was 2.5% per year after adjusting for age and sex (adjusted incidence rate ratio, 1.025 [95% CI, 1.023–1.026]; P < .001). The incidence of HZ significantly increased among all age groups and both sexes. We found no change in the rate of increase before vs after the introduction of the varicella vaccination program.
Conclusions. The incidence of HZ has increased >4-fold over the last 6 decades. This increase is unlikely to be due to the introduction of varicella vaccination, antiviral therapy, or change in the prevalence of immunocompromised individuals.
Herpes zoster (HZ) results from reactivation of varicella zoster virus (VZV) after a latency period following primary infection [1]. HZ causes a painful, blistering rash and can lead to serious complications, such as a long-lasting, painful postherpetic neuralgia and HZ ophthalmicus with eye involvement [2–5]. The lifetime risk of HZ is estimated to be approximately 30% [3]. Risk of HZ increases with waning of cell-mediated immunity, which occurs with aging or immunosuppressive medical conditions or therapies [2, 6].
Temporal increases in the incidence of HZ have been reported previously in the United States, Canada, the United Kingdom, Spain, Japan, Taiwan, and Australia [7–18]. However, the majority of prior studies are limited to the last 2 decades and relatively short study periods of 5–10 years. Most studies relied on administrative claims data without medical records review for confirmation of the diagnosis [19]. Reasons for the increase remain unclear, but hypotheses include introduction of antiviral therapy, which might increase patients’ willingness to come for HZ care; the widespread use of childhood varicella vaccination, which might decrease the boosting of immunity from wild-type VZV exposure; and the increasing use of immunosuppressive therapies for multiple chronic conditions, which might increase susceptibility [8, 9, 12, 15]. The US Advisory Committee on Immunization Practices recommended routine use of varicella vaccination for children in 1996 and HZ vaccination for older adults in 2006.
The objective of our study is to examine the long-term temporal trend of incidence of HZ from 1945 to 2007 in a community population beginning prior to the introduction of antiviral medications (mid-1980s) and extending beyond the introduction of childhood varicella vaccinations (1996). We built on the original work of Ragozzino et al, who were among the first to identify an increase in the rate of HZ in adults using Olmsted County data from 1945 to 1959 [20], and also the work of Yawn et al from 1996 to 2001 [3]. The ability to use medical records review also allowed assessment of the percentage of immunosuppression among patients with HZ. This study should improve understanding of HZ trends to help direct the search for etiologies of the increase.
METHODS
Setting
Olmsted County, Minnesota, provides an opportunity for a population-based study of HZ using the Rochester Epidemiology Project (REP). The REP is a unique resource that links and indexes the records of virtually all providers of medical care to Olmsted County residents [21–24]. This linkage system provides investigators the ability to electronically search for all patients who received a specific diagnosis in a particular time period. Once patients with a diagnosis code of interest have been identified, investigators may then retrieve and review all medical records for each identified patient. The diagnostic indexes are all updated and available to be searched electronically, with reference to site where the medical records from each visit are available. Medical records including details of every outpatient visit to offices, clinics, and emergency departments, as well as hospitalizations and all correspondence concerning each patient, are available from all sources of medical care within the county. Medical records data before 2004 are paper based and either available in the original paper format or have been scanned for electronic viewing. Previous studies have shown that >98% of all care provided to Olmsted County residents is provided within the county and therefore retrievable by REP [21]. Thus, the REP provides community population–based medical data.
Subjects for Potential Inclusion
All Olmsted County children and adults who had a diagnostic code of HZ or an HZ complication (International Classification of Diseases, Ninth Revision codes 053.xx and equivalent codes from earlier coding systems) and have not refused research authorization (required by Minnesota statute after 1997) were included as potential HZ cases. To date, >95% of Olmsted County residents have not refused that authorization [21, 25]. Patients of all ages were identified during the periods between 1 January 1945 and 31 December 1960 and between 1 January 1980 and 31 December 2007. For the period 1945–1959, the original case list of HZ patients was available from the Ragozzino et al study [20] and was used as the basis for data from that period.
The medical records of each potential HZ patient, including all subjects included in the initial Ragozzino et al study, were abstracted or reabstracted using the same methodology and case definition to assure comparability across the entire time period. All cases were based on the first episode of HZ. The criteria for confirmation of the HZ diagnosis have been reported previously [3]. In brief, HZ confirmation required 1 or more of the following:
Physician-confirmed diagnosis of HZ with typical rash and pain;
Signs or symptoms consistent with HZ (acute onset of dermatomal pain and vesicular rash);
Signs or symptoms consistent with HZ complication and laboratory tests (viral culture, polymerase chain reaction, direct fluorescent antibody test, or other) consistent with VZV infection.
Medical Records Abstraction
Experienced nurse abstractors collected data directly from the paper or electronic medical records associated with each visit for HZ for each patient. For quality assurance, 5% reabstraction was completed and reviewed. Intrarater agreement was >95% for confirmation of the diagnosis and month and year of diagnosis [25]. The information collected from each HZ diagnostic visit included the visit site, the visit date, recorded diagnoses, and prescription of antiviral medication. Patient demographic data abstracted included birth date and sex.
Immunostatus of each patient at the time of the HZ episode was assessed by the presence of any of a list of diseases and drugs that are associated with immunosuppression including human immunodeficiency virus, AIDS, actively treated malignancies (systemic chemotherapy only), all hematological malignancies, and any other type of immunosuppressive therapy such as that given for rheumatoid arthritis or solid organ transplant recipients [3]. Corticosteroid medications were considered immunosuppressive if they were given at levels equivalent to 10 mg of prednisone daily for >30 days in the period just prior to the HZ diagnosis (Supplementary Material). If none of the conditions or medication types listed above were documented at the time of or during the year prior to the HZ diagnosis, the patient was presumed to be immunocompetent.
Statistical Analysis
The temporal changes in the prevalence of patient's immunostatus and use of antiviral therapy were examined by year. Incidence rates were estimated by dividing the cases of HZ by person-years (PY) at risk. We first estimated the incidence rates of HZ by sex, age (<50, 50–59, 60–69, 70–79, ≥80 years), and specific time period (1945–1949, 1950–1960, 1980–1989, 1990–1999, and 2000–2007). Incidence rates were age- and sex-standardized to the US population in 2007. Poisson regression modeling with a log-link function was conducted to examine temporal trends in the incidence rates of HZ by entering calendar year as a continuous variable after adjusting for the patients' age and sex. We estimated incidence rate ratios (IRRs) and 95% confidence intervals (CIs). We also examined the relative change in the temporal trend in the incidence rates of HZ before and after the introduction of the varicella vaccination program in 1996 by entering an interaction term between calendar year and indicator for post–varicella vaccination era (before 1996 vs 1997 and beyond). Analyses were conducted using SAS version 9.3 and R statistical software.
RESULTS
In total, 8017 cases of medical records–confirmed HZ were identified (Table 1). The mean age of patients with HZ was 51.8 (SD, 23.7) years, ranging from 0.3 to 101.7 years of age. Overall, 58.7% of cases were female. The population in Olmsted County grew from approximately 44 000 in 1945 to 140 000 in 2007 with only a modest increase in racial and ethnic mixture, from <1% nonwhite in 1945 to 7.3% nonwhite in 2007 and <1% Hispanic in 1945 to 3.8% in 2007. About 6.6% of patients with HZ were immunocompromised and 35.4% received antiviral therapy (Supplementary Table 1). The percentage of immunocompromised status in patients with HZ ranged between 1.4% in 1945–1949 to 8.8% in 1990–1999 and 6.0% in 2000–2007. The use of antiviral therapy increased from 11.8% in 1980–1989 to 46.8% in 2000–2007.
Table 1.
Characteristics of Patients With Herpes Zoster (N = 8017)
| Characteristic | Cases of Herpes Zoster, No. (%) |
|---|---|
| Sex | |
| Male | 3313 (41.3) |
| Female | 4704 (58.7) |
| Age category | |
| <50 y | 3432 (42.8) |
| 50–59 y | 1242 (15.5) |
| 60–69 y | 1245 (15.5) |
| 70–79 y | 1168 (14.6) |
| ≥80 y | 924 (11.5) |
| Location of rash | |
| Head or neck | 1755 (21.9) |
| Trunk, abdomen, or pelvis | 4641 (57.9) |
| Arm or leg | 2000 (25.0) |
| Year category | |
| 1945–1949 | 144 (1.8) |
| 1950–1960 | 503 (6.3) |
| 1980–1989 | 1701 (21.2) |
| 1990–1999 | 2465 (30.7) |
| 2000–2007 | 3204 (40.0) |
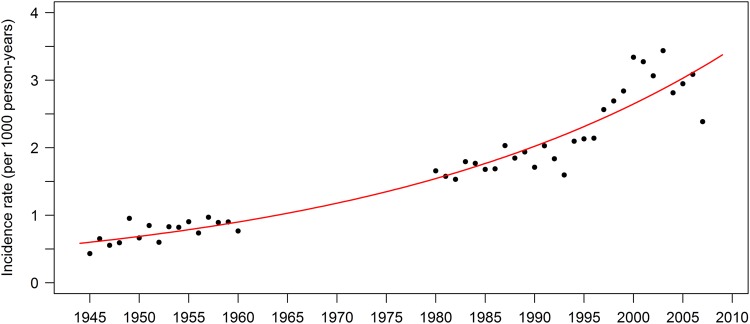
The age- and sex-adjusted incidence rate of HZ increased from 0.76 (95% CI, .63–.89) per 1000 PY in 1945–1949 to 3.15 (95% CI, 3.04–3.26) per 1000 PY in 2000–2007 (Table 2 and Figure 1). The incidence rate of HZ increased for both sexes and for all age groups. Each subsequent year was associated with 1.025 (95% CI, 1.023–1.026; P < .0001) times greater risk of HZ compared with the prior year after adjusting for age and sex (Table 3). This yearly increase corresponds to a 4.5-fold increase in the incidence rate of HZ between 1945 and 2007 (adjusted IRR, 4.51 [95% CI, 4.09–4.97]). The risk of HZ was significantly higher in women than in men (adjusted IRR, 1.21 [95% CI, 1.16–1.27]) and increased with age.
Table 2.
Incidence Rates of Herpes Zoster (Cases per 1000 Person-Years), 1945–2007, Olmsted County, Minnesota
| Variable | Incidence Rate (95% CI) |
Incidence Rate Ratio (95% CI)a | P Value | ||||
|---|---|---|---|---|---|---|---|
| 1945–1949 | 1950–1960 | 1980–1989 | 1990–1999 | 2000–2007 | |||
| Total | |||||||
| Crude | 0.64 (.54–.75) | 0.83 (.76–.90) | 1.76 (1.67–1.84) | 2.18 (2.10–2.27) | 3.03 (2.93–3.14) | 1.027 (1.026–1.029) | <.0001 |
| Adjustedb | 0.76 (.63–.89) | 1.01 (.92–1.10) | 2.04 (1.94–2.13) | 2.39 (2.29–2.49) | 3.15 (3.04–3.26) | … | |
| Sex | |||||||
| Male | 0.60 (.47–.77) | 0.70 (.61–.81) | 1.50 (1.39–1.62) | 1.81 (1.70–1.92) | 2.61 (2.48–2.76) | 1.027 (1.024–1.029) | <.0001 |
| Female | 0.68 (.54–.84) | 0.94 (.84–1.05) | 1.99 (1.87–2.12) | 2.53 (2.41–2.67) | 3.44 (3.29–3.60) | 1.028 (1.026–1.030) | <.0001 |
| Age category | |||||||
| <50 y | 0.38 (.30–.48) | 0.44 (.38–.50) | 0.92 (.86–1.00) | 1.27 (1.19–1.34) | 1.76 (1.67–1.85) | 1.029 (1.027–1.032) | <.0001 |
| 50–59 y | 1.29 (.90–1.86) | 1.65 (1.35–2.02) | 2.44 (2.13–2.80) | 3.37 (3.04–3.74) | 4.49 (4.13–4.88) | 1.022 (1.018–1.026) | <.0001 |
| 60–69 y | 1.89 (1.32–2.72) | 2.61 (2.16–3.16) | 4.62 (4.11–5.20) | 4.76 (4.28–5.29) | 6.52 (5.96–7.12) | 1.019 (1.015–1.023) | <.0001 |
| 70–79 y | 1.70 (1.01–2.87) | 2.40 (1.85–3.12) | 7.13 (6.36–7.99) | 7.60 (6.87–8.39) | 8.35 (7.59–9.19) | 1.022 (1.018–1.026) | <.0001 |
| ≥80 y | 1.64 (.68–3.95) | 3.51 (2.47–4.98) | 8.91 (7.82–10.15) | 7.95 (7.06–8.95) | 10.70 (9.69–11.82) | 1.021 (1.015–1.026) | <.0001 |
Abbreviation: CI, confidence interval.
a Incidence rate ratio, 95% CI, and P value based on the Poisson regression model including year as a continuous variable.
b Age- and sex-standardized to the US population in 2007.
Figure 1.
Incidence of herpes zoster in Olmsted County, Minnesota, 1945–2007, showing the temporal trend beginning prior to the introduction of antiviral medications (mid-1980s) and extending beyond the introduction of childhood varicella vaccinations (1996).
Table 3.
Multivariable Analysis of Predictors for Risk of Herpes Zoster
| Variable | Adjusted IRR | 95% CI | P Value |
|---|---|---|---|
| Year as a continuous variable | 1.025 | 1.023–1.026 | <.0001 |
| Sex | |||
| Men | Reference | ||
| Women | 1.21 | 1.16–1.27 | <.0001 |
| Age category | |||
| <50 y | Reference | ||
| 50–59 y | 2.70 | 2.53–2.88 | <.0001 |
| 60–69 y | 4.12 | 3.86–4.39 | <.0001 |
| 70–79 y | 5.68 | 5.31–6.07 | <.0001 |
| ≥80 y | 6.64 | 6.17–7.14 | <.0001 |
Based on the Poisson regression model that includes year, sex, and age category.
Abbreviations: CI, confidence interval; IRR, incidence rate ratio.
We found no overall evidence of change in the rate of increase in HZ before vs after introduction of varicella vaccination (P = .18 for relative change in IRR; IRR for annual increase during pre–varicella vaccination era, 1.026 [95% CI, 1.023–1.028]; IRR during postvaccination era, 1.020 [95% CI, 1.014–1.027]). However, we observed a decline in HZ rates in children <10 years of age following the introduction of the varicella vaccination program (P < .0001 for relative change). The incidence rate declined from 0.83 (95% CI, .66–1.04) per 1000 PY in 1990–1994 to 0.35 (95% CI, .22–.53) per 1000 PY in 2005–2007 among children <10 years of age.
We found no evidence of seasonality of HZ when examining the association between month and risk of HZ after adjusting for sex, age, and year (P = .20; Supplementary Table 2).
DISCUSSION
The incidence of HZ increased >4-fold over the 60-year period in this population-based cohort study in Olmsted County, Minnesota. The incidence of HZ steadily increased among all age groups and both sexes. We found no change in the rate of increase before vs after the introduction of varicella vaccination. Furthermore, the increase of HZ is unlikely to be due to increased patient healthcare-seeking related to the availability of antiviral therapies, as we observed a similar rate of increase before and after their introduction in the mid-1980s.
To our knowledge, our study has the longest study period reported to date, significantly extending available information on HZ trends and demonstrating an increase in the age- and sex-adjusted incidence rate from 0.76 per 1000 PY in 1945–1949 to 3.15 per 1000 PY in 2000–2007. Other studies also report increasing rates but over shorter periods. In the 14 years from 1993 through 2006, the US MarketScan administrative claims database reported an HZ incidence rate increase from 1.7 to 4.4 per 1000 PY among all ages [9]. Similarly, in Spain, the incidence increased from 2.5 to 3.6 per 1000 PY in the 8 years between 1997 and 2004 in a study population of all ages [11]. In Japan, the incidence increased from 3.8 to 4.5 per 1000 PY in the 10 years between 1997 and 2006 among all ages [12]. Most studies concluded that the incidence increased among all age groups and both sexes [8, 9, 11–13, 15, 16].
We found no evidence of an impact of varicella vaccination on the incidence of HZ. Our findings are consistent with other shorter-term studies examining the trend during the pre- and post-era of varicella vaccination in the United States, Canada, and Taiwan [8, 9, 13, 16, 26]. In a recent study of older adults (≥65 years) using Medicare claims data, age- and sex-standardized HZ incidence rates increased from 10.0 to 13.9 per 1000 PY in the 19 years between 1992 and 2010 without any evidence of change in the rate of increase after the introduction of the varicella vaccination program [8]. Additionally, prior studies have found that the increase occurred before the introduction of a national varicella vaccination program [7–9, 11–16]. However, our study is the first to provide a longer baseline and evidence of a longer and more robust trajectory of increasing HZ, further confirming the unlikely impact of decreased childhood varicella on a possible boosting immunity against VZV in adults [27, 28].
The introduction of the varicella vaccine did, however, appear to affect rates of HZ in preteen children. Consistent with prior studies, we observed a decline in the incidence of HZ following the introduction of varicella vaccination among children <10 years of age [26, 29]. Our reported decline among children <10 years of age in the decade after widespread use of varicella vaccine (from 0.83 to 0.35 per 1000 PY between 1990–1994 and 2005–2007) is similar to the decline from 0.75 to 0.33 per 1000 PY reported between 2000 and 2004 in another US study [29]. This decline is likely a result of the reported lower risk of HZ in children vaccinated with the live attenuated varicella vaccine (Oka strain) than in children with naturally acquired varicella [30, 31]. The possible later risk of HZ in vaccinated children remains unknown; however, our data are reassuring.
The cause of the increase of HZ remains unknown. The increase is unlikely to be due to the introduction of antiviral therapies. Furthermore, the increase cannot be fully explained by changes in the prevalence of immunocompromised individuals, as the percentage of immunosuppression among patients with HZ has not greatly changed over the time period. We cannot rule out the possibility of changes in healthcare-seeking over time. However, Leung et al used a national dataset to test this explanation as a cause for increasing HZ rates and found that it was unlikely in their setting [9]. In addition, studies within the Olmsted County population provided evidence that acute conditions have not necessarily increased over time [32]. A study of ocular herpes simplex virus (HSV) found no increase in incidence during 32 years of observation [32]. Primordial VZV emerged about 70 million years ago and VZV is considered genetically stable, although a genetic recombination between different strains could occur [33]. To our knowledge, there is no evidence of viral changes contributing to the increase.
Studies from Poland, Japan, Taiwan, and Australia reported that incidence of HZ is higher in the summer [12, 14, 15, 34]; however, our study, along with other studies, reported no seasonal difference [6, 10]. It has been speculated that high exposure to ultraviolet (UV) radiation may suppress cell-mediated immunity and possibly reactivate latent VZV during periods of high sun exposure such as the summer [34, 35]. HSV, which belongs to the same member of the Herpesviridae family as VZV, has been demonstrated to be reactivated by UV light [35]. We observed no seasonal variations in HZ rates but cannot rule out the possible role of other environmental factors in reactivation. More research is warranted to understand potential environmental factors associated with risk of HZ.
Our study has several limitations as well as strengths. We did not capture patients with HZ who did not seek medical care or who sought care outside the county, but both groups are anticipated to be small. The increase in healthcare professionals may contribute to the awareness about the disease; however, the percentage of adults employed in healthcare did not change sufficiently to explain the increase. The majority of Olmsted County residents are white, which may limit the generalizability of our findings. It is noteworthy that similar, shorter-term temporal increases were also observed across 7 countries with more diverse racial and ethnic populations [7–18]. We were not able to assess the incidence of HZ from 1961 to 1979 due to insufficient funding. However, given the steady increase over our study period, it is unlikely that a lack of data during those periods would have affected our findings. Major strengths of our population-based study include medical records validation of HZ cases and complete case ascertainment through a long-standing, well-established medical records linkage system [24]. The long-term racial homogeneity of the population is another strength. Given the higher incidence of HZ in white vs black individuals [8], our population's homogeneous racial composition mitigates the possibility that the observed temporal increase is due to racial demographic shifts. Furthermore, our study extends prior literature by demonstrating increasing trends for more than half a century.
The increasing public health burden of HZ is concerning and our study emphasizes the importance of preventing HZ and its complications through vaccination in older adults, as recommended by the US Advisory Committee on Immunization Practices [36]. Uptake has been low, and enhanced efforts are needed to increase the uptake of this vaccine, including healthcare providers recommending vaccine to their patients. The childhood varicella vaccination program has substantially reduced the risk of varicella morbidity and mortality in the United States [37, 38], but is not widely used in many other countries, in part due to the concerns that varicella vaccination may increase the incidence of HZ in adults [39]. The present and prior studies found no evidence of such effects and, therefore, support wider use of varicella vaccination [8, 9, 13, 16].
In conclusion, our population-based cohort study showed that the incidence of HZ has significantly increased in both sexes and among all age groups over the past 6 decades. This increase is unlikely to be due to the introduction of varicella vaccination, antiviral therapies, or change in the prevalence of immunocompromised individuals. The increasing incidence of HZ is a public health concern, and more research is required to elucidate the underlying cause of the 4-fold increase.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or the National Institutes of Health (NIH).
Financial support. This work was supported by the CDC (contract number 200-2009-32409); an investigator-initiated research grant from Merck & Co, Inc (to B. P. Y.); and the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the NIH (award number R01AG034676).
Potential conflicts of interest. K. K. was previously a consultant for Merck & Co, Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med 1965; 58:9–20. [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen JI. Clinical practice: herpes zoster. N Engl J Med 2013; 369:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007; 82:1341–9. [DOI] [PubMed] [Google Scholar]
- 4.Yawn BP, Wollan PC, St Sauver JL, Butterfield LC. Herpes zoster eye complications: rates and trends. Mayo Clin Proc 2013; 88:562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc 2011; 86:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis 2004; 4:26–33. [DOI] [PubMed] [Google Scholar]
- 7.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4:e004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med 2013; 159:739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993–2006: evaluation of impact of varicella vaccination. Clin Infect Dis 2011; 52:332–40. [DOI] [PubMed] [Google Scholar]
- 10.Brisson M, Edmunds WJ, Law B et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect 2001; 127:305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez-Farinós N, Ordobás M, García-Fernández C et al. Varicella and herpes zoster in Madrid, based on the Sentinel General Practitioner Network: 1997–2004. BMC Infect Dis 2007; 7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toyama N, Shiraki K; Society of the Miyazaki Prefecture Dermatologists. Epidemiology of herpes zoster and its relationship to varicella in Japan: a 10-year survey of 48,388 herpes zoster cases in Miyazaki prefecture. J Med Virol 2009; 81:2053–8. [DOI] [PubMed] [Google Scholar]
- 13.Chao DY, Chien YZ, Yeh YP, Hsu PS, Lian IB. The incidence of varicella and herpes zoster in Taiwan during a period of increasing varicella vaccine coverage, 2000–2008. Epidemiol Infect 2012; 140:1131–40. [DOI] [PubMed] [Google Scholar]
- 14.Wu PY, Wu HD, Chou TC, Sung FC. Varicella vaccination alters the chronological trends of herpes zoster and varicella. PLoS One 2013; 8:e77709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jardine A, Conaty SJ, Vally H. Herpes zoster in Australia: evidence of increase in incidence in adults attributable to varicella immunization? Epidemiol Infect 2011; 139:658–65. [DOI] [PubMed] [Google Scholar]
- 16.Russell ML, Schopflocher DP, Svenson L, Virani SN. Secular trends in the epidemiology of shingles in Alberta. Epidemiol Infect 2007; 135:908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esteban-Vasallo MD, Gil-Prieto R, Domínguez-Berjón MF, Astray-Mochales J, Gil de Miguel A. Temporal trends in incidence rates of herpes zoster among patients treated in primary care centers in Madrid (Spain), 2005–2012. J Infect 2014; 68:378–86. [DOI] [PubMed] [Google Scholar]
- 18.Rimland D, Moanna A. Increasing incidence of herpes zoster among veterans. Clin Infect Dis 2010; 50:1000–5. [DOI] [PubMed] [Google Scholar]
- 19.Yawn BP, Wollan P, St Sauver J. Comparing shingles incidence and complication rates from medical record review and administrative database estimates: how close are they? Am J Epidemiol 2011; 174:1054–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ragozzino MW, Melton LJ, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982; 61:310–6. [DOI] [PubMed] [Google Scholar]
- 21.St Sauver JL, Grossardt BR, Yawn BP et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012; 41:1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012; 87:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol 2011; 173:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 2012; 87:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yawn BP, Wollan P. Interrater reliability: completing the methods description in medical records review studies. Am J Epidemiol 2005; 161:974–7. [DOI] [PubMed] [Google Scholar]
- 26.Tanuseputro P, Zagorski B, Chan KJ, Kwong JC. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine 2011; 29:8580–4. [DOI] [PubMed] [Google Scholar]
- 27.Donahue JG, Kieke BA, Gargiullo PM et al. Herpes zoster and exposure to the varicella zoster virus in an era of varicella vaccination. Am J Public Health 2010; 100:1116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaillat J, Gajdos V, Launay O et al. Does monastic life predispose to the risk of Saint Anthony's fire (herpes zoster)? Clin Infect Dis 2011; 53:405–10. [DOI] [PubMed] [Google Scholar]
- 29.Civen R, Chaves SS, Jumaan A et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J 2009; 28:954–9. [DOI] [PubMed] [Google Scholar]
- 30.Hardy I, Gershon AA, Steinberg SP, LaRussa P. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. Varicella Vaccine Collaborative Study Group. N Engl J Med 1991; 325:1545–50. [DOI] [PubMed] [Google Scholar]
- 31.Tseng HF, Smith N, Marcy SM, Sy LS, Jacobsen SJ. Incidence of herpes zoster among children vaccinated with varicella vaccine in a prepaid health care plan in the United States, 2002–2008. Pediatr Infect Dis J 2009; 28:1069–72. [DOI] [PubMed] [Google Scholar]
- 32.Young RC, Hodge DO, Liesegang TJ, Baratz KH. Incidence, recurrence, and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976–2007: the effect of oral antiviral prophylaxis. Arch Ophthalmol 2010; 128:1178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norberg P, Depledge DP, Kundu S et al. Recombination of globally circulating varicella zoster cirus. J Virol 2015; 89:7133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zak-Prelich M, Borkowski JL, Alexander F, Norval M. The role of solar ultraviolet irradiation in zoster. Epidemiol Infect 2002; 129:593–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norval M. The effect of ultraviolet radiation on human viral infections. Photochem Photobiol 2006; 82:1495–504. [DOI] [PubMed] [Google Scholar]
- 36.Harpaz R, Ortega-Sanchez IR, Seward JF;. Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57(RR-5):1–30. quiz CE2-4. [PubMed] [Google Scholar]
- 37.Nguyen HQ, Jumaan AO, Seward JF. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. N Engl J Med 2005; 352:450–8. [DOI] [PubMed] [Google Scholar]
- 38.Marin M, Meissner HC, Seward JF. Varicella prevention in the United States: a review of successes and challenges. Pediatrics 2008; 122:e744–51. [DOI] [PubMed] [Google Scholar]
- 39.Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly Epidemiol Rec 2014; 89:265–87. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.