Abstract
Objective: The efficacy of low-level laser therapy for noninvasive body contouring has been previously demonstrated in clinical trials leading to its market clearance. Subjects achieved these beneficial effects following three weekly low-level laser therapy treatments for two weeks. The objective of this study was to determine if the same aesthetic benefit can be achieved following one weekly low-level laser therapy treatment for six weeks. Setting: Two private dermatology practices. Participants: Healthy adults with a body mass index of 25 to 40kg/m2 (N=54). Measurements: Subjects underwent one weekly low-level laser therapy procedure for six consecutive weeks using a device consisting of six 17mW, 635nm red diodes. Waist, hip, thigh, and upper abdomen circumference were measured weekly. Study success criteria was a 4.5-inch mean decrease in combined body circumference. Results: The mean decrease in combined circumference reduction at six weeks was 5.4 inches (p<0.001), and most subjects (72.2%) achieved a ≥4.5-inch decrease. Most subjects (81.0%) were Satisfied (27%) or Very Satisfied (54%) with the aesthetic results they achieved. There were no adverse events. Conclusion: One weekly low-level laser therapy treatment for six weeks is clinically effective for reducing waist, hip, thigh, and upper abdomen circumference and may be more effective than the previous two-week treatment protocol. ClinicalTrials.gov Identifier: NCT02109107.
Low level laser therapy (LLLT) has become established as an effective method for reducing waist, hip, thigh, and upper arm circumference of overweight, but non-obese individuals (body mass index [BMI] <30kg/m2).1-4 Because it is a completely noninvasive procedure, the use of LLLT for body contouring is not associated with adverse effects, such as pain or infection and no adverse changes in serum lipids.5 LLLT has no adverse effects on the skin or underlying musculofascial system and may improve the appearance of cellulite.6 There is no required recovery period following treatment.
Clinical studies demonstrating the effectiveness of LLLT have used red diodes emitting light at a wavelength of 635nm applied for 40 minutes (20 minutes to front and back) three times weekly for two weeks1-3 or green diodes emitting light at a wavelength of 532nm for 30 minutes (15 minutes to front and back) three times weekly for two weeks.4 A similar treatment protocol has demonstrated the effectiveness of the 635nm wavelength for reducing upper arm circumference.7
The purpose of this clinical study was to test the hypothesis that the effectiveness of 635nm red laser LLLT for noninvasive body contouring of the waist, hips, and thighs can be increased by changing the treatment protocol from three treatment weekly for two weeks to one treatment weekly for six weeks. In addition, this study assessed the effectiveness of LLLT for decreasing the circumference of the upper abdomen.
MATERIALS AND METHODS
Participants. Eligible subjects were 18 to 65 years old, had a BMI of 25 to 40kg/m2, and met criteria for the use of liposuction for removing localized deposits of adipose tissues that do not respond to diet and exercise, and specifically for body contouring in the areas of the hips, waist, thighs, and upper abdomen.8 Each subject expressed their willingness to abstain from treatments designed to promote weight loss including herbal supplements, appetite suppressants, diet plans, or other treatments that promote body contouring or circumference reduction during the study and maintain their regular normal diet and exercise regimen.
Reasons for study exclusion included known cardiac disease, prior cardiac surgery or presence of a pacemaker; prior surgical intervention for body sculpting or weight loss such as liposuction, abdominoplasty, stomach stapling, or lap band surgery; current use of a medication known to affect body weight, cause bloating or swelling, and which could not be safely discontinued; medical conditions known to affect weight levels and/or to cause bloating or swelling; diagnosis of, and/or taking medication for, irritable bowel syndrome; active infection, wound, or other external trauma to the target treatment areas; known photosensitivity disorder; current active cancer or currently receiving treatment for cancer; current pregnancy or planned pregnancy prior to the end of the study; serious mental health illness or psychiatric hospitalization during the past two years; developmental disability or cognitive impairment that would preclude adequate comprehension of the informed consent form or jeopardize the objectives of the study; involvement in litigation, worker’s compensation claim, or receiving disability benefits for weight- or body shape-related issues; or participation in a clinical study or other type of research in the past 30 days.
Ethics. Each subject provided informed consent prior to participating in any study-related activities. The study protocol was approved by an institutional review board (Western Institutional Review Board®, Puyallup, Washington) and conformed to the Good Clinical Practice guidelines of the International Conference on Harmonization.9 ClinicalTrials.gov Identifier: NCT02109107.
Study device. The device used in this study comprises six independent 17mW, 635nm red laser diodes (Erchonia® Zerona 6-Headed Scanner (EZ6); Erchonia Corporation, McKinney, Texas). The device utilizes internal mechanics, which collects the light emitted from each laser diodes and processes it through a proprietary lens system that redirects the beam with a line refractor. The refracted light is then bent into a circular pattern that is completely random and independent of other individual diodes. Each diode emits a line of light approximately 3mm wide and 9cm long having an energy of approximately 0.0002 joules per cm2/minute/treated area at a distance of 3 inches (7.6cm) and approximately 0.0001 joules per cm2/min/treated area at a distance of 4 inches (10.2cm).
Study procedures. The first LLLT treatment was performed during clinic Visit 1 after baseline body measurements were obtained. With subjects laying on their back on a procedure table, the device was positioned 6 inches (15.2cm) above their lower and upper abdomen, stomach, hips, and bilateral thighs area and centered along the body midline, and was activated for exactly 30 minutes. The subjects were then asked to lay on their stomach and the corresponding dorsal areas were treated for an additional 30 minutes. The procedure was repeated weekly for six weeks. Circumference measurements and BMI determinations were made at baseline, after each weekly procedure, and two weeks after the final procedure. The investigators and subjects were required to wear specialty safety glasses, which provide protection against 635nm red laser light.
To ensure consistency, the same trained individual at each study site performed all circumference measurements. Hip circumference was measured such that both hip bones were encircled. For the waist (mid-abdomen) measurement, the distance from the hip bone to the point at which the waist circumference was measured was recorded and used as a reference point for each subsequent measurement. Similarly, the distance from the natural waist to the point at which the upper abdomen was measured was recorded and used as a reference point for subsequent upper abdomen measurements. The distance from the hip bone to the point at which the circumference of the thighs was measured and used as a reference point for subsequent thigh measurements.
Outcome measures. The primary outcome measure was the change in combined bilateral thigh, hip, waist, and upper abdomen circumference from baseline to completion of the sixth and final procedure and two weeks after completing the final procedure. The pre-established study success criteria was a minimum mean decrease in combined circumference of 4.5 inches (11.4cm). This criterion was determined by the United States Food and Drug Administration (FDA) to be statistically significant and clinically meaningful based on previous LLLT clinical studies.
At six weeks, subjects were asked to rate their satisfaction with the aesthetic results they achieved using a 5-point Likert scale: Very satisfied, Somewhat satisfied, Neither satisfied nor dissatisfied, Not very satisfied or Not at all satisfied. Safety assessments included examination of the treatment areas by the investigators and reports of adverse events.
Data analysis. The intent-to-treat (ITT) population analysis includes all enrolled subjects who had circumference measurements recorded at baseline. Inclusion of data from subjects that dropped out of the study was done according to a pre-determined last observation carried forward procedure. The per-protocol population analysis was based on data from subjects who completed the entire study protocol through to study endpoint evaluation. The purpose of the per-protocol population analysis was to corroborate and support the conclusions drawn from the analysis of data based on the ITT population. A t-test analysis for two independent samples was performed to evaluate the difference in the cumulative mean change (waist, hips, bilateral thigh) between the 2- and 6-week treatment protocols.
RESULTS
Demographics. Fifty-four subjects were enrolled in the study and 52 completed the treatment phase and two-week post-treatment study endpoint. Two subjects dropped out after the fifth treatment visit. The demographic characteristics of the enrolled subjects are summarized in Table 1.
TABLE 1.
Baseline demographic characteristics
| N (%) | |
|---|---|
| Gender | |
| Female | 51 (94) |
| Male | 3(6) |
| Race/Ethnicity | |
| Caucasian | 47 (87) |
| African American | 4(7) |
| Hispanic | 2(4) |
| Asian | 1 (2) |
| MEAN (SD) MIN, MAX | |
| Body weight, kg | 78.95 (11.59) 57.73,121.18 |
| BMI, kg/m2 | 29.3 (3.6) 25.1,38.8 |
| Hip circumference, in | 42.1 (2.0) 37.0, 47.0 |
| Waist circumference, in | 39.7 (2.9) 33.5, 49.8 |
| Upper abdomen circumference, in | 36.1 (3.9) 28.5, 47.5 |
| Right Thigh Circumference, in | 24.5 (1.6) 20.0, 29.0 |
| Left thigh circumference, in | 24.3 (1.7) 19.0, 29.5 |
| Combined areas circumference, in | 166.7 (8.8) 144.5, 193.3 |
ITT population, N=54
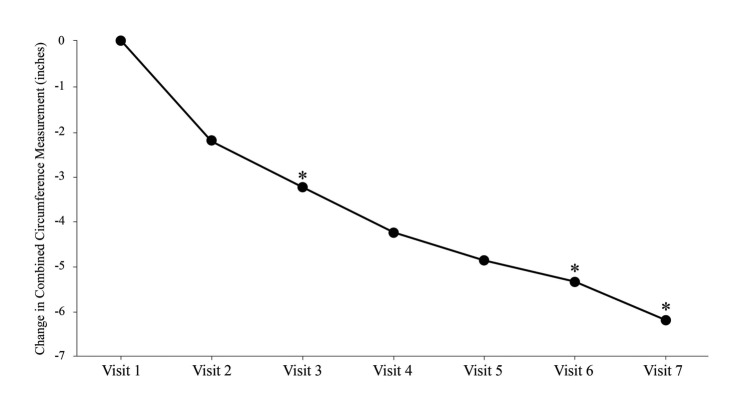
The mean combined decrease in circumference measurement at six weeks was 5.4 inches (13.7cm; p<0.0001). In addition, 40 subjects (72.2%) attained a combined circumference measurement decrease of ≥4.5 inches (11.4cm). The total baseline circumference measurements decreased progressively at each of the three evaluation points culminating in a mean decrease of 6.2 inches (15.7cm) by the two-week post-treatment follow-up evaluation at Visit 7 (Table 2 and Figure 1).
TABLE 2.
Mean total circumference measurements
| MEAN COMBINED CIRCUMFERENCE, cm | SIGNIFICANCE* | |
|---|---|---|
| Baseline | 423.34 | -- |
| Midpoint (Week 3) | 415.01 | p<0.01 |
| Endpoint (Week 6) | 409.72 | p<0.01 |
| 2-week follow-up | 407.54 | p<0.01 |
Change from baseline measurement, ITT population, N=54
Figure 1.
Change in combined body circumference. Treatment 1 was performed at Visit 1 immediately after baseline circumference measures were obtained. The last treatment was performed at Visit 6 (Endpoint). Visit 7 was 2 weeks after the last treatment. Data represent the ITT population, (N=54). *denotes p<0.01 vs. baseline.
The change in mean circumference measurements for the right and left thighs, hips, waist, and upper abdomen are summarized in Table 3. The maximum decrease in total mean circumference was achieved at the two-week post-treatment evaluation for the hips (1.2 inches; 3.0cm), waist (1.8 inches; 4.6cm), upper abdomen (1.3 inches; 3.3cm), right thigh (1.1 inches; 2.8cm), and left thigh (0.9 inches; 2.3cm) (for each, p<0.01 vs. Baseline). Similar to the combined circumference reduction measurements, each of the individual baseline anatomical area circumference measurements progressively decreased at each evaluation visit.
TABLE 3.
Change in mean combined circumference of each anatomical area
| BASELINE | 3-WEEK MIDPOINT | 6-WEEK ENDPOINT | 2-WEEK FOLLOW-UP | SIGNIFICANCE* | |
|---|---|---|---|---|---|
| Hips, cm | 106.91 | 105.00 | 103.93 | 103.99 | p<0.01 |
| Waist, cm | 100.81 | 98.50 | 97.16 | 96.32 | p<0.01 |
| Left thigh, cm | 61.75 | 60.71 | 59.79 | 59.41 | p<0.01 |
| Right thigh, cm | 62.20 | 61.03 | 59.99 | 59.46 | p<0.01 |
| Upper abdomen, cm | 91.67 | 89.79 | 88.87 | 88.34 | p<0.01 |
Change vs. baseline, ITT population, N=54
Mean (SD) body weight decreased from 79.0 (11.6) kg at Visit 1 to 78.3 (11.9) kg at Visit 6 while the mean BMI decreased from 29.3 (3.6) kg/m2 at Visit 1 to 29.0 (3.7) kg/m2 at Visit 6. Although these changes were statistically significant (for each, p<0.005), they were not clinically meaningful. An overall positive satisfaction rating following the final LLLT procedure was given by 81 percent of subjects (Table 4). There were no reports of adverse events and no evidence of skin changes in the treated areas.
TABLE 4.
Subject satisfaction
| N (%) | |
|---|---|
| Very satisfied | 28 (54) |
| Somewhat satisfied | 14 (27) |
| Neither satisfied nor dissatisfied | 4(7) |
| Not very satisfied | 3(6) |
| Not at all satisfied | 3(6) |
DISCUSSION
The results of this study confirm the results of previous studies demonstrating the effectiveness of LLLT for reducing circumference measurements of the bilateral thighs, hips, and waist circumference.1-4 In addition, it has extended these results to include significant reductions in upper abdomen circumference. The mean combined baseline circumference measurements decreased from 166.9 inches (423.9cm) to 161.4 inches (410.0cm) following the last treatment, a mean decrease of 5.4 inches (13.7 cm). This surpassed the pre-determined study success criteria of a minimum mean decrease in combined circumference of 4.5 inches (10.1cm) by 0.9 inches (2.3cm; p<0.0001). The mean body weight decreased by 0.7kg and the mean BMI decreased by 0.33kg/m2, but were considered negligible.
The change in mean cumulative circumference of subjects previously treated with LLLT using the two-week protocol1 was compared to the results obtained using the six-week protocol (waist, hips, bilateral thigh only) (Table 5). The mean combined circumference decreased by 4.9 inches (12.4cm) for the six-week protocol vs. 3.2 inches (8.1cm) for the two-week protocol (N=35;p<0.005). It should be noted that the subjects enrolled in the two-week protocol study had a mean BMI of 25.7kg/nr vs. 29.4kg/m2 in the current study.
TABLE 5.
Change in Mean Circumference of Each Treated Anatomical Area for Two LLLT Treatment Protocols
| 6-WEEK PROTOCOL | 2-WEEK PROTOCOL | |
|---|---|---|
| Waist, cm | ||
| Baseline | 100.79 | 86.21 |
| 2-week follow-up | 96.32 | 83.46 |
| Hips, cm | ||
| Baseline | 106.91 | 99.03 |
| 2-week follow-up | 103.99 | 97.26 |
| Right thigh, cm | ||
| Baseline | 62.20 | 60.45 |
| 2-week follow-up | 59.49 | 58.47 |
| Left thigh, cm | ||
| Baseline | 61.75 | 59.92 |
| 2-week follow-up | 59.41 | 58.22 |
| Cumulative mean change | -12.46 | -8.20 |
Although tumescent liposuction remains the gold standard for the removal of unwanted adipose tissue, there is a strong public demand for less invasive techniques. In addition to LLLT, several marketed devices are currently available that utilize thermal10 and mechanical ultrasound,11 cryotherapy,12 and various forms of radiofrequency13 for noninvasive body sculpting. Ultrasound devices are specifically indicated for the reduction of waist and abdominal circumference while cryotherapy is cleared for the reduction of abdomen, flank, and thigh circumference. The experience with radiofrequency for body contouring remains limited.14-16
Subjects undergoing treatment with thermal ultrasound commonly experience adverse events including mild or moderate procedural pain and ecchymosis.17 Cryotherapy is better tolerated although most subjects in one study reported minimal to tolerable procedural discomfort.18 In contrast, and in agreement with previous studies,1-4,7 subjects undergoing LLLT experienced no treatment-related discomfort or other post-treatment adverse effects. In addition, the six-week protocol will allows for a more convenient treatment plan, further increasing the likelihood of overall patient satisfaction.
Study limitations included a relatively small number of subjects and an open-label study design. In addition, the study population consisted primarily of Caucasian females. Future studies should enroll a great number of male and more racially diverse subjects. The use of dietary supplements with weight control claims were permitted, but their use was not recorded. Subjects were encouraged to use a diary for recording changes in lifestyle that included exercise and physical labor five days per week, but returning it to the investigators was not required.
Based on the results of this study, the device used here received marketing clearance from the FDA on May 21, 2015. The device is indicated for use as a non-invasive dermatological aesthetic treatment for the reduction of circumference of hips, waist, thighs and upper abdomen when applied to individuals with body mass index between 25 kg/m2 and 40 kg/m2.
CONCLUSION
The use of LLLT for body sculpting has been well-established. The results of the current study indicate that one weekly LLLT treatment for six weeks is more effective for reducing waist, hip, thigh, and upper abdomen circumference than the previous two-week treatment protocol, making LLLT therapy more convenient for subjects wishing to undergo body sculpting. It also further extends the known safety profile of LLLT for body sculpting.
ACKNOWLEDGMENT
This study was sponsored by Erchonia Corporation, McKinney, Texas. The authors acknowledge the careful manuscript review by Stephanie Shanks and the statistical support of Elvira Walls, Regulatory Insight, Inc.
Footnotes
DISCLOSURE:This study was funded by Erchonia Corporation, McKinney, Texas.
REFERENCES
- 1.Jackson RF, Dedo DD, Roche GC, et al. Low-level laser therapy as a non-invasive approach for body contouring: a randomized, controlled study. Lasers Surg Med. 2009;41:799–809. doi: 10.1002/lsm.20855. [DOI] [PubMed] [Google Scholar]
- 2.Jackson RF, Stern FA, Neira R, et al. Application of low-level laser therapy for noninvasive body contouring. Lasers Surg Med. 2012;44:211–217. doi: 10.1002/lsm.22007. [DOI] [PubMed] [Google Scholar]
- 3.McRae E, Boris J. Independent evaluation of low-level laser therapy at 635nm for noninvasive body contouring of the waist, hips, and thighs. Lasers Surg Med. 2013;45:1–7. doi: 10.1002/lsm.22113. [DOI] [PubMed] [Google Scholar]
- 4.Suarez DP, Roche GC, Jackson RF. A double-blind, sham-controlled study demonstrating the effectiveness of low-level laser therapy using a 532 nm green diode for contouring the waist, hips and thighs. Am J Cosmet Surg. 2013 in press. [Google Scholar]
- 5.Jackson RF, Roche GC, Wisler K. Reduction in cholesterol and triglyceride serum levels following low-level laser irradiation: a noncontrolled, nonrandomized pilot study. Am J Cosmet Surg. 2010;27:177–184. [Google Scholar]
- 6.Jackson RF, Roche GC, Shanks SC. A double-blind, placebo-controlled randomized trial evaluating the ability of low-level laser therapy to improve the appearance of cellulite. Lasers Surg Med. 2013;45:141–147. doi: 10.1002/lsm.22119. [DOI] [PubMed] [Google Scholar]
- 7.Nestor MS, Zarraga MB, Park H. Effect of 635nm low-level laser therapy on upper arm circumference reduction: a double-blind, randomized, sham-controlled trial. J Clin Aesthet Dermatol. 2012;5:42–48. [PMC free article] [PubMed] [Google Scholar]
- 8.American Academy of Cosmetic Surgery. 2006 Guidelines for Liposuction Surgery. [April 2015]. http://www.cosmeticsurgery.org
- 9.http://www.ich.org/products/guidelines/efficacy/efficacy-single/article/good-clinical-practice.html International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. E6 Good Clinical Practice: Consolidated Guidance. Available:
- 10.Jewell ML, Baxter RA, Cox SE, et al. Randomized sham-controlled trial to evaluate the safety and effectiveness of a high-intensity focused ultrasound device for noninvasive body sculpting. Plast Reconstr Surg. 2011;128:253–262. doi: 10.1097/PRS.0b013e3182174278. [DOI] [PubMed] [Google Scholar]
- 11.Ascher B. Safety and efficacy of UltraShape Contour I treatments to improve the appearance of body contours: multiple treatments in shorter intervals. Aesthet Surg J. 2010;30:217–224. doi: 10.1177/1090820X09360692. [DOI] [PubMed] [Google Scholar]
- 12.Krueger N, Mai SV, Luebberding S, et al. Cryolipolysis for noninvasive body contouring: clinical efficacy and patient satisfaction. Clin Cosmet Investig Dermatol. 2014;7:201–205. doi: 10.2147/CCID.S44371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beasley KL, Weiss RA. Radiofrequency in cosmetic dermatology. Dermatol Clin. 2014;32:79–90. doi: 10.1016/j.det.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Beasley KL, Weiss RA, Weiss MA. Dynamic monopolar reduction of arm fat by duplex ultrasound imaging and 3D imaging. Lasers Surg Med. 2013;45:20–21. [Google Scholar]
- 15.Weiss RA. Noninvasive radio frequency for skin tightening and body contouring. Semin Cutan Med Surg. 2013;32:9–17. [PubMed] [Google Scholar]
- 16.Anolik R, Chapas AM, Brightman LA, et al. Radiofrequency devices for body shaping: a review and study of 12 patients. Semin Cutan Med Surg. 2009;28:236–243. doi: 10.1016/j.sder.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Jewell ML, Weiss RA, Baxter RA, et al. Safety and tolerability of high-intensity focused ultrasonography for noninvasive body sculpting: 24-week data from a randomized, sham-controlled study. Aesthet Surg J. 2012;32:868–876. doi: 10.1177/1090820X12455190. [DOI] [PubMed] [Google Scholar]
- 18.Dierickx CC, Mazer JM, Sand M, et al. Safety, tolerance, and patient satisfaction with noninvasive cryolipolysis. Dermatol Surg. 2013;39:1209–1216. doi: 10.1111/dsu.12238. [DOI] [PubMed] [Google Scholar]