Introduction
Sudden unforeseen disturbances to balance, such as slips or trips, may evoke startle responses representing reflexive reactions to intense tactile, vestibular, or acoustic stimuli [26]. Startle responses are characterized by rapid bilateral sternocleidomastoid muscle activation within ~80ms of stimulus onset that propagates distally to limb muscles [9–11], marked reduction of neuromuscular and movement response amplitudes between first and subsequent repeated trials due to habituation [8, 23], and large first trial electromyographic responses with co-contracting muscles throughout the body. Rapid and exaggerated postural responses during first trial reactions (FTRs) triggered by transient external perturbations resemble startle responses and are seen during sudden events which disturb posture while standing [5, 21], walking [17, 20] and sitting [6, 8]. Studies of FTRs following standing balance perturbations have mainly focused on responses that stabilize the body in forward-backward or sideways directions, i.e., horizontally. Common to events which disrupt balance and result in falls is the gravity-driven downward motion of the body. Limited data from studies of human freefall where participants are hoisted above the ground and suddenly dropped, showed rapid and exaggerated muscle activation in FTRs resembling generalized muscle activity evoked by strong sensory stimuli such as a loud sound that triggers a startle reaction [13, 14]. The validity of this approach is questionable given the initial lack of ground support which is present prior to naturally occurring falls. If freefall FTR is characteristic of FTR to other threats to balance experienced during daily activities, then understanding whether FTRs aid or interfere with balance recovery is needed.
Whether or not repeated standing freefall perturbations habituate is unknown. Moreover, modulating the exaggerated FTR component through participant awareness and motor prediction (central set) may occur on voluntary self-activated freefall perturbations compared with externally imposed perturbations, indicating externally triggered FTRs include a startle reaction [12, 19]. Thus, the aim of the present study was to determine whether whole-body postural responses following repeated standing freefall perturbations from stable ground support resembled startle-induced reactions.
Participants and Methods
Nine healthy participants (five female) were enrolled (age 25.44 ± 2.3 years) and gave their informed consent to participate. The study was conducted in accordance with the standards of the Declaration of Helsinki and approved by the University of Maryland, Baltimore Institutional Review Board. Participants stood atop a moveable platform (45.6 cm wide, 50.5 cm long) secured to a fixed rigid frame using four electromagnets, (12 V DC, Magnetech Corp) allowing a 20 cm freefall onto a padded surface. The platform was soundlessly released via computer at randomized time intervals for externally-triggered (EXT) reactive trials at random time intervals, or by push-button remote during self-activated (SLF) predictive trials once a ready signal for trial start was given by the investigator. The time delay between trigger and release of electromagnets was approximately 100ms.
Each session consisted of twelve trials of EXT and SLF condition freefalls with EXT trials preceding SLF. Participants were fitted with a safety harness with adequate slack to avoid interfering with postural responses. Participants were positioned consistently across both conditions and instructed to look straight ahead and react naturally. Thirty seconds were allotted between trials within each condition. Freefall onset occurred without warning in EXT trials, and was determined by participants who initiated it at a self-selected time once the signal for the trial was given during SLF trials. To minimize habituation participants received no practice trials, and twenty minutes were allotted between the end of EXT trials and the start of SLF trials [16]. To evaluate the effectiveness of the washout interval in minimizing possible order effects, a different cohort of five (one female) untested participants (age 23.6 ± 2.51 years) received sequential EXT trial blocks with a 20 minute seated rest period between them..
Kinematic responses were recorded via a six camera motion capture system (VICON, Los Angeles, CA). Reflective markers were placed on the head, shoulders, elbows, wrists, and the drop platform to determine freefall onset. Data were sampled at 120Hz for 5s following perturbation onset. Coordinate data were smoothed with a 4th order low-pass Butterworth filter with a cutoff frequency of 7 Hz [25]. Pilot studies indicated the presence of robust arm flexion-abduction postural/startle responses to externally imposed freefalls. Thus, initial peak shoulder abduction acceleration defined as the maximum amplitude of acceleration elicited by initial shoulder abduction in response to platform release was used as a representative kinematic marker. Reflective markers placed on the acromia and lateral humeral epicondyles bilaterally formed a two segment model for determining the shoulder abduction angle relative to vertical and shoulder abduction angular acceleration was determined by double differentiation of the angular positon data.
Electromyographic (EMG) activity was recorded (NORAXON, Scottsdale, AZ) bilaterally over the deltoid (DLT), biceps brachii (BIC), medial gastrocnemius (GAS), tibialis anterior (TA) muscles, as well as sternocleidomastoid (SCM) given the high probability of this muscle’s activation following a startling stimuli [3, 9, 24]. Data were recorded at 1500Hz from 2s before to 3s following trigger signal and filtered online with a 10–500Hz band-pass filter before being high pass filtered (20Hz), rectified, and smoothed using a digital 4th order Butterworth filter.
Customized graphical analysis programs (The MathWorks, Inc., Natick, MA) were used during EMG processing. EMG Onset was defined relative to perturbation onset as the time when the rectified EMG value exceeded mean plus 3 standard deviations (SD) from baseline (100ms before trigger signal) for 30ms. Peak EMG activity was defined as the maximal EMG value recorded within the initial phasic response and was normalized as a percentage of the maximal root mean square (RMS) amplitude obtained during FTR. Prior to statistical analysis, peak EMG amplitude values were log-transformed to correct for skewed distributions and heteroscedasticity.
The overall incidence of SCM activation within 100ms in FTR and subsequent trials between conditions was determined using Fisher’s Exact Test. Wilcoxon Signed-Rank Test was used to assess significance of temporal organization in FTR for EXT condition. If significance was found, post-hoc analysis was performed using Friedman’s test. To evaluate the effectiveness of a 20 minute washout period in minimizing habituation carryover, the successive trial block EXT FTRs before washout (Pre20min) and after washout (Post20min) were compared using Wilcoxon signed-rank test. The effects of repeated freefall perturbations on response parameters (EMG onsets and peak amplitude, and peak shoulder acceleration) were compared between initial and trials 2–5 for each condition using a one-way repeated measures ANOVA with four levels (EXTFTR, EXT2-5, SLFFTR, SLF2-5) on the log transformed values with Bonferroni post-hoc analyses. Significance for all statistical tests was set at p <0.05. Data are presented as mean ± SEM.
Results
Neuromuscular responses
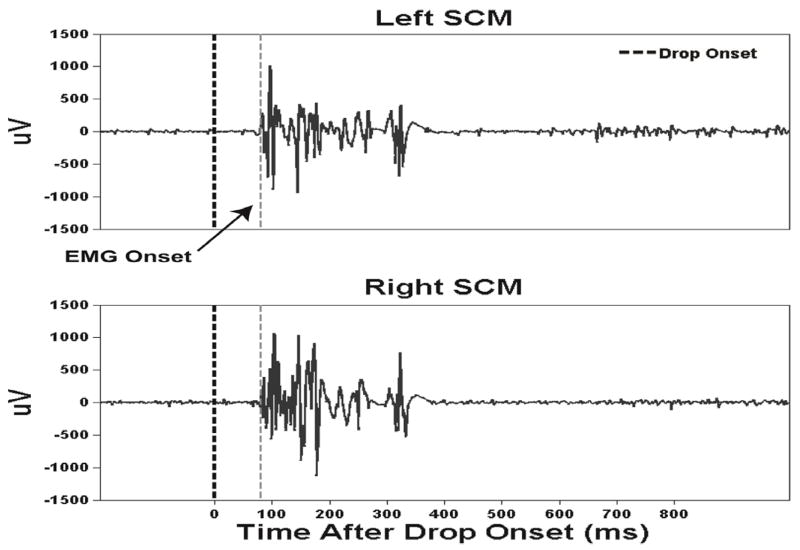
Representative FTR bilateral SCM EMG activity during the EXT condition is shown in Fig. 1. Rapid phasic bilateral and synchronous SCM activity within 100ms after stimulus onset is a hallmark of a startle response [7] (Table 1). EMG FTR amplitudes for EXT (Pre20min) and EXT (Post20min) trial blocks showed that the rest period resulted in statistically non-significant differences in EMG amplitudes during FTR for SCM, DLT, and BIC (p > .05) indicating that the EXT and SLF FTR comparisons were not likely attributable to the carryover effects of habituation (Table 2).
Fig. 1.
Representative example trial of electromyographic (EMG) responses from bilateral sternocleidomastoid neck muscles following an externally triggered freefall perturbation of the standing support surface in one subject.
Table 1.
Group mean (±SEM) EMG onset latencies (ms) for Externally-Triggered (EXT) and Self-triggered (SLF) freefall perturbations.
| EXT | SLF | |||
|---|---|---|---|---|
|
|
||||
| First Trial | Trials 2–5 | First Trial | Trials 2–5 | |
| SCM | 62.49 ± 4.75 | 73.33 ± 6.28 | 69.35 ± 4.96 | 75.53 ± 6.04 |
| DLT | 67.19 ± 4.39 | 76.54 ± 8.50 | 81.37 ± 11.49 | 102.82 ± 21.38 |
| BIC | 72.42 ± 7.91 | 87.00 ± 9.90 | 80.76 ± 13.95 | 102.09 ± 24.58 |
| GAS | 92.12 ± 9.85 | 95.83 ± 11.00 | 90.73 ± 7.49 | 86.13 ± 13.28 |
| TA | 65.71 ± 4.43 | 68.84 ± 3.97 | 75.37 ± 6.88 | 78.26 ± 5.92 |
EXT = externally activated SLF = self-activated drop perturbations
SCM= sternocleidomastoid, DLT= deltoid, BIC=biceps brachii, GAS= medial gastrocnemius, TA=tibialis anterior
Table 2.
Group mean (±SEM) log-transformed peak EMG response amplitudes (μV) during sequential Externally-Triggered (EXT) trial blocks and for EXT versus Self-triggered (SLF) freefall perturbations.
| EXT 1 vs. EXT 2 First Trial | EXT | SLF | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Pre20min | Post20min | First Trial | Trials 2–5 | First Trial | Trials 2–5 | |
| SCM | 5.78 ±0.25 | 5.60 ± 0.33 | 5.62 ± 0.11† | 4.79 ± 0.24 | 4.51± 0.24 | 3.81 ± 0.21* |
| DLT | 6.00 ± 0.18 | 5.45 ± 0.17 | 5.67 ± 0.26† | 4.91 ± 0.31 | 4.67 ± 0.31 | 3.82 ± 0.36* |
| BIC | 5.60 ± 0.28 | 5.91 ± 0.40 | 5.77 ± 0.16† | 4.74 ± 0.29 | 4.78 ± 0.27 | 3.91 ± 0.26* |
| GAS | 5.47± 0.30 | 4.90 ± 0.11 | 5.03 ± 0.23 | 4.92 ± 0.25 | 4.77 ± 0.23 | 4.92 ± 0.19 |
| TA | 5.80 ± 0.05 | 5.70 ± 0.05 | 5.52 ± 0.17 | 5.21 ± 0.10 | 5.11 ± 0.16 | 4.71 ± 0.22 |
SCM= sternocleidomastoid, DLT= deltoid, BIC=biceps brachii, GAS= medial gastrocnemius, TA=tibialis anterior EXT1 and EXT2 = consecutive externally (EXT) activated drop perturbation trials pre and post 20 minute rest period.
Denotes significant differences between first trial response and averaged trials 2–5, p < 0.05.
Denotes significant differences between externally triggered (EXT) and self-triggered (SLF) trials, p < 0.05.
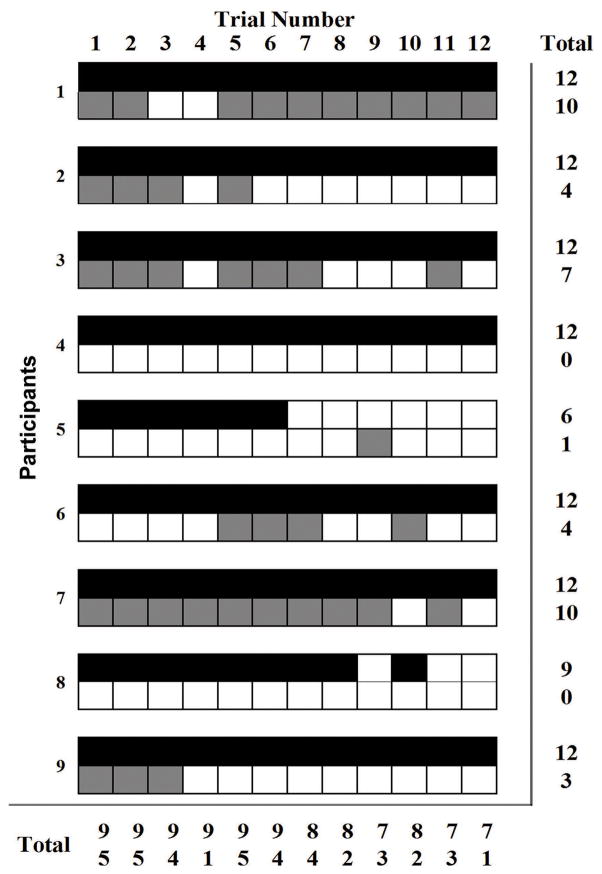
A larger proportion of early SCM activation (100% trials) from all participants during EXTFTR occurred compared to 56% of trials from five participants during SLF (p < 0.01). Across all trials, a larger proportion of SCM activity was present in the EXT condition (92%) compared with the SLF condition (36%) (p < 0.01). Incidence of SCM muscle activation decreased from first trial exposure to the last trial for both EXT and SLF conditions (Fig. 2). By the 12th trial, seven participants produced detectable SCM onsets during EXT compared to one participant for SLF.
Fig. 2.
Presence of detectable sternocleidomastoid neck muscle responses for externally triggered (EXT, top rows black cells) freefall perturbation trials and self-triggered (SLF, bottom rows grey cells) perturbation trials for all subjects and trials. Broken vertical line indicates the instant of drop perturbation onset.
Mean EMG onset latencies for EXT and SLF conditions for all muscles generally occurred within 100ms after perturbation onset and ANOVAs for all muscle onset times were not significant (p > 0.05) indicating no differences between conditions or time points (Table 1). No significant difference in temporal organization was found for EXTFTR (p =f121). SCM, DLT, and BIC onset latencies during EXTFTR were generally earlier for at least 57% of participants compared to SLFFTR. A common pattern between EXT vs. SLF trials was observed for SCM, DLT, and BIC. EXTFTR was larger than EXT2-5, SLFFTR was larger than SLF2-5 and EXTFTR condition was larger than the SLFFTR condition (Table 2). These differences were significant with p < 0.01. TA amplitude was greater in EXTFTR compared to SLFFTR (p < 0.01) and between SLFFTR and SLF2-5 (p = 0.02) but no differences were found between EXTFTR and EXT2-5 (p = 0.07) and EXT2-5 and SLFFTR (p = 0.29). GAS amplitude was unmodified (p > 0.05) between first and later trials during both EXT and SLF conditions.
Arm movement kinematics
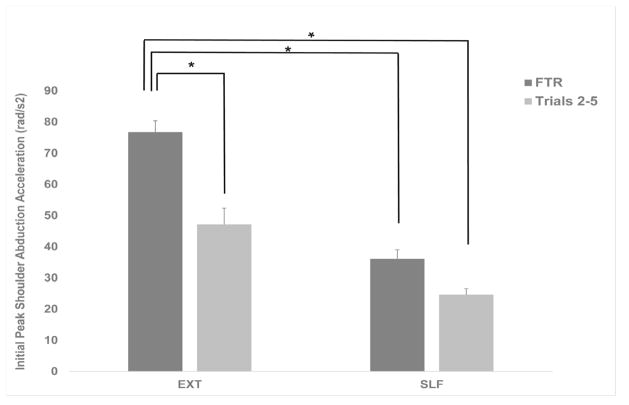
Figure 3 shows the means and standard errors (SEM) of initial peak shoulder abduction acceleration under each condition for FTR and trials 2–5. A significant main effect (p <0.01) and post-hoc analyses indicated differences between EXTFTR and EXT2-5 (76.73 rad/s2 ± 8.32 vs. 47.12 rad/s2 ± 11.79 rad/s2, mean ± SEM, p <0.05) and between EXTFTR and SLFFTR (36.15 rad/s2 ± 6.46 ) and SLFLTR (24.63 rad/s2 ± 4.41 p < 0.05).
Fig. 3.
Group means and standard error of means (SEM) of initial shoulder abduction angular acceleration following EXT and SLF triggered freefall perturbations. Black bars denote 1st trial response and grey bars represent trials 2–5 response. Significant differences in the response within and between conditions are indicated with asterisks (*, p <.05).
Discussion
In the present study, rapid and exaggerated neuromuscular and kinematic first trial responses and subsequent attenuated responses to an externally imposed freefall perturbation of human standing balance resembled the spatiotemporal characteristics of previously reported startle responses incorporated into postural reactions during standing [6, 22], walking [18, 21], and sitting [7, 9]. To our knowledge, our findings are among the first to show differences in the incidence and magnitude of FTRs in neck and upper limb muscles during freefall, resembling those classically associated with startle elicited by other strong stimuli such as a loud sound, as well as in the habituation behavior between externally triggered reactive perturbations compared with self-activated perturbations. These observations suggested that reactive responses to falls include a startle component, especially during the first unpracticed perturbation exposure.
Consistent with the presence of a startle response during reactive freefalls was the high incidence of rapid (<100ms), bilateral and synchronous SCM neck muscle activation: a hallmark motor characteristic for identifying the presence of a startle reaction. SCM activation occurred for all participants on the reactive freefall FTR and in 92% of the total trials compared with only five participants for predictive FTRs and 36% of the total trials. When participants had temporal certainty about the perturbation’s occurrence, they likely suppressed startling themselves through feedforward mechanisms. Overall neck and arm muscle response amplitudes were greater for reactive trials compared with predictive trials. These differences between the FTR and subsequent responses of the EXT and SLF conditions further support the probability that the differences observed were, at least in part, attributable to a startle component.
Another distinguishing feature of the classical startle response is its tendency to habituate with repeated stimulus exposure as indicated by a reduced incidence and/or magnitude of response. Our findings showed reductions in EMG response amplitudes between FTR and trials 2–5 for SCM, DLT, and BIC during both EXT and SLF trials. However, the group mean reductions in amplitude calculated from the original peak EMG values for SCM, DLT, and BIC with EXT exceeded those for SLF by 13%, 4%, and 20% respectively. These greater reductions, and the arm abduction amplitude reduction between FTR and trials 2–5 for EXT but not SLF (see below), were consistent with a habituated startle component additional to postural adaptations that occurred for both conditions. This further supports the likelihood of the incorporation of a startle component into reactive but not predictive freefalls. The onset timing latencies were unchanged over repeated trials for each condition, which is consistent with other past studies of postural and startle reactions [1].
Despite habituation-like changes in neck and upper limb muscles during EXT trials, EMG amplitudes remained unchanged for the lower limb muscles TA and GAS while TA responses for SLF were reduced over repeated trials. This may reflect the need to consistently maximize joint stabilization on landing impact when temporal unpredictability about the perturbation onset limits the use of predictive control strategies. The observed difference in modulation patterns between EXT and SLF EMG amplitudes for TA suggested that ankle muscle co-activation may play a role in balance stabilization to freefall which can be modulated with repeated exposures when such perturbations are more predictable possibly resulting in a less stiff ankle joint and smaller landing impact force [12].
To further probe startle contributions to protective balance responses, we studied upper limb movements which are known to accompany startle reactions to different forms of high intensity stimuli [15, 18]. Similar to arm EMG responses for the EXT condition, robust shoulder abduction FTRs resembling those described for the parachute reaction seen during normal infant development were observed and subsequently habituated in amplitude by half during trials 2–5. Smaller amplitude SLF FTRs were unmodified during subsequent trials reflecting a lack of adaptation or habituation when perturbations were self-activated. This finding further supported the likelihood of a startle contribution to EXT triggered postural responses. Parachute reactions are characterized by abduction of the arms with extension of the elbows and wrists and represent a normal protective reflex elicited when an infant is held in ventral suspension and tilted abruptly forward toward the floor [27]. However, it remains unknown whether or not the response is disruptive or protective during falls among adults. If the presence of the parachute response is a common occurrence during FTRs to sudden balance disturbances, then further investigation of older adults and other clinical populations at risk for falls should be conducted in order to understand potential abnormalities in protective arm responses during falls. This could help shed light on underlying neurophysiological mechanisms for fall recovery in at risk individuals.
The neural processes underlying startle and postural responses involve brainstem structures including the ponto-medullary reticular formation and vestibular nuclei stimulated by multisensory inputs with activation of spinal motor neurons over the reticulospinal and vestibulospinal pathways [26]. During a freefall perturbation, the sudden removal of mechanoreceptive stimulation to the soles of the feet, an abrupt loss of load proprioceptive input to the lower limbs, or downward vertical acceleration of the head engaging the otoliths may have contributed to triggering and adapting the rapid neuromuscular responses. Feedforward modulation of brainstem and spinal circuits over corticofugal and corticospinal pathways during the SLF condition may also have accounted for differences in responses between the conditions [12]. Cerebellar involvement in the adaptation of postural response patterns may be further implicated in changes occurring with trial and error practice [2].
A study limitation was that no direct comparison was performed between freefall induced response profiles indicative of startle and those triggered by a known standard reference stimulus such as a loud sound [8, 22]. Studies comparing neuromuscular responses to a startling acoustic stimulus with sudden tilts of a standing platform [22] or freefall of an angled mattress while recumbent [3, 4] showed similarities between the different forms of eliciting stimuli. We also did not randomize the presentation order of the freefall perturbation stimuli, and as a result it is possible that habituation effects obtained during EXT trials carried over to SLF condition trials rather than the latter being attributable to a feedforward modulation of response incidence or amplitude associated with the SLF condition. To minimize such effects, 15–20 minutes were allotted between two successive EXT trial blocks and comparisons between them showed no differences in neck and arm muscle FTR amplitudes indicating no habituation carryover effects between conditions with this rest interval. [16].
Conclusion
Presence of rapid neck and limb muscle activation, larger FTRs, and subsequent habituated responses suggested that especially FTRs to externally imposed freefall include a startle component together with balance stabilizing responses. EXT FTR incidence and EMG response amplitudes for neck, shoulder, and elbow muscles decreased after repeated perturbation exposures to a greater extent overall than for SLF activated responses, likely indicating the presence of startle habituation in addition to postural adaptation. EXT triggered neuromuscular responses were accompanied by arm abduction movements resembling the parachute reaction of normal infant development which were reduced with trial repetition and were smaller overall during the SLF condition without habituation. These findings have neurophysiological implications for understanding fall recovery mechanisms for at risk individuals such as older adults and those with clinical conditions.
Highlights.
First trial posture responses (FTR) to external (EXT) and self (SLF) set freefall.
Greater incidence of rapid EXT FTR neck muscle activation than SLF condition.
Incidence and amplitude of EXT neck and arm FTRs habituated with repeated trials.
EXT FTR freefall includes a startle incorporated with postural responses.
Acknowledgments
Supported by National Institutes of Health grants R01AG033607 and R01AG033607-S1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allum JH, Tang KS, Carpenter MG, Oude Nijhuis LB, Bloem BR. Review of first trial responses in balance control: influence of vestibular loss and Parkinson’s disease. Human movement science. 2011;30:279–295. doi: 10.1016/j.humov.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Current opinion in neurobiology. 2006;16:645–649. doi: 10.1016/j.conb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Bisdorff AR, Bronstein AM, Gresty MA. Responses in neck and facial muscles to sudden free fall and a startling auditory stimulus. Electroencephalography and clinical neurophysiology. 1994;93:409–416. doi: 10.1016/0168-5597(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 4.Bisdorff AR, Bronstein AM, Wolsley C, Gresty MA, Davies A, Young A. EMG responses to free fall in elderly subjects and akinetic rigid patients. Journal of neurology, neurosurgery, and psychiatry. 1999;66:447–455. doi: 10.1136/jnnp.66.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloem BR, van Vugt JP, Beckley DJ. Postural instability and falls in Parkinson’s disease. Advances in neurology. 2001;87:209–223. [PubMed] [Google Scholar]
- 6.Blouin JS, Descarreaux M, Belanger-Gravel A, Simoneau M, Teasdale N. Self-initiating a seated perturbation modifies the neck postural responses in humans. Neuroscience letters. 2003;347:1–4. doi: 10.1016/s0304-3940(03)00632-3. [DOI] [PubMed] [Google Scholar]
- 7.Blouin JS, Inglis JT, Siegmund GP. Startle responses elicited by whiplash perturbations. The Journal of physiology. 2006;573:857–867. doi: 10.1113/jphysiol.2006.108274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blouin JS, Siegmund GP, Timothy Inglis J. Interaction between acoustic startle and habituated neck postural responses in seated subjects. Journal of applied physiology. 2007;102:1574–1586. doi: 10.1152/japplphysiol.00703.2006. [DOI] [PubMed] [Google Scholar]
- 9.Brown P, Day BL, Rothwell JC, Thompson PD, Marsden CD. The effect of posture on the normal and pathological auditory startle reflex. Journal of neurology, neurosurgery, and psychiatry. 1991;54:892–897. doi: 10.1136/jnnp.54.10.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlsen AN, Dakin CJ, Chua R, Franks IM. Startle produces early response latencies that are distinct from stimulus intensity effects. Experimental brain research. 2007;176:199–205. doi: 10.1007/s00221-006-0610-8. [DOI] [PubMed] [Google Scholar]
- 11.Carlsen AN, Hunt MA, Inglis JT, Sanderson DJ, Chua R. Altered triggering of a prepared movement by a startling stimulus. Journal of neurophysiology. 2003;89:1857–1863. doi: 10.1152/jn.00852.2002. [DOI] [PubMed] [Google Scholar]
- 12.Fu S, Hui-Chan C. Mental set can modulate response onset in the lower limb muscles to falls in humans. Neuroscience letters. 2002;321:77–80. doi: 10.1016/s0304-3940(02)00060-5. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood R, Hopkins A. Muscle responses during sudden falls in man. The Journal of physiology. 1976;254:507–518. doi: 10.1113/jphysiol.1976.sp011242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones GM, Watt DGD. Muscular control of landing from unexpected falls in man. The Journal of physiology. 1971;219:729–737. doi: 10.1113/jphysiol.1971.sp009685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maki BE, McIlroy WE. The role of limb movements in maintaining upright stance: the “change-in-support” strategy. Physical therapy. 1997;77:488–507. doi: 10.1093/ptj/77.5.488. [DOI] [PubMed] [Google Scholar]
- 16.Mang DW, Siegmund GP, Inglis JT, Blouin JS. The startle response during whiplash: a protective or harmful response? Journal of applied physiology. 2012;113:532–540. doi: 10.1152/japplphysiol.00100.2012. [DOI] [PubMed] [Google Scholar]
- 17.Marigold DS, Bethune AJ, Patla AE. Role of the unperturbed limb and arms in the reactive recovery response to an unexpected slip during locomotion. Journal of neurophysiology. 2003;89:1727–1737. doi: 10.1152/jn.00683.2002. [DOI] [PubMed] [Google Scholar]
- 18.McIlroy WE, Maki BE. Early activation of arm muscles follows external perturbation of upright stance. Neuroscience letters. 1995;184:177–180. doi: 10.1016/0304-3940(94)11200-3. [DOI] [PubMed] [Google Scholar]
- 19.Nakazawa K, Kawashima N, Akai M. Effect of different preparatory states on the reflex responses of ankle flexor and extensor muscles to a sudden drop of support surface during standing in humans. Journal of Electromyography and Kinesiology. 2009;19:782–788. doi: 10.1016/j.jelekin.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Nieuwenhuijzen PH, Duysens J. Proactive and reactive mechanisms play a role in stepping on inverting surfaces during gait. Journal of neurophysiology. 2007;98:2266–2273. doi: 10.1152/jn.01226.2006. [DOI] [PubMed] [Google Scholar]
- 21.Oude Nijhuis LB, Allum JH, Borm GF, Honegger F, Overeem S, Bloem BR. Directional sensitivity of “first trial” reactions in human balance control. Journal of neurophysiology. 2009;101:2802–2814. doi: 10.1152/jn.90945.2008. [DOI] [PubMed] [Google Scholar]
- 22.Oude Nijhuis LB, Allum JH, Valls-Sole J, Overeem S, Bloem BR. First trial postural reactions to unexpected balance disturbances: a comparison with the acoustic startle reaction. Journal of neurophysiology. 2010;104:2704–2712. doi: 10.1152/jn.01080.2009. [DOI] [PubMed] [Google Scholar]
- 23.Siegmund GP, Blouin JS, Inglis JT. Does startle explain the exaggerated first response to a transient perturbation? Exercise and sport sciences reviews. 2008;36:76–82. doi: 10.1097/JES.0b013e318168f1ce. [DOI] [PubMed] [Google Scholar]
- 24.Siegmund GP, Inglis JT, Sanderson DJ. Startle response of human neck muscles sculpted by readiness to perform ballistic head movements. The Journal of physiology. 2001;535:289–300. doi: 10.1111/j.1469-7793.2001.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winter DA. Biomechanics and motor control of human movement. John Wiley & Sons; 2009. [Google Scholar]
- 26.Yeomans JS, Li L, Scott BW, Frankland PW. Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neuroscience and biobehavioral reviews. 2002;26:1–11. doi: 10.1016/s0149-7634(01)00057-4. [DOI] [PubMed] [Google Scholar]
- 27.Zafeiriou DI. Primitive reflexes and postural reactions in the neurodevelopmental examination. Pediatric neurology. 2004;31:1–8. doi: 10.1016/j.pediatrneurol.2004.01.012. [DOI] [PubMed] [Google Scholar]