Abstract
Phosphatase enzymes cleave an inorganic phosphate from a substrate. Phosphatase enzyme histochemistry followed by flat-embedding in glycol methacrylate is extremely useful in studying retinal and choroidal vascular development and loss, since only viable blood vessels have these enzyme activities. Sites of occlusion and remodeling can be identified and analysed, resulting in new insights into the cause of occlusion. The phosphatase activities are elevated in neovascularization making possible high resolution analysis of neovascularization, the feeder vessels, and the retinal milieu in which angiogenesis occurs. Adenosine diphosphatase (ADPase) catalyzes ADP to an inorganic phosphate plus adenosine monophosphate, preventing accumulation of ADP, one of the most potent stimuli for platelet aggregation. The ADPase technique can be used in any species but this report highlights its use in dog and human retinas. The ADPase technique has yielded important insights into vaso-occlusive and vasoproliferative processes in retinopathy of prematurity, sickle cell and diabetic retinopathies. The alkaline phosphatase flatembedding technique is useful in evaluating dog, cat, and human choroidal vasculatures. It has permitted quantification of the loss of choriocapillaris in diabetic choroidopathy and of the RPE and choriocapillaris in geographic atrophy and exudative age-related macular degeneration.
Keywords: Phosphatase, Enzyme histochemistry, Adenosine diphosphatase, Alkaline phosphatase, Blood vessels
1. Adenosine diphosphatase
Phosphatase enzymes cleave an inorganic phosphate from a substrate. Adenosine diphosphatase (ADPase) catalyzes ADP to an inorganic phosphate plus adenosine monophosphate and, like many other phosphatases, requires a divalent cation (magnesium). For enzyme histochemistry, the tissue is incubated with ADP, lead nitrate, and magnesium chloride in a TRIS maleate buffer (pH 7.2) resulting in a lead phosphate reaction product wherever ADPase is present in the tissue (Lutty & McLeod, 1992). Lessel and Kuwabara were the first to notice that this enzyme was specific for the blood vessels in retina (Lessell & Kuwabara, 1964). We have also observed the activity in endothelial cell precursors (angioblasts) in retina as well (Lutty & McLeod, 1992). ADPase is an ectoenzyme (present on the external surface of the plasma membrane) on endothelial cells, which is necessary to prevent platelet aggregation in the vascular lumen because ADP is one of the most potent stimuli for platelet aggregation. Recently, it has been determined that ectoADPase is CD39 (Marcus et al., 1997).
It was our desire to not just view the retinal vasculature as in a flat mount, but be able to perform a two dimensional analysis of the blood vessels in their normal neural retina milieu. So, we embedded retinas in the transparent polymer glycol methacrylate (JB4, Polysciences) after incubation for ADPase activity and fixing them in a flattened position (Lutty & McLeod, 1992). We could then view the retinal vasculature with darkfield illumination en bloc in the flat perspective and observe the lead ADPase reaction product in retinal blood vessels. We then could cut 2 μm sections of the retina through blood vessels documented in the flat perspective and achieve a second dimension in cross sections of the same blood vessels in their retinal milieu. The technique has proven invaluable in studying retinal vascular development as well as retinal vascular pathology that occurs in diabetes and sickle cell disease. The technique works in all species but the examples presented in this chapter are limited to dog and human retinas.
2. Retinal vascular development
As mentioned previously, ADPase is present in angioblasts as well as endothelial cells, so the differentiation of angioblasts into endothelial cells can be seen dramatically in dog and human inner retina using ADPase enzyme histochemistry. In the one-day-old dog, the retina is only 60% vascularized so vascular development is still ongoing at birth. ADPase+ angioblasts are present throughout the entire avascular peripheral retina, and can be seen organizing into vascular cords and primordial blood vessels (McLeod, Brownstein, & Lutty, 1996; McLeod, Crone, & Lutty, 1996). In sections of one-day old dog, ADPase+ angioblasts (McLeod et al., 1996; McLeod & Crone et al., 1996) differentiate from round to spindle shaped in the cell free spaces formed by innermost Muller cell processes. At the top of the spaces, angioblasts aggregate to form cords; eventually a lumen will form as the cord matures. We interpreted this to be the process of vasculogenesis, in situ formation of blood vessels from precursors, because it occurs in the absence of proliferation, as would occur in angiogenesis (McLeod et al., 1996; McLeod & Crone et al., 1996). This interpretation was recently confirmed in fetal human retinal vascular development, where ADPase+ angioblasts are present well in advance of the forming retinal vasculature and their position actually predicts where the initial retinal vasculature will form (Chan-Ling et al., 2004) (Figs. 1 and 2). More recently, using an antibody against CD39/ADPase and the confocal microscope, we have observed that CD39+ angioblasts do not proliferate (Ki67−) when they form the initial human retinal vasculature. Furthermore, they do not express CD34 or CD31, traditional endothelial cell markers, as individual angioblasts or when present in cords, only when they are present on lumens of newly formed blood vessels (Lutty, Hasegawa, Prow, Merges, & McLeod, 2005).
Fig. 1.
ADPase incubated retina from a 12-week gestation (WG) human fetus. (A) Darkfield illumination of the retina shows lead ADPase reaction product in the small retinal vasculature and ADPase+ angioblasts in advance of the formed blood vessels. (B) At higher magnification of the retina in part “A”, the ADPase+ angioblasts are more apparent in advance of the blood vessels (double arrow). Angioblasts are spindle shaped (arrows) in inner retina (C) and more round (arrowheads) in deeper retina (D). This is apparent in a section through this are when viewed with Nomarski optics (E) and when ADPase activity is developed with ammonium sulfide in the same section (F). Ammonium sulfide converts the lead to a brown reaction product. (A–D) Darkfield illumination of ADPase reaction product in flat-embedded retinas; (E) Nomarski optics of section; (F) transmitted light of brown ADPase reaction product and thionin counterstain (Republished with permission of the Association for Research in Vision and Ophthalmology, Fig. 1 from Chan-Ling T, McLeod DS, Hughes S, Baxter L, Chu Y, Hasegawa T, Lutty GA. Astrocyte-endothelial cell relationships during human retinal vascular development, Invest. Opthalmol. Vis. Sci. 2004;45:2020–2032; permission conveyed through Copyright Clearance Center, Inc.).
Fig. 2.
ADPase incubated retina from a 16WG human fetus. (A) The retinal vasculature has expanded considerably at this later age and the four vascular arcades are well defined. (B) Area indicated with double arrows in part “A” at higher magnification shows a field of angioblasts in advance of the forming blood vessels. The letters indicate where cross sections in parts “D–F” were taken from. (C) At even higher magnification, it is apparent that a primordial vessel (arrow) has formed distant from the rest of the retinal vasculature suggesting vasculogenesis. (D) In a section developed with ammonium sulfide, there are spindle-shaped angioblasts (arrow) above, around, and in advance of the formed blood vessel and round-shaped angioblasts below the vessel (arrowheads). (E–F) In this area, the spaces made by the inner Muller cell processes are obvious and ADPase+ angioblasts appear to moving through the spaces. (A–C) Darkfield illumination of flat-embedded retina; (D–F) ADPase reaction product and thionin counterstain (Republished with permission of the Association for Research in Vision and Ophthalmology, Fig. 3 from Chan-Ling T, McLeod DS, Hughes S, Baxter L, Chu Y, Hasegawa T Lutty GA. Astrocyte-endothelial cell relationships during human retinal vascular development, Invest. Opthalmol. Vis. Sci. 2004;45:2020–2032; permission conveyed through Copyright Clearance Center, Inc.).
3. Oxygen-induced retinopathy: A model for retinopathy of prematurity (ROP)
When the developing retinal vasculature is exposed to hyperoxia, endothelial cells die and vasculature is lost, which is referred to as vaso-obliteration. Using ADPase histochemistry, we have measured a 77% reduction in capillary density after 4 days exposure to 100% oxygen (McLeod et al., 1996; McLeod & Crone et al., 1996) (Fig. 3). This assessment of vascularity or percent vascular area is easily accomplished because the lead ADPase reaction product viewed with darkfield illumination is an almost a black and white image (white ADPase+ blood vessels against a black background). Digital images of the preparation are made binary and the number white pixels is calculated by NIH Image software (Lutty & McLeod, 1992). This analysis could not be accomplished with a vascular tracer because many of the vascular segments after vaso-obliteration are highly constricted or do not have blood flow at all.
Fig. 3.
ADPase-incubated retinas from a normal (A) and an oxygen-exposed animal at 5 days of age. There is a 77% reduction in ADPase+ retinal vessels after 4 days exposure to hyperoxia. (Flat mounts of ADPase reaction product developed with ammonium sulfide and viewed with brightfield illumination) (Republished with permission of the Association for Research in Vision and Ophthalmology, Fig. 2 from McLeod DS, Brownstein, R Lutty GA. Vaso-obliteration in the canine model of oxygen-induced retinopathy, Invest. Opthalmol. Vis. Sci. 1996;37:300–311; permission conveyed through Copyright Clearance Center, Inc.).
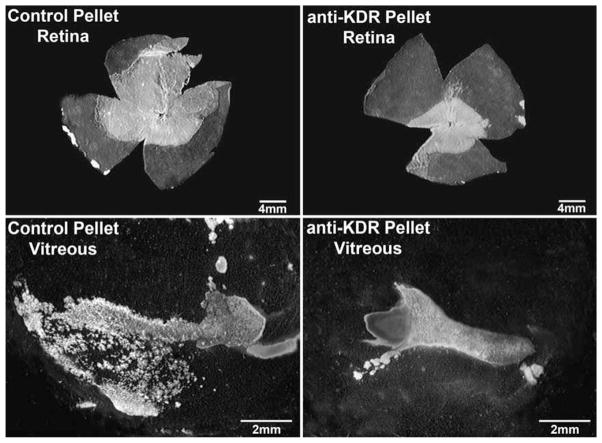
When the animals are returned to room air after four days of hyperoxia, the retina is ischemic and VEGF is produced, which stimulates angiogenesis. Neovascularization is easily discerned with ADPase since new vessels have much higher levels of ADPase. We have used the dog model of oxygen-induced retinopathy (OIR) to assess the effects of antiangiogenic agents on the vasoproliferation that ensues. Slow release polymers with antiangiogenic agents or control substances are implanted in vitreous on postnatal day 7 (P-7) and then the animals sacrificed at P-21. The vitreous is removed from the eye and then retina gently teased off of the retinal pigment epithelium. Both tissues are incubated for ADPase activity and the area of vasculature calculated from digital images again using NIH Image (McLeod, Taomoto & Cao et al., 2002). The experiment shown in Fig. 4 demonstrates that an antibody against VEGF receptor-2 delivered by slow release pellet from vitreous inhibits the growth of preretinal neovascularization but has the undesirable effect of inhibiting the regrowth of the retinal vasculature after vaso-obliteration.
Fig. 4.
ADPase-incubated retinas (top) and vitreous bodies (bottom) from a 22-day old oxygen-treated dog. The eye on the left was treated with a control pellet and the right eye with a pellet-containing antibody against VEGF-R2 (KDR). Although both eyes have dense mats of retinal blood vessel, the antibody-treated eye has fewer retinal blood vessels and less intravitreal neovascularization. (ADPase activity viewed with darkfield illumination) (Republished with permission of the Association for Research in Vision and Ophthalmology, Inc. Fig. 2 from McLeod DS, Taomoto M, Cao J, Zhu Z, Witte L, Lutty GA. Localization of VEGF receptor-2 (KDR/FLK-1) and effects of blocking it in oxygen-induced retinopathy. Invest. Opthalmol. Vis. Sci. 2002;43:474–482; permission conveyed through Copyright Clearance Center, Inc.).
4. Diabetic retinopathy
ADPase enzyme histochemistry was particularly useful in studying diabetic retinopathy, because a hallmark of this disorder is endothelial cell dysfunction and retinal capillary dropout. Blood vessels loose ADPase activity when the endothelial cells are dysfunctional or die, so areas of non-perfusion can easily be found in the retinal vasculature (Lutty & McLeod, 1992). We found blood vessels in which ADPase activity ended abruptly and, upon sectioning the blood vessel, found that areas lacking ADPase activity lacked endothelial cells. We have incubated the ADPase retinas for nonspecific esterase activity and demonstrated that neutrophils are often present at these sites where loss of ADPase activity occurs, suggesting that neutrophils initiate the occlusions and injure the endothelial cells resulting in loss of ADPase activity (Fukushima, McLeod, Merges, & Lutty, 1997; Lutty & Ogura, 2003). Blood vessels in which ADPase activity was elevated were always vascular formations in which endothelial cell proliferation occurred. These included micro- and macro-aneurysms, which many investigators consider aborted attempts at neovascularization. It also included intraretinal microvascular abnormalities (IRMA), which we interpret to intraretinal neovascularization due to the proliferation of endothelial cells within them (Lutty & McLeod, 1992).
5. Sickle cell retinopathy
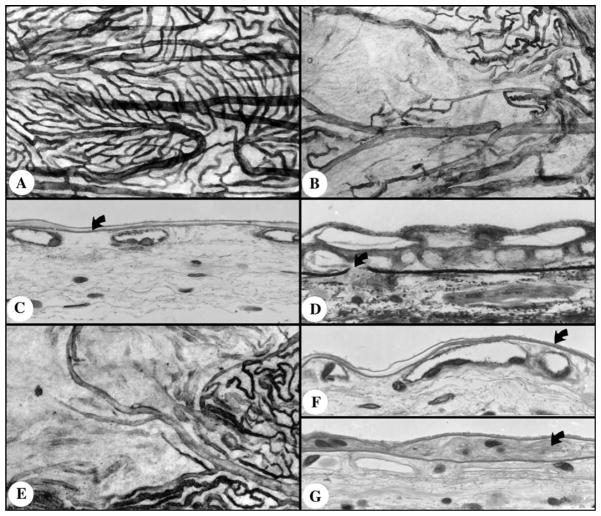
The application of ADPase enzyme histochemistry has yielded many insights into sickle cell retinopathy. A point mutation in the β globin gene causes hemoglobin to polymerize in hypoxic and acidic conditions and erythrocytes to sickle. The rigid, abnormal erythrocytes become trapped in the microvasculature causing endothelial dysfunction and capillary loss, which is apparent from the loss of ADPase activity in these segments. ADPase incubation of sickle cell retinas shows the loss of peripheral retinal vasculature that begins with capillary occlusions in children and ends with major artery and vein loss in adulthood (McLeod, Goldberg, & Lutty, 1993) (Fig. 5). Hairpin loops and vascular segments extruded from retina can be identified en bloc and serial sectioning demonstrated the nature of these structures and their relationship to sites of occlusion (McLeod et al., 1993). Hairpin loops are sites of major vessel occlusion where a new vascular segment has formed in the wall of the occluded vessel (McLeod, Merges, Fukushima, Goldberg, & Lutty, 1997). Because ADPase is elevated in angiogenesis, the earliest buds of neovascularization can be identified as they breach the internal limiting membrane of retina (McLeod et al., 1997). The large preretinal neovascularization formations that result at the border of perfused and nonperfused retina are called sea fan formations (Fig. 5). A study of these structures in ADPase-incubated retinas demonstrated that they occur most often at arteriovenous crossings and not at arteriovenous anastomoses as reported in clinical studies (Fig. 6). We have also documented up to five feeding arterioles and four draining venules in one sea fan formation when the ADPase-incubated retinas were serial sectioned (McLeod et al., 1997). The analysis permitted us to demonstrate that a given sea fan can have channels forming by angiogenesis, mature blood vessels (endothelial cells and pericytes), and autoinfarcted segments (Fig. 6).
Fig. 5.
Superior half of an ADPase-incubated retina from a 40-year-old sickle cell anemia patient. The bright field illumination makes the ADPase reaction product look black. Many sea fan formations are present at the border of nonperfused (top) and perfused (bottom) retina. The arrow indicates the sea fan formation present in Fig. 6. (From McLeod et al., Am. J. Ophthalmol. 1997;124:455–472).
Fig. 6.
Sea fan formation indicated by the arrow in Fig. 5. (A) This sea fan has formed at the crossing of an artery (a) and vein (v) with the feeding arteriole indicated by the open curved arrow and venule indicated by the closed curved arrow. (B) The draining venule (curved arrow) as it traverses the internal limiting membrane (ILM) (arrowhead). (C) The feeding arteriole as it breaches the ILM (arrowhead). In this sea fan there are newly forming blood vessels (D) that appear as angioblastic masses, fully formed blood vessels (E) with pericytes (arrow) and endothelial cells (arrowhead), and autoinfarcted capillaries (F). ((A) Darkfield illumination of flat-embedded ADPase retina; (B–F). cross sections stained with periodic acid-Schiff and hematoxylin) (From McLeod et al., Am. J. Ophthalmol. 1997;124:455–472).
6. Alkaline phosphatase
A second phosphatase that has proven useful for studying the ocular vasculatures is alkaline phosphatase (APase). Less is known about the actual function of this enzyme but some isoforms are involved in calcium transport and deposition. Like ADPase, it is restricted to the vasculature but unlike ADPase, it is not present in all blood vessels. APase is mostly on the arterial side in retina (arteries, arterioles, capillaries on the arterial side) (unpublished data) but in choroid it is present in all blood vessels, with arteries having the least activity (McLeod & Lutty, 1994). With our technique, choroid is dissected from the eyecup, fixed briefly, and incubated for enzyme histochemical demonstration of APase: napthol AS-MX phosphate is used as substrate and fast blue RR as indicator in TRIS buffer at pH 9.2 (McLeod & Lutty, 1994). The blue reaction withstands the bleaching of choroidal pigment with 30% hydrogen peroxide and subsequent embedment in glycol methacrylate. The lobular patterns of the choriocapillaris are apparent in the flat perspective and the relationship of choroidal vasculature to RPE, Bruch’s membrane, and other choroidal structures was possible in sections (Fig. 7). Using this technique, we have demonstrated that there is a fourfold greater loss in diabetic choriocapillaris than in normal aged choriocapillaris; this was possible because APase is only present in viable blood vessels (Cao, McLeod, Merges, & Lutty, 1998) (Fig. 7). If the choroids were also incubated with nonspecific esterase, we observed that capillaries lacking APase were associated with neutrophils, suggesting the involvement of these leukocytes with capillary loss in diabetic choroid (Lutty, Cao, & McLeod, 1997). We also found a significant number of peripheral choroidal neovascular structures in diabetic choroid, but they were often autoinfarcted (Fig. 7) (McLeod & Lutty, 1994).
Fig. 7.
Alkaline phosphatase incubated choroids from an 84-year-old subject without diabetes (A and C) and an 89-year-old subject with diabetes (B and D–G). (A) APase reaction product is present in all capillaries, arteries and veins in the flat perspective in this area in nondiabetic peripheral choroid. (B) Area of peripheral choroid in the diabetic where the majority of the capillaries lack APase activity. (C) A section through the normal choroid in “A” demonstrates APase+ positive choriocapillaris near Bruch’s membrane (curved arrow) at the top. (D) A section through the center of the area in the diabetic choroid shown in “B” shows that there are no choriocapillaris lumens, only nonviable choroidal neovascularization (CNV) above Bruch’s membrane (curved arrow). (E) Another field in the diabetic choroid showing viable capillaries on right. (F) Section through the viable capillary area shown in “E.” (G) Section through the nonviable capillary area in “E” shows only collagenous tubes where choriocapillaris had been and some free, APase-cells (curved arrow) in a deposit within Bruch’s membrane. (A–C, E) APase activity in flat-embedded choroid viewed with brightfield illumination; (D) section stained with toluidine-basic fuchsin; (F–G) periodic acid-Schiff and hematoxylin (Republished with permission of the Association for Research in Vision and Ophthalmology, Fig. 6 from McLeod DS and Lutty GA. High-resolution histologic analysis of the human choroidal vasculature. Invest. Opthalmol. Vis. Sci. 1994;3799–3811; permission conveyed through Copyright Clearance Center, Inc.).
We have recently modified the technique and only partially bleached the pigment, and used the APase flat-embedding technique to quantify the loss of choriocapillaris and RPE in age-related macular degeneration (McLeod, Taomoto & Otsuji et al., 2002). We found that RPE degeneration appears to precede the loss of choriocapillaris in geographic atrophy. Interestingly, there were surviving choriocapillaris lumens (APase+) even in areas with complete loss of RPE, although surviving vessels were highly constricted (McLeod, Taomoto & Cao et al., 2002; McLeod, Taomoto & Otsuji et al., 2002).
7. Summary
Phosphatase enzyme histochemistry followed by flat-embedding is extremely useful in studying retinal and choroidal vascular development and loss, since only viable blood vessels have these enzyme activities. Sites of occlusion and remodeling can be identified and analysed, resulting in new insights into the cause of occlusion, whether it is erythrocytes or leukocytes or calcific emboli (Lutty & McLeod, 1992). The phosphatase activities are elevated in neovascularization making possible high resolution analysis of neovascularization, the feeder vessels, and the retinal milieu in which angiogenesis occurs.
Acknowledgments
This work was supported by NIH Grants EY 01765 (Wilmer Institute), HL45922 (GL) and EY09357 (GL), the ROPARD Foundation (GL), Research to Prevent Blindness (Wilmer), the Reginald Lewis Foundation, and the Brownstein Foundation. Gerard A. Lutty is an American Heart Association Established Investigator and the recipient of a Research to Prevent Blindness Lew Wasserman Merit Award.
References
- Cao J, McLeod S, Merges CA, Lutty GA. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch Ophthalmol. 1998;116:589–597. doi: 10.1001/archopht.116.5.589. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, McLeod DS, Hughes S, Baxter L, Chu Y, Hasegawa T, Lutty GA. Astrocyte-endothelial cell relationships during human retinal vascular development. Invest Ophthalmol Vis Sci. 2004;45:2020–2032. doi: 10.1167/iovs.03-1169. [DOI] [PubMed] [Google Scholar]
- Fukushima I, McLeod DS, Merges C, Lutty GA. Relationship of neutrophils to capillary dropout and adhesion molecules in diabetic retina. Invest Ophthalmol Vis Sci. 1997;38(suppl):S769. [Google Scholar]
- Lessell S, Kuwabara T. Phosphatase histochemistry of the eye. Arch Ophthalmol. 1964;71:851–860. doi: 10.1001/archopht.1964.00970010867015. [DOI] [PubMed] [Google Scholar]
- Lutty GA, Cao J, McLeod DS. Relationship of polymorphonuclear leukocytes (PMNs) to capillary dropout in the human diabetic choroid. Am J Pathol. 1997;151:707–714. [PMC free article] [PubMed] [Google Scholar]
- Lutty GA, Hasegawa T, Prow T, Merges C, McLeod DS. Development of the fetal retinal vasculature. Invest Ophthalmol Vis Sci. 2005;(suppl) [Google Scholar]
- Lutty GA, McLeod DS. A new technique for visualization of the human retinal vasculature. Arch Ophthalmol. 1992;110:267–276. doi: 10.1001/archopht.1992.01080140123039. [DOI] [PubMed] [Google Scholar]
- Lutty GA, Ogura Y. Involvement of leukocytes in diabetic retinopathy and choroidopathy. In: Schmid-Schonbein GW, Granger DN, editors. Molecular basis for microcirculatory disorders. Paris: Springer-Verlag; 2003. pp. 559–569. [Google Scholar]
- Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Alyonycheva TN, Safier LB, Hajjar KA, Posnett DN, Schoenborn MA, Schooley KA, Gayle RB, Maliszewski CR. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod D, Goldberg M, Lutty G. Dual perspective analysis of vascular formations in sickle cell retinopathy. Arch Ophthalmol. 1993;111:1234–1245. doi: 10.1001/archopht.1993.01090090086026. [DOI] [PubMed] [Google Scholar]
- McLeod DS, Brownstein R, Lutty GA. Vaso-obliteration in the canine model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 1996;37:300–311. [PubMed] [Google Scholar]
- McLeod DS, Crone SN, Lutty GA. Vasoproliferation in the neonatal dog model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 1996;37(7):1322–1333. [PubMed] [Google Scholar]
- McLeod DS, Lutty GA. High resolution histologic analysis of the human choroidal vasculature. Invest Ophthalmol Vis Sci. 1994;35:3799–3811. [PubMed] [Google Scholar]
- McLeod DS, Merges C, Fukushima A, Goldberg MF, Lutty GA. Histopathological features of neovascularization in sickle cell retinopathy. Am J Ophthalmol. 1997;124:473–487. doi: 10.1016/s0002-9394(14)70862-1. [DOI] [PubMed] [Google Scholar]
- McLeod DS, Taomoto M, Cao J, Zhu Z, Witte L, Lutty GA. Localization of VEGF receptor-2 (KDR/FLK-1) and effects of blocking it in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2002;43:474–482. [PubMed] [Google Scholar]
- McLeod DS, Taomoto M, Otsuji T, Green WR, Sunness JS, Lutty GA. Quantifying changes in RPE and choriocapillaris in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002;43:1986–1993. [PubMed] [Google Scholar]