Abstract
As part of an investigation to generate optimized drug leads from marine natural pharmacophores for the treatment of neoplastic and infectious diseases, a series of novel isoaaptamine analogs were prepared by coupling acyl halides to the C9 position of isoaaptamine (2) isolated from the Aaptos sponge. This library of new semisynthetic products was evaluated for biological activity against HIV-1, Mtb, AIDS-OI, tropical parasitic diseases, and cancer. Compound 4 showed potent activity against HIV-1 (EC50 0.47 μg/mL), compound 19 proved to possess remarkable activity against Mycobacterium intracellulare with an IC50 and MIC value of 0.15 and 0.31 μg/mL, while compounds 4 and 17 possessed anti-leishmanial activity with IC50 values of 0.1 and 0.4 μg/mL, respectively. Compounds 16 and 17 showed antimalarial activity with EC50 values of 230 and 240 ng/mL, respectively, and compound 14 exhibited an EC50 of 0.05 μM against the Leukemia cell line K-562.
Keywords: Isoaaptamine derivatives, Anti-cancer leads, Anti-HIV-1 activity, Marine natural products
1. Introduction
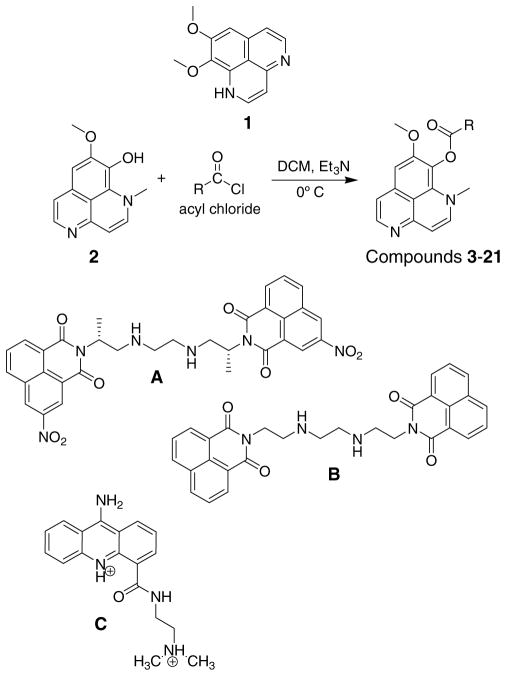
The development of resistance and the toxicity associated with current chemotherapeutic agents has led to an increasing failure of existing drugs utilized in the treatment of various microbial, viral, and neoplastic disorders. The marine environment, and the unique natural products contained therein, remains a relatively untapped source of possibilities for novel drug development. As seen in Figure 1, the marine natural product aaptamine (1), first isolated by Nakamura,1 has been reported to have antineoplastic and α-adrenoceptor blocking activity.1,2 The closely related compound isoaaptamine (2) was first isolated by Fedoreev3 from a sponge in the genus Suberites and later by two other groups from the sponge Aaptos aaptos.4,5 Recently, isoaaptamine (2) was isolated from a sponge belonging to the genus Hymeniacidon.6 Isoaaptamine (2) has also been reported to be a PKC inhibitor.7 However, the recent investigation by Pettit et al. of PKC inhibition and tubulin polymerization showed only minimal activity.8 However, further investigation by this group revealed that inhibition of the S-Phase of the cell cycle may be involved in the observed cytotoxicity which suggests a possible interaction with DNA or topoisomerase. The closely related bis(naphthalimide) derivative DMP 840 (A) has been reported to be a topoisomerase II inhibitor9 and is currently undergoing phase I clinical trials.10 Additionally, the analog LU 79553 (B) has shown efficacy in vivo against tumor xenographs.11 The related acridine anticancer agent AAC (C) has also been shown to elicit its activity through modulation of topoisomerase II.12–14 Additional SAR investigation suggests a positive correlation between nonelectrostatic binding free energy and anti-cancer potency of acridine derivatives.15
Figure 1.
Aaptamine related structures.
Aaptamine, isoaaptamine, and demethylated aaptamine have shown antifouling activity in the zebra mussel assay with EC50 values of 24.2, 11.6, and 18.6 μM respectively.16 Isoaaptamine (2) has shown activity against the protozoan that causes malaria, Plasmodium falciparum, with an IC50 of 1.8 and 0.6 μg/mL for the D6 and W2 clones, respectively. Pettit et al. recently reported dibenzyl aaptamine derivatives with activity against Mycobacterium tuberculosis.17 Isoaaptamine has been shown to exhibit remarkable activity against cancer cell lines including P-3384,6,8 (murine lymphocytic leukemia), KB16 (human mouth epidermoid carcinoma), A549 (human lung adenocarcinoma), and HT-29 (human colon adenocarcinoma).4 The synthesis of aaptamine18 (1) and isoaaptamine19 (2) has recently been reported. The first SAR study of these compounds by Shen et al.20 concluded that the C-9 hydroxyl position was important for cytotoxic activity and acylation causes a decrease in activity. Recently, the studies by Pettit et al.6,8,17 with modifications at the hydroxyl and nitrogen positions of aaptamine have aided to further elucidate the SAR of these unique compounds. Specifically, para substituted phenyl substituents at one or both of the nitrogen positions increased activity.
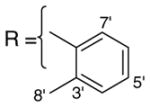
In order to further investigate the SAR of side-chain attachment at the C9 hydroxyl position of isoaaptamine (2), which is readily available from the sponge Aaptos sp. in gram quantities, a series of analogs was generated. Presented here are 19 derivatives (Table 1) of isoaaptamine (2) generated with various acyl halides coupled at the C9 position of isoaaptamine (2) and their biological activity against a number of pathogens and cancer cell lines.
Table 1.
Isoaaptamine derivatives Entry Product Yield (%)
| Entry | Product | Yield (%) |
|---|---|---|
| 3 |
|
89 |
| 4 |
|
91 |
| 5 |
|
85 |
| 6 |
|
85 |
| 7 |
|
84 |
| 8 |
|
91 |
| 9 |
|
90 |
| 10 |
|
92 |
| 11 |
|
94 |
| 12 |
|
82 |
| 13 |
|
93 |
| 14 |
|
94 |
| 15 |
|
83 |
| 16 |
|
93 |
| 17 |
|
92 |
| 18 |
|
89 |
| 19 |
|
94 |
| 20 |
|
86 |
| 21 |
|
91 |
2. Results and discussion
The products 3–21 have been evaluated for activity against malaria, AIDS-OI pathogens, leishmania, cancer, HIV-1, and Mtb. The results from the in vitro assays and preliminary SAR are presented in Tables 2–4.
Table 2.
Anti-microbial activity data
| Compound | IC50/MIC
|
||||||
|---|---|---|---|---|---|---|---|
| Candida albicans | Cryptococcus neoformans | Staphylococcus aureus | Methicillin resistant Staphylococcus | Pseudomonas aeruginosa | Mycobacterium intracellulare | Aspergillus fumigatus | |
| 2 | — | — | — | — | — | — | — |
| 3 | — | 15/20 | — | — | — | 5.0/10 | — |
| 4 | 10/20 | 5.5/10 | 8.0/20 | 8.0/20 | — | 0.8/2.5 | — |
| 5 | — | — | — | — | — | 3.5/10 | — |
| 6 | — | — | — | — | — | — | — |
| 7 | — | — | — | — | — | — | — |
| 8 | — | — | — | — | — | — | — |
| 9 | — | — | — | — | — | — | — |
| 10 | — | — | 6.5/20 | 6.5/20 | — | 5.0/10 | — |
| 11 | — | 15/20 | — | — | — | 4.0/10 | — |
| 12 | — | 6.5/10 | — | — | — | 1.0/5.0 | — |
| 13 | 3.0/5.0 | 1.5/2.5 | 1.5/2.5 | 1.5/2.5 | — | 0.5/1.3 | 5 |
| 14 | 4.5/10 | 3.0/5.0 | 3.0/5.0 | 3.0/5.0 | — | 1.0/2.5 | 10.0 |
| 15 | — | — | — | — | — | 5.5/20 | — |
| 16 | — | 10/20 | — | — | — | 1.0/5.0 | — |
| 17 | 6.5/10 | 1.5/2.5 | 3.0/5.0 | 2.5/5.0 | — | 0.5/1.3 | 10.0 |
| 18 | — | — | — | — | — | — | — |
| 19 | — | — | — | — | — | 0.2/0.3 | — |
| 20 | — | — | 10/20 | 10/20 | — | — | — |
| 21 | — | — | — | — | — | 10/20 | — |
| Amphotericin B | 0.3/1.3 | 0.6/2.5 | — | — | — | — | — |
| Ciprofloxacin | 0.1/0.6 | 0.1/0.6 | 0.1/1.3 | 0.3/1.3 | — | ||
IC50 is the concentration (μg/mL) that affords 50% inhibition of growth. IC50 ≤ 15 μg/mL is considered active. For compounds that were considered active (≤15 μg/mL), a MIC (minimum inhibitory concentration (μg/mL); lowest tested concentration that allows no detectable growth) was calculated. The screens were run at concentrations of 50, 10, and 2 μg/mL.
Table 4.
Anti-cancer data
| 1° Screening | Prostate DU-145 (μM) |
Ovary IGROV-ET (μM) |
Breast SK-BR3 (μM) |
Melanoma SK-MEL-28 (μM) |
Lung A549 (μM) |
Leukemia K-562 (μM) |
Pancreas PANC1 (μM) |
Colon LOVO (μM) |
Cervix HELA-APL (μM) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | GI50 | >28.9 | 13.8 | >28.9 | 15.9 | >28.9 | 15.0 | 8.5 | 10.4 | 14.3 |
| TGI | >28.9 | >28.9 | >28.9 | >28.9 | >28.9 | 20.2 | 24.3 | 28.9 | >28.9 | |
| LC50 | >28.9 | >28.9 | >28.9 | >28.9 | >28.9 | 27.0 | 28.9 | >28.9 | >28.9 | |
| 4 | GI50 | 5.4 | 4.1 | 7.3 | 5.3 | 5.5 | 6.0 | 3.2 | 4.3 | 4.8 |
| TGI | 9.2 | 8.4 | 14.4 | 9.1 | 9.5 | 9.6 | 6.6 | 8.3 | 8.9 | |
| LC50 | 15.8 | 17.1 | 17.8 | 15.5 | 16.5 | 15.3 | 13.4 | 16.3 | 16.5 | |
| 5 | GI50 | 13.5 | 9.6 | 9.4 | 7.5 | 9.6 | 10.0 | 4.8 | 5.1 | 6.9 |
| TGI | >28.5 | >28.5 | 20.8 | 23.2 | 25.2 | 14.3 | 9.3 | 11.9 | 17.9 | |
| LC50 | >28.5 | >28.5 | >28.5 | >28.5 | >28.5 | 20.3 | 18.3 | 27.6 | >28.5 | |
| 6 | GI50 | 15.8 | 10.7 | 9.3 | 4.8 | 12.2 | 7.1 | 5.1 | 4.9 | 10.0 |
| TGI | >27.1 | >27.1 | 18.3 | 9.1 | >27.1 | 11.0 | 10.8 | 11.4 | 27.1 | |
| LC50 | >27.1 | >27.1 | >27.1 | 17.1 | >27.1 | 17.1 | 23.1 | 26.4 | >27.1 | |
| 7 | GI50 | 6.9 | 5.7 | 6.2 | 4.9 | 6.1 | 8.8 | 4.6 | 4.6 | 5.7 |
| TGI | 15.0 | 10.0 | 11.0 | 8.4 | 12.5 | 12.5 | 8.3 | 8.3 | 10.8 | |
| LC50 | >27.1 | 17.4 | 19.5 | 14.3 | 25.4 | 17.9 | 15.0 | 14.9 | 20.2 | |
| 8 | GI50 | >30.5 | 25.7 | >30.5 | 29.4 | >30.5 | 26.5 | 14.1 | 18.1 | 30.5 |
| TGI | >30.5 | >30.5 | >30.5 | >30.5 | >30.5 | >30.5 | >30.5 | >30.5 | >30.5 | |
| LC50 | >30.5 | >30.5 | >30.5 | >30.5 | >30.5 | >30.5 | >30.5 | >30.5 | >30.5 | |
| 9 | GI50 | 8.3 | 9.2 | 10.4 | 5.6 | 9.6 | 7.9 | 5.4 | 7.5 | 6.8 |
| TGI | 18.9 | 18.2 | >26.4 | 9.5 | 26.4 | 11.8 | 9.3 | 15.2 | 12.4 | |
| LC50 | >26.4 | >26.4 | >26.4 | 16.2 | >26.4 | 17.9 | 16.0 | >26.4 | 22.7 | |
| 10 | GI50 | 4.8 | 3.4 | 5.0 | 5.0 | 5.0 | 6.4 | 2.8 | 3.5 | 4.5 |
| TGI | 8.4 | 6.8 | 8.6 | 8.7 | 9.0 | 10.0 | 6.1 | 7.0 | 8.3 | |
| LC50 | 14.7 | 13.8 | 14.8 | 15.1 | 16.2 | 15.6 | 13.0 | 13.8 | 15.2 | |
| 11 | GI50 | 14.8 | 10.8 | 15.8 | 9.8 | >28.9 | 8.1 | 5.3 | 7.9 | 6.6 |
| TGI | >28.9 | >28.9 | >28.9 | 28.9 | >28.9 | 13.4 | 9.9 | 17.6 | 14.0 | |
| LC50 | >28.9 | >28.9 | >28.9 | >28.9 | >28.9 | 22.2 | 18.4 | >28.9 | 28.9 | |
| 12 | GI50 | 5.6 | 4.4 | 11.3 | 4.2 | 11.9 | 2.8 | 3.1 | 3.0 | 3.8 |
| TGI | 14,1 | 9.0 | 24.0 | 7.9 | >24.0 | 5.6 | 6.3 | 6.2 | 8.3 | |
| LC50 | >24.0 | 18.3 | >24.0 | 15.0 | >24.0 | 11.0 | 12.9 | 12.8 | 17.8 | |
| 13 | GI50 | 4.4 | 8.1 | 7.3 | 3.9 | 4.7 | 1.7 | 2.8 | 2.9 | 3.6 |
| TGI | 8.0 | 12.0 | 11.2 | 7.4 | 8.3 | 4.3 | 5.9 | 5.9 | 7.0 | |
| LC50 | 14.4 | 17.8 | 17.3 | 14.0 | 14.5 | 9.8 | 12.4 | 12.2 | 13.6 | |
| 14 | GI50 | 3.8 | 2.9 | 6.5 | 3.0 | 4.5 | 0.1 | 1.1 | 1.5 | 1.0 |
| TGI | 7.6 | 6.1 | 10.1 | 6.0 | 8.0 | 0.5 | 3.7 | 4.5 | 3.3 | |
| LC50 | 15.5 | 12.6 | 15.8 | 12.0 | 14.3 | 1.1 | 9.6 | 10.8 | 9.2 | |
| 15 | GI50 | >27.1 | 16.2 | 21.9 | 10.5 | 27.1 | 3.6 | 6.0 | 9.4 | 13.9 |
| TGI | >27.1 | >27.1 | >27.1 | 27.1 | >27.1 | 7.5 | 13.4 | 27.1 | >27.1 | |
| LC50 | >27.1 | >27.1 | >27.1 | >27.1 | >27.1 | 15.5 | >27.1 | >27.1 | >27.1 | |
| 16 | GI50 | 6.2 | 5.8 | 6.5 | 4.9 | 5.8 | 5.8 | 3.8 | 3.4 | 4.8 |
| TGI | 10.7 | 10.5 | 10.7 | 8.8 | 10.8 | 9.6 | 7.6 | 6.9 | 8.9 | |
| LC50 | 18.6 | 18.9 | 17.8 | 15.6 | 20.3 | 15.9 | 14.8 | 14.0 | 16.6 | |
| 17 | GI50 | 4.9 | 4.3 | 5.8 | 4.8 | 4.9 | 1.9 | 2.8 | 3.2 | 4.7 |
| TGI | 8.7 | 7.9 | 9.5 | 8.4 | 8.6 | 4.2 | 6.0 | 6.5 | 8.2 | |
| LC50 | 15.0 | 14.6 | 15.5 | 14.5 | 15.0 | 9.6 | 12.4 | 13.1 | 14.4 | |
| 18 | GI50 | >30.7 | 23.2 | >30.7 | 27.0 | 28.2 | >30.7 | 16.8 | 12.3 | 30.7 |
| TGI | >30.7 | >30.7 | >30.7 | >30.7 | >30.7 | >30.7 | >30.7 | >30.7 | >30.7 | |
| LC50 | >30.7 | >30.7 | >30.7 | >30.7 | >30.7 | >30.7 | >30.7 | >30.7 | >30.7 | |
| 19 | GI50 | 3.6 | 9.1 | 5.6 | 3.7 | 7.4 | 5.7 | 3.1 | 3.2 | 3.6 |
| TGI | 6.4 | >20.3 | 10.2 | 6.5 | 20.3 | 8.6 | 5.8 | 5.9 | 6.6 | |
| LC50 | 11.3 | >20.3 | 18.4 | 11.4 | >20.3 | 13.0 | 10.8 | 10.9 | 11.9 | |
| 20 | GI50 | 9.1 | 4.9 | 6.9 | 4.8 | 6.1 | 4.4 | 4.4 | 4.5 | 4.9 |
| TGI | 24.3 | 8.9 | 12.1 | 8.7 | 10.9 | 8.0 | 8.3 | 8.1 | 8.9 | |
| LC50 | >28.0 | 16.2 | 21.1 | 15.5 | 19.3 | 14.6 | 15.6 | 14.6 | 16.3 | |
| 21 | GI50 | 19.5 | 11.0 | >27.6 | 6.1 | 20.8 | 6.7 | 5.2 | 5.0 | 5.7 |
| TGI | >27.6 | 25.6 | >27.6 | 11.5 | >27.6 | 12.6 | 10.5 | 9.4 | 10.4 | |
| LC50 | >27.6 | >27.6 | >27.6 | 21.6 | >27.6 | 23.8 | 21.2 | 17.7 | 18.9 | |
GI50 is the concentration at which 50% growth inhibition was observed. TGI is the concentration at which total growth inhibition was observed. LC50 is the lethal concentration at which 50% cell death occurred.
2.1. Anti-HIV-1
The anti-HIV-1 activity of isoaaptamine (2, 0.6 μM) and aaptamine (1.30 μM) has previously been reported.21 The structure–activity relationship of fluorine substitution on an indole heterocyclic system has recently been reported to increase the potency of anti-HIV-1 activity. 22 Compounds 5, 6, 7, and 15 contained fluorine at different positions on the phenyl substituent. Substitution of fluorine at the ortho position (7, EC50 2.1 μg/mL), relative to substitution at other positions on the ring, increased activity except where there was adjacent fluorine (6, EC50 37.7 μg/mL) in the meta position. Substitution of fluorine in the para position (5, EC50 10.9 μg/mL; 15, EC50 16.6 μg/mL) was not as dramatic except where the substituent was a tri-fluorinated O-methyl group (12, EC50 2.3 μg/mL). The position of fluorine substitution on the phenyl ring appears to be the most important aspect for anti-HIV-1 activity. Methyl substitution at the ortho, para, and meta positions (3, 11, and 16) reflects the same pattern seen in the fluorinated derivatives with highest activity in ortho (3, EC50 1.3 μg/mL) substitution. Substitution at the para position with carbon chains of varying length shows a dramatic increase in activity with ethyl (4, EC50 0.47 μg/mL) having better activity than methyl (16, EC50 9.2 μg/mL) and a decrease as the chain is lengthened (13, EC50 3.8 μg/mL; 14, EC50 18.8 μg/mL). Taking these results into consideration, short chain para substitution provides the best structure for anti-HIV-1 activity in this series of compounds.
2.2. AIDS opportunistic pathogens
Isoaaptamine (2) alone did not show toxic activity (IC50 ≤ 15 μg/mL) against any of the tested microbes. However, several derivatives (4, 13, 14, and 17) showed significant activity against Mycobacterium intracellulare with alkyl para substitution on the phenyl ring being the dominant pattern. Most interesting was the remarkable activity of compound 19 (IC50 0.2 μg/mL, MIC 0.3 μg/mL) against M. intracellulare being more active than ciprofloxacin (IC50 0.3 μg/mL, MIC 1.3 μg/mL). Compound 19, deviating from the aforementioned pattern, consists of a 17 carbon acyl ester chain with an unsaturation between C9′ and C10′.
Tuberculosis, malaria, and leishmaniasis
Isoaaptamine (2) did not show activity against Mycobacterium tuberculosis. However, the derivative (21) was active with IC50 values of 41.03 μg/mL. There was no evident congruence in the derivative structure and the activity reported.
The activity of isoaaptamine (2, IC50 0.68 μg/mL) was more active than both pentamidine (IC50 1.6 μg/mL) and amphotericin B (IC50 1.1 μg/mL) against Leishmania donovani. Modification of isoaaptamine (2) resulted in an increase in activity with the most active derivative 4 (IC50 0.1 μg/mL) containing a para ethyl-substituted phenyl ring and 17 (IC50 0.4 μg/mL) containing a para tert-butyl-substituted phenyl ring. Substitution of a group longer than two carbons in the para position decreases the activity against Leishmania. Isoaaptamine (2) shows remarkable activity against the W2 clone and mild activity against the D6 clone of P. falciparum prior to modification (380, 1100 ng/mL, respectively). All modifications had a negative impact on the activity against the W2 clone. However, an increase in D6 activity of many of the derivatives was observed (4, 5, 10–17, 20, and 21) with para substitution on the phenyl ring of short carbon chains for compounds 4 (IC50 330 ng/mL), 16 (IC50 230 ng/mL), and 17 (IC50 240 ng/mL) being the most potent. A decrease in activity corresponding to extension of the chain was also observed. Although additional derivatives showed activity, no other obvious patterns were evident.
2.3. Cancer cell cytotoxicity
Isoaaptamine (2) has shown remarkable activity against a range of different cancer cell lines with activity against the murine leukemia cell line P388 as low as 0.28 μg/mL.6 All 21 compounds were evaluated against 14 different cancer cell lines, many of which can be found in Table 4. The compounds found to have broad activity against the tested cell lines (4, 10, 12–14, 16, 17, 19, and 20) contained para substituted phenyl rings. Most notable were the activities of 13 (GI50 1.66 μM), 14 (GI50 0.05 μM), and 17 (GI50 1.9 μM) against the leukemia cell line K-562. Compound 14 was substituted with a 5 carbon chain at the para position while compounds 13 and 17 were substituted with a 4 carbon chain and a t-butyl group, respectively. A decrease in activity was observed with a methyl (16, 5.8 μM) or an ethyl (4, 6.0 μM) substituent at the para position.
The prevalence of para substitution of a phenyl ring attached via an ester linkage to the C9 hydroxyl position of isoaaptamine (2) appears to be the underlying SAR evident in the data presented in this paper. The substituent at the para position determined the selectivity toward the target of interest. Substitution of a phenyl ester moiety at the C9 position of isoaaptamine (2) was more effective than the acylation previously reported at this position by Shen et al. A further point of observation reveals the congruence of the attachment of para substituted cyclic moieties in this group of compounds with and overall increase in activity. This observation reflects the results found in the present study and those previously published by Pettit et al.
In conclusion, this is the first reported SAR investigation for anti-HIV-1 activity of the aaptamine alkaloids thus far. In addition, we are now able to report several optimized potential antitumor (12–14, and 17) and anti-infective leads (4, 13, 14, 17, and 19), suggesting that further investigations of this class of marine natural products maybe fruitful. Natural products have been credited with as many as 75% of the treatments of infectious diseases and 60% of treatments for cancer.23 However only 5–6% represent the unmodified natural product indicating the importance of optimizing novel marine natural product structural classes with reasonable drug-like properties.
3. Experimental
3.1. General
1D and 2D NMR spectra were recorded on a Bruker Avance DRX-400 spectrometer. Chemical shift (δ) values expressed in parts per million (ppm) are referenced to the residual solvent signals with resonances at δH/δC 7.26/77.00 (CDCl3). ESI-FTMS analyses were measured on a Bruker-Magnex BioAPEX 30es ion cyclotron HR HPLC-FT spectrometer by direct injection into an electrospray interface. TLC was performed on aluminum sheets (Si gel 60 F254, Merck KGaA, Germany) with an acetone/hexane (80:20) solvent system. All acyl halides were purchased from Sigma–Aldrich, USA.
Isoaaptamine (2) (150 mg, 0.657 mmol) was dissolved in dry methylene chloride (2 mL) at 0 °C, 0.5 mL of tri-ethyl amine was added, and the reaction mixture was stirred for 30 min. An excess of the acyl halide was added dropwise over a period of 15 min. The reaction was allowed to stir at 0 °C for 30 min, slowly warmed to room temperature, and the progress was monitored on TLC. The reaction was stopped when the TLC showed the reaction was completed (2–24 h). The residue was fractionated on silica gel G254 2000 μm using MeOH/CHCl3 (80:20) and yielded products of various colors for compounds 3–21.
3.1.1. 9-O-2-methylbenzoylisoaaptamine (3)
Brownish amorphous solid; 1H and 13C NMR (CDCl3) data see Table 5; ESI-MS m/z 347 (M+, 100). High resolution EI-MS calculated for C21H18N2O3 (M+) m/z 347.1396, observed m/z 347.1396.
Table 5.
1H and 13C NMR spectral data for compounds 3, 4, and 5
| Position | 3
|
4
|
5
|
|||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 2 | 6.9, br d | 133.6, d | 7.2, d(7.6) | 130.1, d | 6.9, d(6.8) | 133.2, d |
| 3 | 6.8, br d | 100.9, d | 6.4, d(7.2) | 101.5, d | 6.8, br d | 113.3, d |
| 3a | 148.9, s | 151.9, s | 146.1, s | |||
| 5 | 7.5, br d | 147.2, d | 7.9, d(7.2) | 148.1, d | 7.5, d(6.4) | 146.1, d |
| 6 | 7.3, br d | 113.5, d | 7.7, d(7.2) | 113.6, d | 7.5, d(6.4) | 116.4, d |
| 6a | 136.3, s | 136.4, s | 136.6, s | |||
| 7 | 6.8, br s | 100.3, d | 7.0, s | 98.8, d | 6.8, s | 99.9, d |
| 8 | 157.2, s | 157.9, s | 156.7, s | |||
| 9 | 126.9, s | 124.4, s | 134.7, s | |||
| 9a | 124.0, s | 127.3, s | 124.3, s | |||
| 9b | 117.8, s | 117.6, s | 117.9, s | |||
| NCH3 | 3.8, s | 45.4, q | 4.0, s | 44.8, q | 3.9, s | 45.0, q |
| OCH3 | 3.9, s | 56.8, q | 3.9, s | 56.2, q | 3.9, s | 56.6, q |
| 1′ | 165.4, s | 164.9, s | 164.0, s | |||
| 2′ | 134.9, s | 125.6, s | 123.3, s | |||
| 3′ | 141.9, s | 8.1, d(8) | 130.4, d | 8.2, br d | 133.1, d | |
| 4′ | 7.1, br d | 130.9, d | 7.46, d(8) | 128.3, d | 7.2, br d | 114.5, d |
| 5′ | 7.5, br d | 132.2, d | 149.1, s | 167.8, s | ||
| 6′ | 7.3, br d | 126.3, d | 7.46, d(8) | 128.3, d | 7.2, br d | 114.5, d |
| 7′ | 8.1, d(7.6) | 131.5, d | 8.1, d(8) | 130.4, d | 8.2, br d | 133.1, d |
| 8′ | 2.6, s | 21.8, q | 2.7, q(7.6) | 28.6, t | F | |
| 9′ | 1.3, t(7.6) | 14.3, t | ||||
Measured in CDCl3 at 400 MHz for 1H and 100 MHz for 13C, respectively. J values in Hz.
3.1.2. 9-O-4-ethylbenzoylisoaaptamine (4)
Brownish powder; 1H and 13CNMR(CDCl3) data see Table 5; ESI-MS m/z 361 (M+, 100). High resolution EI-MS calculated for C22H20N2O3 (M+) m/z 361.1552, observed m/z 361.1551.
3.1.3. 9-O-4-fluorobenzoylisoaaptamine (5)
Yellow amorphous solid; 1H and 13C NMR (CDCl3) data see Table 5; ESI-MS m/z 351 (M+, 100). High resolution EI-MS calculated for C20H15N2O3F (M+ ) m/z 351.1145, observed m/z 351.1145.
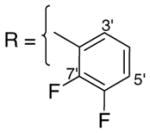
3.1.4. 9-O-2,3-difluorobenzoylisoaaptamine (6)
Brownish amorphous solid; 1H and 13C NMR (CDCl3) data see Table 6; ESI-MS m/z 369 (M+, 100). High resolution EI-MS calculated for C20H14N2O3F2 (M+) m/z 369.1051, observed m/z 369.1059.
Table 6.
1H and 13C NMR spectral data for compounds 6, 7, and 8
| Position | 6
|
7
|
8
|
|||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 2 | 7.5, d(8) | 124.6, d | 7.3, br d | 123.0, d | 7.3, br d | 130.0, d |
| 3 | 6.8, br d | 113.5, d | 5.3, br d | 113.5, d | 6.7, br d | 101.1, d |
| 3a | 149.0, s | 156.7, s | 148.7, s | |||
| 5 | 7.9, br d | 127.5, d | 7.0, br d | 123.3, d | 7.6, d(5.2) | 147.4, d |
| 6 | 7.3, br d | 118.3, d | 6.8, br d | 118.9, d | 6.9, d(5.2) | 113.3, d |
| 6a | 136.8, s | 148.9, s | 135.7, s | |||
| 7 | 6.8, s | 100.5, d | 6.8, s | 100.6, d | 6.8, s | 99.7, d |
| 8 | 156.7, s | 156.9, s | 153.8, s | |||
| 9 | 134.6, s | 136.6, s | 135.0, s | |||
| 9a | 118.5, s | 134.4, s | 125.1, s | |||
| 9b | 100.9, s | 118.2, s | 117.6, s | |||
| NCH3 | 3.9, s | 45.2, q | 3.9, s | 45.1, q | 3.9, s | 45.3, q |
| OCH3 | 3.9, s | 56.8, q | 3.8, s | 56.8, q | 3.9, s | 56.8, q |
| 1′ | 161.7, s | 161.6, s | 42.7, t | |||
| 2′ | 117.7, s | 118.7, d | 3.4, br q | 14.1, q | ||
| 3′ | 7.9, br d | 123.2, d | 7.8, s | 157.1, s | 1.2, t(6.8) | 42.3, t |
| 4′ | 7.0, br t | 123.1, d | 119.1, d | 3.4, br q | 13.3, q | |
| 5′ | 7.1, br d | 118.4, d | 7.0, br d | 117.5, d | 1.3, t(6.8) | |
| 6′ | 146.6, s | 7.0, br d | 158.8, s | |||
| 7′ | 149.9, s | N | ||||
Measured in CDCl3 at 400 MHz for 1H and 100 MHz for 13C, respectively. J values in Hz.
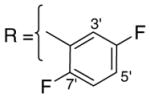
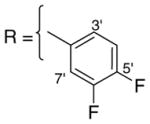
3.1.5. 9-O-2,5-difluorobenzoylisoaaptamine (7)
Reddish brown amorphous solid; 1H and 13C NMR (CDCl3) data see Table 6; ESI-MS m/z 369 (M+, 100). High resolution EI-MS calculated for C20H14N2O3F2 (M+) m/z 369.1051, observed m/z 369.1054.
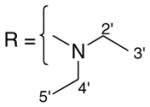
3.1.6. 9-O-diethylcarbamoylisoaaptamine (8)
Dark red amorphous solid; 1H and 13C NMR (CDCl3) data see Table 6; ESI-MS m/z 328 (M+, 100). High resolution EI-MS calculated for C18H21N3O3 (M+) m/z 328.1661, observed m/z 328.1653.
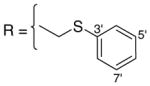
3.1.7. 9-O-2-(thiophenyl)acetylisoaaptamine (9)
Greenish black amorphous solid; 1H and 13C NMR (CDCl3) data see Table 7; ESI-MS m/z 379 (M+, 100). High resolution EI-MS calculated for C21H18N2O3S (M+) m/z 379.1116, observed m/z 379.1113.
Table 7.
1H and 13C NMR spectral data for compounds 9, 10, and 11
| Position | 9
|
10
|
11
|
|||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 2 | 7.3, d(7.2) | 128.8, d | 7.4, d(7) | 128.6, d | 6.7, br d | 131.8, d |
| 3 | 7.3, br d | 113.4, d | 6.8, d(7) | 101.1, d | 6.5, br d | 101.1, d |
| 3a | 148.8, s | 148.7, s | 148.7, s | |||
| 5 | 7.5, d(7.6) | 128.9, d | 7.6, br d | 130.1, d | 7.3, br d | 147.7, d |
| 6 | 7.3, br d | 125.2, d | 7.1, d(6) | 113.5, d | 7.3, br d | 113.5, d |
| 6a | 134.9, s | 136.4, s | 136.2, s | |||
| 7 | 6.7, s | 101.3, d | 6.8, s | 100.1, d | 6.8, s | 100.1, d |
| 8 | 157.2, s | 157.3, s | 157.0, s | |||
| 9 | 134.3, s | 134.8, s | 134.6, s | |||
| 9a | 126.5, s | 123.9, s | 123.8, s | |||
| 9b | 117.7, s | 117.6, s | 117.5, s | |||
| NCH3 | 3.7, s | 44.7, q | 3.9, s | 45.6, q | 3.7, s | 45.5, q |
| OCH3 | 3.9, s | 56.1, q | 3.9, s | 56.9, q | 3.7, s | 57.0, q |
| 1′ | 4.0, s | 168.3, s | 164.4, s | 165.0, s | ||
| 2′ | 46.4, t | 127.8, s | 127.9, s | |||
| 3′ | 7.3, br d | 136.3, s | 8.2, d(8) | 130.9, d | 7.8, br d | 127.7, d |
| 4′ | 7.2, br t | 128.5, d | 7.6, d(8) | 129.1, d | 7.4, br t | 128.9, d |
| 5′ | 7.1, t(6.8) | 128.7, d | 144.0, s | 7.4, br d | 135.2, d | |
| 6′ | 7.2, br t | 127.7, d | 7.6, d(8) | 129.1, d | 138.9, s | |
| 7′ | 7.3, br d | 128.7, d | 8.2 d(8) | 130.9, d | 7.8, s | 131.0, d |
| 8′ | 128.4, d | 4.7, s | 45.2, t | 2.3, s | 21.2, q | |
| 9′ | Cl | |||||
Measured in CDCl3 at 400 MHz for 1H and 100 MHz for 13C, respectively. J values in Hz.
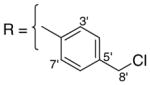
3.1.8. 9-O-4-chloromethylbenzoyl isoaaptamine (10)
Dark yellow amorphous solid; 1H and 13C NMR (CDCl3) data see Table 7; ESI-MS m/z 381 (M+, 100). High resolution EI-MS calculated for C21H17N2O3Cl (M+) m/z 381.1006, observed m/z 381.1000.
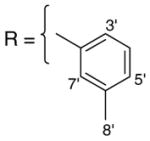
3.1.9. 9-O-3-methylbenzoylisoaaptamine (11)
Brown amorphous solid; 1H and 13C NMR (CDCl3) data see Table 7; ESI-MS m/z 347 (M+, 100). High resolution EI-MS calculated for C21H18N2O3 (M+) m/z 347.1396, observed m/z 347.1391.
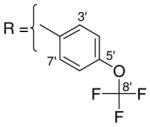
3.1.10. 9-O-4-trifluoromethoxybenzoylisoaaptamine (12)
Yellowish brown amorphous solid; 1H and 13C NMR (CDCl3) data see Table 8; ESI-MS m/z 417 (M+, 100). High resolution EI-MS calculated for C21H15N2O4F3 (M+) m/z 417.1062, observed m/z 417.1069.
Table 8.
1H and 13C NMR spectral data for compounds 12, 13, and 14
| Position | 12
|
13
|
14
|
|||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 2 | 7.2, br d | 119.8, d | 7.0, d(5.6) | 127.8, d | 7.0, d(7.2) | 131.8, d |
| 3 | 6.8, br d | 100.6, d | 6.8, d(5.6) | 103.5, d | 6.8, br d | 113.4, d |
| 3a | 136.6, s | 150.8, s | 150.3, s | |||
| 5 | 7.5, br d | 131.6, d | 8.0, br d | 144.4, d | 7.5, d(6.8) | 147.1, d |
| 6 | 7.5, br d | 113.5, d | 7.2, d(6.8) | 113.4, d | 7.4, br d | 127.8, d |
| 6a | 126.3, s | 136.8, s | 136.1, s | |||
| 7 | 6.8, s | 100.6, d | 6.6, s | 98.6, d | 6.8, s | 100.7, d |
| 8 | 153.7, s | 156.2, s | 156.9, s | |||
| 9 | 134.9, s | 134.9, s | 134.5, s | |||
| 9a | 118.9, s | 123.0, s | 123.6, s | |||
| 9b | 117.9, s | 118.8, s | 117.5, s | |||
| NCH3 | 3.9, s | 45.5, q | 3.7, s | 44.7, q | 3.9, s | 45.3, q |
| OCH3 | 3.9, s | 56.8, q | 3.8, s | 56.4, q | 3.9, s | 56.8, q |
| 1′ | 157.1, s | 165.2, s | 164.9, s | |||
| 2′ | 123.7, s | 125.8, s | 125.4, s | |||
| 3′ | 8.2, d(6.8) | 132.7, d | 8.1, d(8) | 130.6, d | 8.1, d(7.6) | 130.6, d |
| 4′ | 7.4, d(7.2) | 120.7, d | 7.3, d(8) | 129.0, d | 7.3, br d | 129.0, d |
| 5′ | 163.8, s | 150.1, s | 148.9, s | |||
| 6′ | 7.4, d(7.2) | 120.7, d | 7.3, d(8) | 129.0, d | 7.3, br d | 129.0, d |
| 7′ | 8.2, d(6.8) | 132.7, d | 8.1, d(8) | 130.6, d | 8.1, d(7.6) | 130.6, d |
| 8′ | 2.7, t(7.6) | 35.8, t | 2.7, t(8) | 36.0, t | ||
| 9′ | 1.6, m(7.6) | 33.2, t | 1.7, br m | 31.4, t | ||
| 10′ | 1.4, m(7.6) | 22.3, t | 1.3, br m | 30.7, t | ||
| 11′ | 0.9, t(7.6) | 13.9, q | 1.3, br m | 22.4, t | ||
| 12′ | 0.9, br t | 13.9, t | ||||
Measured in CDCl3 at 400 MHz for 1H and 100 MHz for 13C, respectively. J values in Hz.
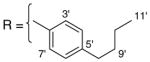
3.1.11. 9-O-4-butylbenzoylisoaaptamine (13)
Brown amorphous solid; 1H and 13C NMR (CDCl3) data see Table 8; ESI-MS m/z 389 (M+, 100). High resolution EI-MS calculated for C24H24N2O3 (M+) m/z 389.1865, observed m/z 389.1875.
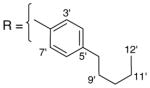
3.1.12. 9-O-4-pentylbenzoylisoaaptamine (14)
Brown amorphous solid; 1H and 13C NMR (CDCl3) data see Table 8; ESI-MS m/z 403 (M+, 100). High resolution EI-MS calculated for C25H26N2O3 (M+) m/z 403.2022, observed m/z 403.2009.
3.1.13. 9-O-3,4-difluorobenzoylisoaaptamine (15)
Greenish powder; 1H and 13C NMR (CDCl3) data see Table 9; ESI-MS m/z 369 (M+, 100). High resolution EI-MS calculated for C20H14N2O3F2 (M+) m/z 369.1051, observed m/z 369.1065.
Table 9.
1H and 13C NMR spectral data for compounds 15, 16, and 17
| Position | 15
|
16
|
17
|
|||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 2 | 7.1, br d | 131.4, d | 6.9, d(6) | 132.1, d | 7.0, br d | 131.5, d |
| 3 | 6.8, br d | 101.0, d | 6.8, d(6) | 101.2, d | 6.8, br d | 113.4, d |
| 3a | 149.2, s | 149.4, s | 149.2, s | |||
| 5 | 7.6, d(6) | 147.0, d | 8.0, d(7.6) | 146.4, d | 7.5, br d | 147.0, d |
| 6 | 7.5, br d | 118.3, d | 7.2, d(7.6) | 113.4, d | 7.5, br d | 124.8, d |
| 6a | 136.7, s | 135.5, s | 136.5, s | |||
| 7 | 6.8, s | 101.0, d | 6.7, s | 101.1, d | 6.8, s | 100.7, d |
| 8 | 151.6, s | 157.2, s | 158.5, s | |||
| 9 | 135.0, s | 135.0, s | 135.0, s | |||
| 9a | 123.8, s | 123.9, s | 124.0, s | |||
| 9b | 117.9, s | 118.1, s | 118.0, s | |||
| NCH3 | 3.9, s | 45.3, q | 3.8, s | 45.4, q | 3.9, s | 45.5, q |
| OCH3 | 3.9, s | 56.8, q | 3.9, s | 56.7, q | 3.9, s | 56.8, q |
| 1′ | 163.2, s | 164.9, s | 164.9, s | |||
| 2′ | 125.0, s | 125.3, s | 125.2, s | |||
| 3′ | 8.0, br d | 127.8, d | 8.1, d(8) | 130.5, d | 8.1, d(8) | 130.5, d |
| 4′ | 7.4, dd(8) | 113.6, d | 7.3, d(8) | 129.7, d | 7.6, d(8) | 126.0, d |
| 5′ | 157.2, s | 145.5, s | 157.3, s | |||
| 6′ | 149.0, s | 7.3, d(8) | 129.7, d | 7.6, d(8) | 126.0, d | |
| 7′ | 8.0, br d | 118.1, d | 8.1, d(8) | 130.5, d | 8.1, d(8) | 130.5, d |
| 8′ | 2.5, s | 21.8, q | 35.3, s | |||
| 9′ | 1.4, s | 31.0, q | ||||
| 10′ | 1.4, s | 31.0, q | ||||
| 11′ | 1.4, s | 31.0, q | ||||
Measured in CDCl3 at 400 MHz for 1H and 100 MHz for 13C, respectively. J values in Hz.
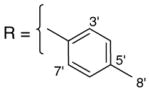
3.1.14. 9-O-4-methylbenzoylisoaaptamine (16)
Brown amorphous solid; 1H and 13C NMR (CDCl3) data see Table 9; ESI-MS m/z 347 (M+, 100). High resolution EI-MS calculated for C21H18N2O3 (M+) m/z 347.1396, observed m/z 347.1378.
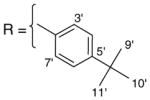
3.1.15
9-O-4-tert-butylbenzoylisoaaptamine (17). brown amorphous solid; 1H and 13C NMR (CDCl3) data see Table 9; ESI-MS m/z 389 (M+, 100). High resolution EI-MS calculated for C24H24N2O3 (M+) m/z 389.1865, observed m/z 389.1877.
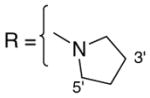
3.1.16. 9-O-1-(pyrrolidine)carbonylisoaaptamine (18)
Pale orange oil; 1H and 13C NMR (CDCl3) data see Table 10; ESI-MS m/z 326 (M+, 100). High resolution EI-MS calculated for C18H19N3O3 (M+) m/z 326.1505, observed m/z 326.1501.
Table 10.
1H and 13C NMR spectral data for compounds 18, 19, and 20
| Position | 18
|
19
|
20
|
|||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 2 | 6.9, d(6.8) | 130.0, d | 7.4, d(7.2) | 130.0, d | 7.8, d(6.4) | 131.7, d |
| 3 | 6.7, d(6.8) | 101.2, d | 7.1, d(7.2) | 113.3, d | 6.8, d(6.4) | 113.6, d |
| 3a | 148.5, s | 148.8, s | 150.5, s | |||
| 5 | 7.6, d(7.2) | 147.6, d | 7.5, d(6.8) | 147.2, d | 7.7, d(7.6) | 144.7, d |
| 6 | 7.3, br d | 113.3, d | 6.8, br d | 129.6, d | 7.0, d(7.6) | 129.9, d |
| 6a | 135.6, s | 136.0, s | 135.0, s | |||
| 7 | 6.8, s | 99.6, d | 6.8, s | 100.6, d | 6.7, s | 99.1, d |
| 8 | 152.6, s | 156.7, s | 156.1, s | |||
| 9 | 134.8, s | 123.7, s | 132.1, s | |||
| 9a | 124.8, s | 134.3, s | 122.8, s | |||
| 9b | 117.4, s | 117.5, s | 117.6, s | |||
| NCH3 | 3.9. s | 45.5, q | 4.0, s | 45.2, q | 3.7, s | 44.8, q |
| OCH3 | 3.9, s | 57.0, q | 3.9, s | 56.7, q | 3.9, s | 56.6, q |
| 1′ | 158.0, s | 172.0, s | 163.7, s | |||
| 2′ | 3.5, br t | 46.9, t | 2.6, t(7.2) | 33.9, t | 137.2, s | |
| 3′ | 2.0, br m | 24.9, t | 1.8, br m | 24.6, t | 8.3, d(8) | 131.0, d |
| 4′ | 2.0, br m | 25.8, t | 1.3, br m | 29.0, t | 7.9, d(8) | 132.8, d |
| 5′ | 3.5, br t | 46.8, t | 1.3, br m | 29.2, t | 118.7, s | |
| 6′ | 1.3, br m | 29.7, t | 7.9, d(8) | 132.8, d | ||
| 7′ | 1.3, br m | 29.2, t | 8.3, d(8) | 131.0, d | ||
| 8′ | 2.0, br q | 27.1, t | 117.7, s | |||
| 9′ | 5.4, br q | 129.7, d | ||||
| 10′ | 5.4, br q | 129.8, d | ||||
| 11′ | 2.0, br q | 27.1, t | ||||
| 12′ | 1.3, br m | 29.2, t | ||||
| 13′ | 1.3, br m | 29.4, t | ||||
| 14′ | 1.3, br m | 29.6, t | ||||
| 15′ | 1.3, br m | 29.1, t | ||||
| 16′ | 1.3, br m | 31.8, t | ||||
| 17′ | 1.3, br m | 22.6, t | ||||
| 18′ | 0.9, br t | 14.1, q | ||||
Measured in CDCl3 at 400 MHz for 1H and 100 MHz for 13C, respectively. J values in Hz.
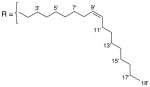
3.1.17. 9-O-Z-oleoylisoaaptamine (19)
Brown amorphous solid; 1H and 13C NMR (CDCl3) data see Table 10; ESI-MS m/z 493 (M+, 100). High resolution EIMS calculated for C31H44N2O3 (M+) m/z 493.3430, observed m/z 493.3412.
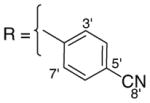
3.1.18. 9-O-4-cyanobenzoylisoaaptamine (20)
Brownish amorphous solid; 1H and 13C NMR (CDCl3) data see Table 10; ESI-MS m/z 358 (M+, 100). High resolution EI-MS calculated for C21H15N3O3 (M+) m/z 358.1192, observed m/z 358.1192.
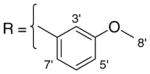
3.1.19. 9-O-3-methoxybenzoylisoaaptamine (21)
Brown amorphous solid; 1H and 13C NMR (CDCl3) data see Table 11; ESI-MS m/z 363 (M+, 100). High resolution EI-MS calculated for C21H18N2O4 (M+) m/z 363.1219, observed m/z 363.1224.
Table 11.
1H and 13C NMR spectral data for compound 21
| Position | 21
|
|
|---|---|---|
| δH | δC | |
| 2 | 7.1, br d | 131.4, d |
| 3 | 6.8, br d | 100.8, d |
| 3a | 148.9, s | |
| 5 | 7.8, d(7.6) | 147.1, d |
| 6 | 7.4, br d | 115.0, d |
| 6a | 136.4, s | |
| 7 | 6.8, s | 100.6, d |
| 8 | 157.1, s | |
| 9 | 129.2, s | |
| 9a | 123.8, s | |
| 9b | 117.7, s | |
| NCH3 | 3.9, s | 45.5, q |
| OCH3 | 3.9, s | 56.9, q |
| 1′ | 164.8, s | |
| 2′ | 134.8, s | |
| 3′ | 7.7, s | 113.4, d |
| 4′ | 159.9, s | |
| 5′ | 7.1, br d | 120.7, d |
| 6′ | 7.5, t(8.4) | 130.1, d |
| 7′ | 7.5, br d | 122.8, d |
| 8′ | 3.9, s | 55.6, q |
Measured in CDCl3 at 400 MHz for 1H and 100 MHz for 13C, respectively. J values in Hz.
Supplementary Material
Table 3.
Anti-Mtb, anti-HIV-1, anti-malarial, and anti-leishmania data
| Compound | Mycobacterium tuberculosis (H37Rv) MIC (μg/mL) | P. falciparum (D6 clone) IC50 (μg/mL) | P. falciparum (chloroquine-resistant W2 clone) IC50 (μg/mL) | Cytotoxicity (Vero) TC50 (μg/ml) | Anti-HIV-1
|
Leishmania donovani
|
||
|---|---|---|---|---|---|---|---|---|
| EC50 (μM) | EC90 (μM) | IC50 (μg/mL) | IC90 (μg/mL) | |||||
| 2 | — | 1.1 | 0.4 | — | 0.6 | — | 0.7 | 1.1 |
| 3 | >128 | 2.0 | 1.3 | NC | 1.3 | 8.0 | 1.7 | 2.7 |
| 4 | >128 | 0.3 | 1.1 | NC | 0.5 | 3.0 | 0.1 | 0.2 |
| 5 | >128 | 0.4 | 1.5 | NC | 10.9 | 33.5 | 1.3 | 2.5 |
| 6 | >128 | 1.8 | 3.8 | NC | 37.7 | 70.6 | 4.1 | 9.5 |
| 7 | >128 | 1.8 | 4.1 | NC | 2.1 | 10.5 | 1.4 | 2.5 |
| 8 | >128 | 2.9 | NA | NC | 45.9 | 95.4 | 6.8 | 12.0 |
| 9 | >128 | 3.3 | 3.7 | NC | 4.6 | 33.9 | 1.7 | 2.7 |
| 10 | >128 | 0.6 | 0.9 | NC | 4.0 | 25.2 | 1.6 | 2.7 |
| 11 | >128 | 0.4 | 0.8 | NC | 9.9 | 30.6 | 1.8 | 5.5 |
| 12 | >128 | 0.6 | 2.5 | NC | 2.3 | 16.3 | 1.8 | 5.0 |
| 13 | >128 | 0.3 | 1.2 | NC | 3.8 | 7.1 | 1.5 | 2.5 |
| 14 | >128 | 0.4 | 1.3 | NC | 18.8 | 66.4 | 1.4 | 2.6 |
| 15 | >128 | 0.4 | 1.1 | NC | 16.6 | 54.3 | 6.0 | 11.0 |
| 16 | >128 | 0.2 | 1.0 | NC | 9.2 | 29.1 | 1.6 | 2.6 |
| 17 | >128 | 0.2 | 1.0 | NC | 3.7 | 6.9 | 0.4 | 0.7 |
| 18 | >128 | 3.0 | NA | NC | >100 | >100 | 26.0 | 42.0 |
| 19 | >128 | 3.0 | 4.3 | NC | 52.8 | >100 | 6.2 | 12.0 |
| 20 | >128 | 0.8 | 1.5 | NC | 8.2 | 25.3 | 4.8 | 10.0 |
| 21 | 41.03 | 0.3 | 0.8 | NC | 33.7 | 60.1 | 4.3 | 9.5 |
| Pentamidine | — | — | — | — | — | — | 1.6 | 3.5 |
| Amphotericin B | — | — | — | — | — | — | 1.1 | 2.3 |
| Chlorquine | — | 0.0165 | 0.140 | — | — | — | — | — |
IC50 and IC90 are the sample concentrations that kill 50% and 90% cells compared to the solvent controls. For malarial assays, screens were run at 4760, 1587, and 528.8 ng/mL. For Leishmania assays, screens were run at 50, 12.5, and 3.125 μg/mL NA, not active; NT, not tested: NC, not cytotoxic (4.7 μg/mL).
Acknowledgments
We are indebted to S. Wahyuono and A. Mursyidi from Gadjah Mada University, Indonesia, for assistance with sample collection, J. Trott for in vitro malaria assays, B. Tekwani for leishmania assays, S. Franzblau and F. Zhang for Mtb assays, and P. Tharnish for anti-HIV-1 assays. We are also indebted to S. Sanders and B. Smiley, from the National Center for Natural Products Research, for bioassays. We are grateful to A. Holley, A. Murphy, and J. Fiechtl for their help in table preparation and J. Mustafa for valuable suggestions and discussions. This work was supported by NIH (R01A136596, KO2AI01502), Pharma-Mar, R.F.S. is supported by the Emory University Center for AIDS Research and Department of Veterans Affairs. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program (C06 RR-14-503-01) from the National Center for Research Resources (NIH).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bmc.2006.08.042.
References and notes
- 1.Nakamura H, Kobayash J, Ohizumi Y, Hirata Y. Tetrahedron Lett. 1982;23:5555–5558. [Google Scholar]
- 2.Ohizumi Y, Kajiwara A, Nakamura H, Kobayashi J. J Pharm Pharmacol. 1984;36:785–786. doi: 10.1111/j.2042-7158.1984.tb04876.x. [DOI] [PubMed] [Google Scholar]
- 3.Fedoreev S, Prokofeva N, Denisenko V, Rebachuk N. Khim Farm Zh. 1988;22:943. [Google Scholar]
- 4.Kashman Y, Rudi A, Hirsh S, Isaacs S, Green D, Blasberger D, Carmely S. New J Chem. 1990;14:729–740. [Google Scholar]
- 5.Shen Y, Chein C, Hsieh P, Duh C. Taiwan Shuichan Xuehuikan. 1997;24:117. [Google Scholar]
- 6.Pettit GR, Hoffmann H, McNulty J, Higgs KC, Murphy A, Molloy DJ, Herald DL, Williams MD, Pettit RK, Doubek DL, Hooper JNA, Albright L, Schmidt JM, Chapuis J, Tackett LP. J Nat Prod. 2004;67(3):506–509. doi: 10.1021/np0204592. [DOI] [PubMed] [Google Scholar]
- 7.Patil A, Westley J, Mattern M, Freyer A, Hofmann G. WO 95/0584. PCT Int Appl. 1995 Mar;
- 8.Pettit GR, Hoffmann H, Herald DL, McNulty J, Murphy A, Higgs KC, Hamel E, Lewin NE, Pearce LV, Blumberg PM, Pettit RK, Knight JC. J Org Chem. 2004;69:2251–2256. doi: 10.1021/jo0300486. [DOI] [PubMed] [Google Scholar]
- 9.Nitiss J, Zhou J, Rose A, Hsiung Y, Gale K, Osheroff N. Biochemistry. 1998;37:3078–3085. doi: 10.1021/bi9723257. [DOI] [PubMed] [Google Scholar]
- 10.O’Reilly S, Baker S, Sartorius S, Rowinsky E, Finizio M, Lubiniecki G, Grochow L, Gray J, Pieniaszek H, Donehower R. Ann Oncol. 1998;9:101–104. doi: 10.1023/a:1008260515869. [DOI] [PubMed] [Google Scholar]
- 11.Bousquet P, Brana M, Conlon D, Fitzgerald K, Perron D, Cocchiaro C, Miller R, Moran M, George J, Qian X. Cancer Res. 1995;55:1176–1180. [PubMed] [Google Scholar]
- 12.Atwell G, Cain B, Baguley B, Finlay G, Denny W. J Med Chem. 1984;27:1481–1485. doi: 10.1021/jm00377a017. [DOI] [PubMed] [Google Scholar]
- 13.Denny W, Wakelin L. Cancer Res. 1986;46:1719–1725. [PubMed] [Google Scholar]
- 14.Finlay G, Riou J, Baguley B. Eur J Cancer. 1996;32:708–714. doi: 10.1016/0959-8049(95)00604-4. [DOI] [PubMed] [Google Scholar]
- 15.Hutchins R, Crenshaw J, Graves D, Denny W. Biochemistry. 2003;42:13754–13761. doi: 10.1021/bi035434w. [DOI] [PubMed] [Google Scholar]
- 16.Diers JA, Pennaka HK, Peng J, Bowling JJ, Duke SO, Hamann MT. J Nat Prod. 2004;67:2117–2120. doi: 10.1021/np040097t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pettit GR, Hoffmann H, Herald DL, Blumberg PM, Hamel E, Schmidt JM, Chang Y, Pettit RK, Lewin NE, Pearce LV. J Med Chem. 2004;47(7):1775–1782. doi: 10.1021/jm030070r. [DOI] [PubMed] [Google Scholar]
- 18.Sugino E, Choshi T, Hibino S. Heterocycles. 1999;50:543. [Google Scholar]
- 19.Walz A, Sundberg R. J Org Chem. 2000;65:8001. doi: 10.1021/jo001080s. [DOI] [PubMed] [Google Scholar]
- 20.Shen YC, Lin TT, Sheu JH, Duh CY. J Nat Prod. 1999;62:1264–1267. doi: 10.1021/np990156g. [DOI] [PubMed] [Google Scholar]
- 21.Gochfeld D, El Sayed K, Yousaf M, Hu J, Bartyzel P, Dunbar D, Wilkins S, Zjawiony J, Schinazi R, Wirtz S, Tharnish P, Hamann M. Mini Rev Med Chem. 2003;3:401–424. doi: 10.2174/1389557033487962. [DOI] [PubMed] [Google Scholar]
- 22.Wang T, Zhang Z, Wallace O, Deshpande M, Fang H, Yang Z, Zadjura L, Tweedie D, Huang S, Zhao F, Ranadive S, Robinson B, Gong Y, Ricarrdi K, Spicer T, Deminie C, Rose R, Wang H, Blair W, Shi P, Lin P, Colonno R, Meanwell N. J Med Chem. 2003;46:4236–4423. doi: 10.1021/jm034082o. [DOI] [PubMed] [Google Scholar]
- 23.Newman DJ, Cragg GM, Snader KM. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.